Back to Journals » Journal of Hepatocellular Carcinoma » Volume 9
Tumor Response and Nomogram-Based Prognostic Stratification for Hepatocellular Carcinoma After Drug-Eluting Beads Transarterial Chemoembolization
Authors Ji K, Zhu H, Wu W, Li X, Zhan P, Shi Y, Sun J, Li Z
Received 10 February 2022
Accepted for publication 25 May 2022
Published 7 June 2022 Volume 2022:9 Pages 537—551
DOI https://doi.org/10.2147/JHC.S360421
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Ahmed Kaseb
Kun Ji,1,* Hanlong Zhu,2,* Wei Wu,3,* Xin Li,4 Pengchao Zhan,4 Yang Shi,4 Junhui Sun,1 Zhen Li4
1Hepatobiliary and Pancreatic Interventional Treatment Center, Division of Hepatobiliary and Pancreatic Surgery, the First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, 310000, People’s Republic of China; 2Department of Gastroenterology and Hepatology, Jinling Hospital, Medical School of Nanjing University, Nanjing, 210002, People’s Republic of China; 3Department of Medical Oncology, the First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, 310000, People’s Republic of China; 4Department of Interventional Radiology, the First Affiliated Hospital of Zhengzhou University, Zhengzhou, 450052, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Zhen Li, Department of Interventional Radiology, the First Affiliated Hospital of Zhengzhou University, No. 1 East Jian She Road, Zhengzhou, 450052, People’s Republic of China, Tel +86-15837192255, Email [email protected] Junhui Sun, Hepatobiliary and Pancreatic Interventional Treatment Center, Division of Hepatobiliary and Pancreatic Surgery, the First Affiliated Hospital, Zhejiang University School of Medicine, No. 79 Qingchun Road, Hangzhou, 310000, People’s Republic of China, Tel +86-13575725162, Email [email protected]
Purpose: To explore the tumor response and propose a nomogram-based prognostic stratification for hepatocellular carcinoma (HCC) after drug-eluting beads transarterial chemoembolization (DEB-TACE).
Patients and Methods: From the database of two centers, patients who received DEB-TACE as an initial treatment were enrolled and divided into the training and validation sets. The tumor response after DEB-TACE was estimated according to the Modified Response Evaluation Criteria in Solid Tumors. Using the independent survival predictors in the training set, a nomogram was constructed and validated internally and externally by measuring concordance index (C-index) and calibration. A prognostic stratification based on the nomogram was established.
Results: A total of 335 patients met the inclusion criteria for the study. Alkaline phosphatase level, tumor maximum diameter, tumor capsule and portal vein invasion were interrelated with the achievement of complete release after DEB-TACE. Alpha-fetoprotein level, Child-Pugh class, tumor maximum diameter, tumor number, tumor extent and portal vein invasion were integrated into the nomogram. The nomogram demonstrated good calibration and discrimination, with C-indexes of 0.735 and 0.854 and higher area under the curve (AUC) than BCLC and CNLC staging systems in the internal and external validation sets. The prognostic stratification classified patients into three different risk groups, which had significant differences in survival, complete release and objective response rate between any two groups (P < 0.05).
Conclusion: The nomogram-based prognostic stratification has a good distinction and may help to identify the patients benefiting from DEB-TACE.
Keywords: hepatocellular carcinoma, drug-eluting beads, nomogram, prediction, transarterial chemoembolization
Introduction
Hepatocellular carcinoma (HCC) is one of the most common malignancies and the third leading cause of cancer-related death in the world.1 Due to the nonspecific symptoms, most HCCs were at an advanced stage when diagnosed and thus lost the opportunity for surgery and liver transplantation.2 Guidelines have recommended transcatheter arterial chemoembolization (TACE) as a standard therapeutic method for these patients.3 In recent years, drug-eluting beads TACE (DEB-TACE) has gained growing applications in clinical practice with improved drug release and is less toxic than conventional TACE (cTACE).4 Studies have reported the superior efficacy of DEB-TACE in comparison with cTACE for HCC treatment.5,6
However, HCC responds differently to DEB-TACE and thus differs in survival, due to the tumor heterogeneity and considerably various physical conditions, liver function and tumor staging of patients. It is still controversial as to which patients are the best candidates for DEB-TACE. Currently, several commonly used staging systems, such as Barcelona Clinic Liver Cancer (BCLC),7 China liver cancer staging system (CNLC)8 et al, have been applied to estimate the survival of HCC patients. Unfortunately, they are not designed exclusively for HCC following DEB-TACE and fail to provide the specific survival time, consequently with limited ability in identifying the subset of patients who benefit from DEB-TACE. It is proposed that nomogram has advantages over the traditional staging systems, and is even regarded as a new prognostic standard for some tumors.9,10 Therefore, monogram may be an effective and reliable tool to help achieve the aforementioned goal. Nevertheless, up to now, no nomogram has been established for survival prediction of patients with HCC after DEB-TACE.
In this study, we analyzed the preoperative clinical and biological characteristics of HCC patients undergoing DEB-TACE as an initial treatment to develop and validate a nomogram for survival prediction. Furthermore, a prognostic stratification based on nomogram was constructed to help clinicians identify a subset of patients benefiting from DEB-TACE and guide the tailored treatment and follow-up strategies.
Patients and Methods
Patients
Patients with unresectable HCC who received DEB-ACE as an initial therapy at the First Affiliated Hospital of Zhengzhou University from June 2016 to May 2020 were retrospectively enrolled to constitute the training and internal validation sets. Between January 2018 and December 2020, an independent subset of HCC patients ineligible for excision who underwent DEB-TACE as an initial treatment at the First Affiliated Hospital, Zhejiang University School of Medicine formed the external validation set.
The diagnosis of HCC followed clinical guidelines11 and included two imaging techniques showing typical features of HCC or positive findings of one imaging technique together with an alpha fetoprotein (AFP) level of >400 ng/mL or cytological/histological diagnosis of HCC.
The inclusion criteria were as follows: (1) age between 18 and 85 years; (2) Child-Pugh A or B liver function; (3) Eastern Cooperative Oncology Group (ECOG) performance scores 0–2; and (4) HCC without previous treatment. The exclusion criteria were as follows: (1) severe coagulopathy (prothrombin activity <40% or a platelet count of <40,000/mm3); (2) extrahepatic metastasis; (3) presence of hepatic vein or inferior cava vein invasion; and (4) incomplete data on medical records or follow-up.
This was a retrospective study conducted in two centers, approved by the Ethics Committees of the First Affiliated Hospital, Zhejiang University School of Medicine and the First Affiliated Hospital of Zhengzhou University, and in accordance with the Declaration of Helsinki. Informed consents were obtained from all individual participants included in the study.
Data Collection
Each patient underwent a complete medical assessment and collection at admission, including demographics, laboratory data and tumor characteristics. Laboratory data, including serology for hepatitis virus, liver function tests and AFP, were captured. The Child-Pugh class, neutrophil-to-lymphocyte ratio (NLR), aspartate aminotransferase (AST)-to-lymphocyte ratio index (ALRI) and AST-to-platelet ratio index (APRI) were calculated from the clinical and laboratory information. Tumor characteristics captured by CT/MRI included the tumor number, diameter of the largest lesion, tumor capsule, extent of tumor and portal vein invasion. Tumor staging was performed for each patient using the above data in light of the BCLC and CNLN staging criteria.
DEB-TACE Procedure
Using a transfemoral approach, a 5-F RH catheter (Cook) was used to catheterize the celiac trunk or anatomic variant to gain access to the hepatic arteries, which was achieved with a 2.7-F Progreat microcatheter (Terumo). Diagnostic angiographic runs were obtained on the celiac trunk and proper hepatic and right and left hepatic arteries to define tumor arterial supply. Through the microcatheter, a vial of 100–300 or 300–500μm in size CalliSpheres microspheres (Jiangsu Hengrui Callisyn Biomedical Co., Ltd., Suzhou, China) loaded with 60 mg of pirarubicin or epirubicin were injected to occlude each tumor artery. If tumor staining was still present on repeated imaging after all DEB, 560–710 μm gelatin sponge particles or polyvinyl alcohol (ALICON Pharm SCI & TECH) were added until near-stasis was achieved, defined as the disappearance of tumor stain on angiography. The procedures were conducted by two interventional radiologists with more than 15 years of experience.
Tumor Response and Follow-Up
The tumor response was estimated through the difference between pre- and 4 weeks post-operative contrast-enhanced CT/MRI based on the Modified Response Evaluation Criteria in Solid Tumors (mRECIST),12 which was divided into complete release (CR), partial release (PR), stable disease (SD) and progressive disease (PD). Objective response rate (ORR) was (CR+PR)/(CR+PR+SD+PD)×100%; disease control rate (DCR) was (CR+PR+SD)/(CR+PR+SD+PD)×100%. In patients undergoing multiple TACE procedures, we used the best response achieved during the course of treatment to estimate the clinical benefit of the TACE. Repeated DEB-TACE was performed if the tumors showed residual enhancement in contrast-enhanced CT/MRI, until one of the following endpoints was reached: (1) technical impossibility to catheterize the arteries of tumor supply; (2) occurrence of contraindications to TACE; or (3) surgical resection or ablation of tumor. If DEB-TACE could not be continued to work for liver cancer, the patients were recommended to receive treatments such as oral sorafenib, local ablation, radiotherapy, chemotherapy or best supportive treatment, either alone or in combination, based on the residual hepatic functional reserve and general condition of the patient. The patients were followed up via dynamic enhanced CT or MRI 4 weeks post-operatively and once every 3 months thereafter. Overall survival (OS) and survival status were recorded. OS was defined as the interval between the date of initial DEB-TACE and patients’ death or last follow-up. Progression-free survival (PFS) was defined as the time from the date of initial DEB-TACE to disease progression or death from any cause. The follow-up was administered until death, loss to follow-up, or the cutoff date (31/12/2020 for both training and internal validation sets, and 30/9/2021 for the external validation set).
Nomogram Construction and Validation
Univariate and multivariate logistic regressions were applied to analyze the independent factors associated with the achievement of CR at 1 month after DEB-TACE. Then, among the patients from the First Affiliated Hospital of Zhengzhou University, 70% of them were randomly divided into the training set for nomogram construction, while the rest constituted the internal validation set for internal verification. The univariate and multivariate Cox regression analyses were implemented to identify the independent survival factors. A nomogram was developed to predict the probability of 6-month, 1-year and 2-year OS by integrating the independent prognostic factors.
The discrimination and calibration of the nomogram were validated in both internal and external validation sets. The concordance index (C-index) is defined as the proportion of all patient pairs whose predictions are consistent with the actual results.13 The calibration of the nomogram was appraised by a calibration curve that compares nomogram-predicted and actually observed estimates of survival probability. Moreover, the area under the receiver operating characteristic curve (AUC) was employed to compare the prediction accuracy of the nomogram with BCLC and CNLN staging systems. In addition, a nomogram-based prognostic stratification was established to separate the patients into low -, medium -, and high-risk groups. Kaplan–Meier (K-M) curves were applied to further compare the survival differences among the three risk groups. Furthermore, the relationship between tumor response and prognostic stratification was explored.
Statistical Analysis
Statistical analyses were undertaken employing the SPSS software (version 21, IBM Corporation, USA) and the programming language R (version 4.0.3) for Windows. Categorical variables were categorized on account of clinical findings. The Student’s t-test and the Chi-square test, for the continuous and categorical variables, respectively, were utilized to compare their differences. The variables with a P-value below 0.05 in univariate analysis were selected for entering multivariate regression analysis. Independent prognostic factors were identified through stepwise selection in a Cox regression model. Survival curves were depicted using the Kaplan–Meier method and compared using the Log rank test. The C-index, AUC, nomogram, calibration curves and K-M curves were generated in R with packages “rms”, “survival”, “foreign”, “timeROC” and “shiny”. The total score of each patient in the three sets was calculated based on the nomogram. Furthermore, we divided whole patients into three subgroups (low-risk, medium-risk and high-risk) according to the optimal survival cutoff points of the total score using the X-tile 3.6.1 software (Yale University, USA). Statistical significance was set as P < 0.05 in a two-sided test.
Results
Patient Characteristics
Among the 335 HCC patients who received DEB-TACE as an initial treatment, there were 189 cases in the training set, 79 cases in the internal validation set and 67 cases in the external validation set. The tumor capsule and portal vein thrombosis were present in 13.4% and 34.6% of the patients, respectively. More details on patient characteristics are shown in Table 1.
 |
Table 1 Clinical and Biological Characteristics of Patients with Hepatocellular Carcinoma on Admission |
Treatment Results and Adverse Reactions
The size of DEB was 100–300μm in 57.6% of patients and 300–500μm in 42.4% of patients. The main adverse reaction was post-embolism syndrome and 25 (7.5%) patients suffered from liver abscess or bile leakage, which were improved after symptomatic treatment. A history of biliary surgery for gallstone may account for 1 (0.3%) patient suffering from bile leakage, which was improved after administration of percutaneous drainage and antibiotics at last. There were no DEB-TACE-associated deaths.
Independent Factors Associated with CR and OS
Eighty (23.8%) cases were CR, 200 (59.7%) were cases PR, 27 (8.0%) were SD and 28 (8.3%) were PD, with ORR of 83.5% and DCR of 91.6%. Univariate and multivariate logistic regression analyses displayed that ALP level, tumor maximum diameter, tumor capsule and portal vein invasion were independent factors interrelated with the achievement of CR (Table 2). The median OS and PFS were 37.2 and 26.5 months for all patients, respectively. In the training set, the univariate and multivariate Cox regression revealed that AFP, Child-Pugh class, tumor maximum diameter, tumor number, tumor extent and portal vein invasion were independent factors for OS after DEB-TACE in HCC patients (Table 3).
 |
Table 2 Logistic Analysis of Achievement of Complete Release of Tumor After DEB-TACE |
 |
Table 3 Cox Analysis of Prognostic Factors in the Primary Set |
Development and Validation of the Nomogram
Based on independent prognostic factors, the nomogram was built to predict the 6-month, 1-year and 2-year survival rates after DEB-TACE (Figure 1). Each category of the prognostic variables is assigned a score on the Points scale. The sum of these scores is located on the total points scale and a line is drawn downward to determine the specific probability of 6-month, 1-year and 2-year survival rates. The score assignment of the variables and the corresponding survival rate was summarized in Tables 4 and 5. To facilitate the use of the nomogram, a corresponding web calculator was provided (https://jikun.shinyapps.io/deb_tace) (Figure 2).
 |
Table 4 Score Assignment for Variables Included in the Nomogram |
 |
Table 5 Total Points and Survival Rate in the Nomogram |
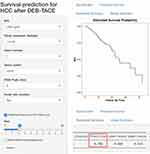 |
Figure 2 Layout of an online version of the nomogram (https://jikun.shinyapps.io/deb_tace). |
The calibration chart demonstrated a high consistency between the predicted and actual survival of the nomogram in two validation sets (Figure 3A and B). The C-indexes of nomogram were 0.735 (95% CI: 0.649–0.821) in the internal validation set and 0.854 (95% CI: 0.740–0.968) in the external validation set. Moreover, the AUCs of the nomogram of two validation sets were higher than those of the CNLC and BCLC staging systems (P< 0.05) (Figure 3C and D).
Prognostic Stratification
Based on the total scores generated from the nomogram, we subsequently developed a prognostic stratification to divide the patients into three subgroups: low-risk group (0–158 points), medium-risk group (159–272 points) and high-risk group (273–491 points), with the median survival time of >48.0, 22.1 and 8.3 months, separately. As shown in the K-M curves, the risk groups presented a clear stratification of prognosis with statistically significant survival difference among the three risk groups (P < 0.001) (Figure 4A). The higher risk was associated with a worst prognosis.
The median survival rate of patients with CR, PR, SD and PD was >48.0, 29.1, 14.0 and 5.8 months, respectively, and statistical differences were discovered between any both (P < 0.05) (Figure 4B). There were 57 (35.0%), 20 (17.9%) and 3 (5.0%) cases of CR in the low-, medium- and high-risk groups, respectively (Figure 5A). There were significant differences in CR, ORR and DCR among the three risk groups (Figure 5B).
Discussion
With the improved drug delivery into the tumor, DEB-TACE has gained increasing application for unresectable HCC. A growing body of evidence suggests a significant advantage of DEB-TACE over cTACE in more extensive tumor necrosis and fewer adverse events.14 The effectiveness and benefit of DEB-TACE varies greatly among patients with HCC due to the heterogeneity of tumor burden and liver function.5 Several attempts have been developed to subdivide the HCC cohort to predict the benefits of TACE using different scoring systems, such as the OKUDA score,15 the MESH score,16 the mHAP-II score,17 as well as the SNACOR risk prediction model.18 However, some of them were validated in western cohorts with alcohol abuse and hepatitis C virus (HCV) infection contributing most of the aetiology; others were not designed exclusively for HCC following DEB-TACE and failed to provide a specific survival time.
Here, we proposed a nomogram for individual survival prediction and further nomogram-based prognostic stratification to identify who might benefit from DEB-TACE for patients with HCC. It is worth noting that the prognostic stratification could be applied to most patients with HCC, not just those treated with DEB-TACE, for risk assessment to identify those suitable for DEB-TACE, as well as to formulate personalized therapeutic strategies for other populations. Since we aimed to develop the pre-treatment nomogram, indicators during DEB-TACE such as the size and the type of loaded drug of DEB were not taken into account in the process of nomogram construction. Furthermore, we found the two factors have no remarkable effect on survival (both p > 0.05). As the nomogram integrated factors including AFP, Child-Pugh class, tumor maximum diameter, tumor number, tumor extent and portal vein invasion, it comprehensively embodied the effect of tumor marker, liver function and tumor biological behavior on the prognosis of HCC patients treated with DEB-TACE. To facilitate clinical use, a web calculator of nomogram (https://jikun.shinyapps.io/deb_tace/) was offered to obtain the survival information only by clicking on your phone or computer, which not only observably improves calculation speed but also avoids the error caused by manual measurement.
Both the Chinese University Prognostic Index (CUPI)19 and Cancer of the Liver Italian Program (CLIP)20 incorporate the preoperative AFP level as a momentous prognostic indicator. As a specific biomarker for HCC, on behalf of tumor burden and invasiveness,21 serum AFP is reported to be associated with survival of HCC patients after TACE,22,23 but the mechanism is still unclear. Scholars considered that increased AFP level might disable the function of antigen-presenting cells and dendritic cells to exert influence on the prognosis of HCC patients.24,25
Due to the biological behavior of HCC, the portal vein system is prone to be invaded to shape portal vein tumor thrombus (PVTT) with an incidence of 44.0%~62.2%.26 Patients with PVTT have only an average survival of 2.7–4.0 months, attributing to consequent portal hypertension, intrahepatic and distant metastasis. According to the BCLC staging system, systemic therapy is recommended for patients with HCC and portal vein invasion. In terms of a potential risk of hepatic infarction or deterioration of liver function, the presence of PVTT was regarded as a relative contraindication of TACE, while evidence exists in support of TACE in this subset of patients. The recently introduced Hong Kong Liver Cancer (HKLC) staging system proposed a more aggressive therapeutic algorithm by recommending TACE in patients with vascular invasion.27 There have been several reports that showed the safety and efficacy of TACE (including cTACE and DEB-TACE) for patients with HCC and PVTT from different countries.28–30 Moreover, there was no patient with complete embolism of the main portal vein with cancer embolus in our study, and it was found that patients with PVTT did not have more complications than those without PVTT. The outcome of HCC patients was also related to the tumor size, tumor extent and Child-Pugh class,31 which have been included in several staging systems.7,15,21,32
The proposed nomogram exhibited an excellent and reliable survival predictive ability in both internal and external validation sets. In terms of calibration, the predictive survival of the nomogram has high consistency with the actual survival; with regard to differentiation, the C-indexes and AUCs were significantly higher than those of BCLC and CNLC staging systems. There is a distinct impact of each variable on the prognosis of HCC patients, but conventional staging systems assign equal weight to each variable improperly.33 On the contrary, our nomogram distributed an unequal score to each factor depending on how much influence it has on prognosis. That is the reason why the nomogram performed well in predicting survival in patients with HCC following DEB-TACE.
The prognostic stratification model based on the nomogram could divide patients into low-, medium- and high-risk groups, which may be helpful to screen out the patients who are suitable for DEB-TACE, as well as to formulate personalized therapeutic strategies depending on the different risk groups. For instance, if a patient has a total score less than 117 at low risk, he/she is inclined to be an appropriate candidate for DEB-TACE; if the total score varies from 118 to 254 at medium risk, radiofrequency ablation or molecular targeted agents might synergize with DEB-TACE to improve anti-tumor control; if the total score is more than 255 at high risk, he/she may be more suitable for radiotherapy or targeted drug rather than DEB-TACE. In addition, this prognostic stratification model also corresponded to the tumor response after DEB-TACE. As the prognostic risk decreased, HCCs gain more sensitivity to DEB-TACE and therefore are more likely to achieve CR, which demonstrated the reliability of the nomogram from another perspective.
On the other hand, the prognostic factors play an important role in the design, conduction and analysis of the prospective clinical trial, and their improper distribution among the cohorts may bring about bias in therapeutic benefit.34 As a result, when there is a prospective Phase II or III clinical trial regarding DEB-TACE for HCC patients, the nomogram could be applied in advance to ensure the consistent distribution of the prognostic factors among the cohorts to reduce survival bias resulting from differences in baseline characteristics.34 Besides, an individualized follow-up plan could also be generated according to the different risk groups. For patients in the high-risk group, their follow-up interval should be shortened and adjusted on the basis of tumor progression; If patients are in the low-risk group, the follow-up interval could be appropriately prolonged to reduce their financial and psychological burdens.
Some limitations exist in our study. The retrospective nature of the study may inevitably result in selection bias. Another limitation is the lack of predictive capability over the last 3 years and beyond, which may be attributed to the fact that DEB-TACE has not been employed in the clinic for a long time. Finally, improved model accuracy often requires greater complexity. There is a common problem that the balance between comprehensiveness and comprehensibility is not easy to achieve during modeling for nomogram.35 Given this, we only selected clinically important and practical variables, which led to uncertainty of the nomogram. Therefore, we provided 95% CI for the C-index to determine the degree of uncertainty and validated the nomogram using a second independent cohort of patients from another medical center as the external validation set.
Conclusions
Taken together, we developed and validated a reliable nomogram with superior survival prediction to stratify prognosis in patients with HCC after DEB-TACE. The nomogram-based prognostic stratification model might help clinicians identify patients who benefit most from DEB-TACE and guide the treatment and follow-up strategies.
Abbreviations
HCC, hepatocellular carcinoma; TACE, transcatheter arterial chemoembolization; DEB, drug-eluting beads; BCLC, Barcelona Clinic Liver Cancer; CNLC, China liver cancer staging; DEB-TACE, drug-eluting beads transarterial chemoembolization; ECOG, Eastern cooperative oncology group; ALRI, alanine aminotransferase-to-lymphocyte ratio index; ROC, receiver operating characteristic; AFP, alpha-fetoprotein; AUC, area under ROC curve; K-M, Kaplan–Meier curve.
Data Sharing Statement
The datasets used in this study are available from the corresponding authors on reasonable requests.
Author Contributions
All authors made a significant contribution to the work whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; agreed to submit to the current journal; and agreed to be accountable for all aspects of the work.
Funding
This study is an independent research supported by the National Natural Science Foundation of China (Grant No. U1904143), the National Science and Technology Major Project, Prevention and Treatment of Major Infectious Diseases such as AIDS and Viral Hepatitis (2018ZX10303502), and the Medical Science and Technology Program of Henan Province (SBGJ202102099).
Disclosure
None of the authors have any conflicts of interest in this work.
References
1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
2. O’Leary C, Mahler M, Soulen MC. Curative-intent therapies in localized hepatocellular carcinoma. Curr Treat Options Oncol. 2020;21(4):31. doi:10.1007/s11864-020-0725-3
3. Heimbach JK, Kulik LM, Finn RS, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67(1):358–380. doi:10.1002/hep.29086
4. Lencioni R, de Baere T, Soulen MC, et al. Lipiodol transarterial chemoembolization for hepatocellular carcinoma: a systematic review of efficacy and safety data. Hepatology. 2016;64(1):106–116. doi:10.1002/hep.28453
5. Chen C, Qiu H, Yao Y, et al. Comprehensive predictive factors for CalliSpheres® microspheres (CSM) drug-eluting bead-transarterial chemoembolization and conventional transarterial chemoembolization on treatment response and survival in hepatocellular carcinoma patients. Clin Res Hepatol Gastroenterol. 2020;45(2):101460. doi:10.1016/j.clinre.2020.05.008
6. Shi Q, Chen D, Zhou C, et al. Drug-eluting beads versus lipiodol transarterial chemoembolization for the treatment of hypovascular hepatocellular carcinoma: a single-center retrospective study. Cancer Manag Res. 2020;12:5461–5468. doi:10.2147/cmar.S255960
7. Hsu CY, Liu PH, Ho SY, et al. Using nomogram of the Barcelona clinic liver cancer system for treatment selection in patients with stage C hepatocellular carcinoma. BMC Cancer. 2018;18(1):289. doi:10.1186/s12885-018-4202-3
8. Zhou J, Sun H, Wang Z, et al. Guidelines for the diagnosis and treatment of hepatocellular carcinoma (2019 edition). Liver Cancer. 2020;9(6):682–720. doi:10.1159/000509424
9. Maurichi A, Miceli R, Eriksson H, et al. Factors affecting sentinel node metastasis in Thin (T1) cutaneous melanomas: development and external validation of a predictive nomogram. J Clin Oncol. 2020;38(14):1591–1601. doi:10.1200/jco.19.01902
10. Chen SH, Wan QS, Zhou D, et al. A simple-to-use nomogram for predicting the survival of early hepatocellular carcinoma patients. Front Oncol. 2019;9:584. doi:10.3389/fonc.2019.00584
11. Bruix J, Sherman M, Llovet JM, et al; Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the study of the liver. J Hepatol. 2001;35(3):421–430. doi:10.1016/s0168-8278(01)00130-1
12. Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30(1):52–60. doi:10.1055/s-0030-1247132
13. Wolbers M, Koller MT, Witteman JC, et al. Prognostic models with competing risks: methods and application to coronary risk prediction. Epidemiology. 2009;20(4):555–561. doi:10.1097/EDE.0b013e3181a39056
14. Li H, Wu F, Duan M, et al. Drug-eluting bead transarterial chemoembolization (TACE) vs conventional TACE in treating hepatocellular carcinoma patients with multiple conventional TACE treatments history: a comparison of efficacy and safety. Medicine. 2019;98(21):e15314. doi:10.1097/md.0000000000015314
15. Okuda K, Ohtsuki T, Obata H, et al. Natural history of hepatocellular carcinoma and prognosis in relation to treatment. Study of 850 patients. Cancer. 1985;56(4):918–928. doi:10.1002/1097-0142(19850815)56:4<918::aid-cncr2820560437>3.0.co;2-e
16. Hsu CY, Liu PH, Hsia CY, et al. Nomogram of the Barcelona Clinic Liver Cancer system for individual prognostic prediction in hepatocellular carcinoma. Liver Int. 2016;36(10):1498–1506. doi:10.1111/liv.13114
17. Peisen F, Maurer M, Grosse U, et al. Predictive performance of the mHAP-II score in a real-life western cohort with hepatocellular carcinoma following trans-arterial chemoembolisation with drug-eluting beads (DEB-TACE). Eur Radiol. 2020;30(7):3782–3792. doi:10.1007/s00330-020-06734-8
18. Mahringer-Kunz A, Weinmann A, Schmidtmann I, et al. Validation of the SNACOR clinical scoring system after transarterial chemoembolisation in patients with hepatocellular carcinoma. BMC Cancer. 2018;18(1):489. doi:10.1186/s12885-018-4407-5
19. Chan SL, Johnson PJ, Mo F, et al. International validation of the Chinese university prognostic index for staging of hepatocellular carcinoma: a joint United Kingdom and Hong Kong study. Chin J Cancer. 2014;33(10):481–491. doi:10.5732/cjc.014.10133
20. Chan AW, Chong CC, Mo FK, et al. Incorporating albumin-bilirubin grade into the cancer of the liver Italian program system for hepatocellular carcinoma. J Gastroenterol Hepatol. 2017;32(1):221–228. doi:10.1111/jgh.13457
21. Shen JY, Li C, Wen TF, et al. Alpha fetoprotein changes predict hepatocellular carcinoma survival beyond the Milan criteria after hepatectomy. J Surg Res. 2017;209:102–111. doi:10.1016/j.jss.2016.10.005
22. Liang B, Xiang H, Ma C, et al. Comparison of chemoembolization with CalliSpheres(®) microspheres and conventional chemoembolization in the treatment of hepatocellular carcinoma: a multicenter retrospective study. Cancer Manag Res. 2020;12:941–956. doi:10.2147/cmar.S187203
23. Tian M, Zhang X, Huang G, et al. Alpha-fetoprotein assessment for hepatocellular carcinoma after transarterial chemoembolization. Abdom Radiol. 2019;44(10):3304–3311. doi:10.1007/s00261-019-02116-x
24. Yamamoto M, Tatsumi T, Miyagi T, et al. α-Fetoprotein impairs activation of natural killer cells by inhibiting the function of dendritic cells. Clin Exp Immunol. 2011;165(2):211–219. doi:10.1111/j.1365-2249.2011.04421.x
25. Um SH, Mulhall C, Alisa A, et al. Alpha-fetoprotein impairs APC function and induces their apoptosis. J Immunol. 2004;173(3):1772–1778. doi:10.4049/jimmunol.173.3.1772
26. Zhang XP, Gao YZ, Chen ZH, et al. An eastern hepatobiliary surgery hospital/portal vein tumor thrombus scoring system as an aid to decision making on hepatectomy for hepatocellular carcinoma patients with portal vein tumor thrombus: a multicenter study. Hepatology. 2019;69(5):2076–2090. doi:10.1002/hep.30490
27. Yau T, Tang VY, Yao TJ, et al. Development of Hong Kong Liver Cancer staging system with treatment stratification for patients with hepatocellular carcinoma. Gastroenterology. 2014;146(7):1691–1700 e1693. doi:10.1053/j.gastro.2014.02.032
28. Gorodetski B, Chapiro J, Schernthaner R, et al. Advanced-stage hepatocellular carcinoma with portal vein thrombosis: conventional versus drug-eluting beads transcatheter arterial chemoembolization. Eur Radiol. 2017;27(2):526–535. doi:10.1007/s00330-016-4445-9
29. Han G, Berhane S, Toyoda H, et al. Prediction of survival among patients receiving transarterial chemoembolization for hepatocellular carcinoma: a response-based approach. Hepatology. 2020;72(1):198–212. doi:10.1002/hep.31022
30. Chung GE, Lee JH, Kim HY, et al. Transarterial chemoembolization can be safely performed in patients with hepatocellular carcinoma invading the main portal vein and may improve the overall survival. Radiology. 2011;258(2):627–634. doi:10.1148/radiol.10101058
31. Wu G, Wu J, Wang B, et al. Importance of tumor size at diagnosis as a prognostic factor for hepatocellular carcinoma survival: a population-based study. Cancer Manag Res. 2018;10:4401–4410. doi:10.2147/cmar.S177663
32. Chun YS, Pawlik TM, Vauthey JN. 8th Edition of the AJCC Cancer Staging Manual: pancreas and Hepatobiliary Cancers. Ann Surg Oncol. 2018;25(4):845–847. doi:10.1245/s10434-017-6025-x
33. Wang Y, Li J, Xia Y, et al. Prognostic nomogram for intrahepatic cholangiocarcinoma after partial hepatectomy. J Clin Oncol. 2013;31(9):1188–1195. doi:10.1200/jco.2012.41.5984
34. Xu L, Peng ZW, Chen MS, et al. Prognostic nomogram for patients with unresectable hepatocellular carcinoma after transcatheter arterial chemoembolization. J Hepatol. 2015;63(1):122–130. doi:10.1016/j.jhep.2015.02.034
35. Zhang ZY, Luo QF, Yin XW, et al. Nomograms to predict survival after colorectal cancer resection without preoperative therapy. BMC Cancer. 2016;16(1):658. doi:10.1186/s12885-016-2684-4
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.