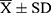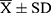Back to Journals » Journal of Inflammation Research » Volume 15
Tumor Necrosis Factor Alpha Level in the Uterine Fluid of Patients with Polycystic Ovary Syndrome and Its Correlation with Clinical Parameters
Authors Ha LX, Li WX, Du YD, Yuan YY, Qu XX
Received 18 July 2022
Accepted for publication 16 September 2022
Published 29 October 2022 Volume 2022:15 Pages 6015—6020
DOI https://doi.org/10.2147/JIR.S382808
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Ling-Xia Ha,1 Wei-Xia Li,2 Yu-Dong Du,3 Ying-Ying Yuan,1 Xiao-Xiao Qu3
1Reproductive Medicine Center, General Hospital of Ningxia Medical University, Yinchuan, 750004, Ningxia, People’s Republic of China; 2Reproductive Center, Maternal and Child Health Care Hospital of Yinchuan City, Yinchuan, Ningxia, 750000, People’s Republic of China; 3Ningxia Medical University, General Hospital of Ningxia Medical University, Yinchuan, Ningxia, 750004, People’s Republic of China
Correspondence: Ling-Xia Ha, Reproductive Medicine Center, General Hospital of Ningxia Medical University, No. 804 of Shengli Road, Xingqing District, Yinchuan, Ningxia, 750004, People’s Republic of China, Tel +86 951 6744033 ; +86 13629500990, Fax +86 951 6743891, Email [email protected]
Objective: This study aimed to analyze tumor necrosis factor alpha (TNF-α) level in the uterine fluid of patients with polycystic ovary syndrome (PCOS) and its correlation with the clinical parameters of PCOS.
Methods: A total of 162 patients treated in the Reproductive Medicine Center of the General Hospital of Ningxia Medical University between December 2019 and November 2021 were enrolled as research subjects, including 80 patients with PCOS and 82 patients with other gynecological disease, who were used as the controls. The patients’ general data, along with blood glucose, blood lipid, insulin, and sex hormone levels and other data, were collected. The TNF-α levels in the patients’ serum and uterine fluid were detected using enzyme-linked immunosorbent assay.
Results: Compared with the patients in the control group, the body mass index (BMI), anti-Müllerian hormone, luteinizing hormone, testosterone (T), fasting insulin (FINS), homeostasis model assessment insulin resistance (HOMA-IR), triglyceride (TG), and low-density lipoprotein (LDL) of patients with PCOS were higher, and high-density lipoprotein was lower (P < 0.05). The TNF-α levels in the serum and uterine fluid of patients with PCOS were higher than those in the control group (P < 0.01), and the TNF-α levels in the uterine fluid of these patients was significantly correlated with BMI, T, FINS, HOMA-IR, serum TNF-α, TG, and LDL (P < 0.05).
Conclusion: There is local inflammation in the uterine cavity of patients with PCOS, and the detection of cytokines in uterine secretions may be a simple and feasible method of understanding the uterine microenvironment of patients with PCOS.
Keywords: polycystic ovary syndrome, uterine fluid, TNF-α, insulin resistance, lipid metabolism
Introduction
Polycystic ovary syndrome (PCOS) is a common gynecological endocrine disease that affects 5–20% of women of reproductive age.1,2 With the in-depth study of PCOS, its correlation with chronic low-grade inflammation has attracted an increasing amount of attention. Previous studies have revealed that the concentrations of tumor necrosis factor alpha (TNF-α) in the serum and follicular fluid of patients with PCOS are elevated and have found that TNF-α is involved in the formation of insulin receptors (IRs) by blocking their tyrosine kinase phosphorylation.3–5 Furthermore, it has been found that the polymorphism of inflammation-related genes, such as TNF- α and tumor necrosis factor receptor 2, is closely related to hyperandrogenism in PCOS.
Most patients with PCOS have ovulation disorders. The ovulation rate of these patients can be increased after ovulation induction treatment, but their pregnancy rate remains low. Previous studies have revealed that the endometrial tissue in patients with PCOS has an inflammatory reaction, and this increase in inflammatory factor level affects endometrial receptivity, which may cause embryo implantation failure and recurrent abortion.6,7 Since van der Gaast et al8 first proposed a new method for evaluating cytokines by analyzing endometrial secretions in 2003, relevant studies have found that TNF-α, interleukin-1, and other cytokines in endometrial secretions could be used as important indicators to predict pregnancy outcome.9–12 However, there are currently no reports on the expression of inflammatory factors in the uterine fluid of patients with PCOS. In the present study, TNF-α levels were detected in the serum and uterine secretions of patients with PCOS, the correlations of cytokine TNF-α level with obesity, IR, and androgen status in these patients were analyzed, and possible mechanisms in the pathogenesis of TNF-α involving PCOS were explored to provide new ideas for the diagnosis and treatment of patients with PCOS.
Data and Methods
Subjects
A total of 162 patients treated in the Reproductive Medicine Center of the General Hospital of Ningxia Medical University between December 2019 and November 2021 were enrolled as the research subjects, including 80 patients with PCOS and 82 patients with other gynecological diseases, who were used as the controls. The diagnosis of PCOS conformed to the 2003 Rotterdam standard,13 namely: (1) long-term ovulation disorder; (2) hyperandrogenism signs and/or biochemical indicators; (3) pelvic ultrasound showed a polycystic ovary (PCO), ie, increased ovarian volume, enhanced capsule echo, and more than 12 small follicles of 2–9 mm in one ovary. The diagnosis was confirmed when a patient met any two of these criteria and other diseases that cause hyperandrogenism were excluded. Hysterosalpingography was used to confirm that all patients had at least one unobstructed fallopian tube, and semen analysis was used to confirm that any male partners had normal sperm or mild asthenospermia. The control group was composed of patients with other infertility factors, regular menstruation, and normal basic endocrine levels. This study followed the Declaration of Helsinki and was approved by the Ethics Committee of the General Hospital of Ningxia Medical University. Written informed consent was obtained from all participants.
Exclusion criteria: (1) Endometrial lesions or previous uterine cavity surgery; (2) other diseases that cause hyperandrogenism (such as Cushing’s syndrome, late-onset congenital adrenal hyperplasia, and androgen-secreting tumors), fever, viral and bacterial illnesses, chronic inflammatory diseases, tumors, or other diseases; (3) patients who had taken hormone drugs in the past three months.
Treatment Scheme
All subjects were screened from outpatient ovulation induction patients, and the general data and biochemical indicators of these patients were collected. On the third day of menstruation, they were treated with menotropin or letrozole to promote ovulation. When the follicle diameter monitored by transvaginal B-ultrasound was 17–20 mm, 10,000 UI of chorionic gonadotropin was injected intramuscularly. On the same day, 5 mL of the patient’s venous blood was drawn and centrifuged at 2000 rpm for 10 min. The supernatant was then collected and placed into a marked 2-mL centrifuge tube. The uterine secretions of the patients were also collected that day, and the treatment method was the same as that of the venous blood. All samples were stored at –80°C for testing.
Research Methods
The TNF-α in the serum and uterine fluid was detected using an enzyme-linked immunosorbent assay. ELISA kit (TNF-α) was purchased from Shenyang Wan Class Biotechnology Co., LTD. The operations were carried out in accordance with the kit instructions as following: (1) The concentration of the coated antibody is 0.5 mg/mL, and the coated antibody is diluted with 1× coating solution to a concentration of 10 μg/mL, and coated overnight at 4°C. (2) Take 100 μL of the diluted standard substance and add it to 7 wells in a row of a 96-well plate in turn, and add 1×PBST to the blank wells. (3) Sample detection: add 100 μL of the sample to be tested, and incubate at 37°C. for 2 hours. (4) Shake off the liquid in the well, do not wash, add 100 μL of the diluted capture antibody, and react at 37°C for 1 h. (5) Washing the plate: wash three times with 300 μL PBST washing solution, soaking for 2 min each time. (6) 100 μL of diluted HRP-Streptavidin was added, and the reaction was carried out at 37°C for 30 min. (7) Washing the plate: wash 5 times with 300 μL PBST washing solution, soaking for 2 min each time. (8) Add 100ul TMB color developing solution to each well and react at 37°C for 15min. (9) Add 50ul of TMB stop solution D to stop the reaction, and read the absorbance value at 450nnm with a microplate reader. The coefficient of variation within an indicator and between two indicators was less than 15%.
Each subject underwent a 75-g oral glucose tolerance test (OGTT). Serum glucose levels at 0 min and 2h after OGTT were measured using oxidase method, while insulin levels were measured by enzymatic and chemiluminescent method. The homeostasis model assessment of insulin resistance (HOMA-IR) was calculated according to the following equation: fasting serum glucose (FPG, mmol/l) × fasting insulin (FINS, mIU/l)/22.5. Follicle stimulating hormone (FSH), luteinizing hormone (LH), prolactin (PRL), total testosterone (T), estradiol (E2) and anti-Müllerian hormone(AMH) were measured using chemiluminescent analyzer (Beckman Coulter Inc, Fullerton, CA, USA). Total cholesterol (TC), triglycerides (TG), low-density lipoprotein (LDL) and high-density lipoprotein (HDL) were measured on an automated biochemistry analyzer (Olympus 600, Clinical Chemistry Analyser; Olympus Diagnostica Gmbh, Ireland).
Statistical Analysis
Data were statistically analyzed using SPSS 25.0 software. Measurement data were expressed as mean ± standard deviation (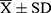 ), and the data were analyzed using an independent sample t-test or one-way analysis of variance. The correlation between factors was evaluated using Spearman correlation analysis. P < 0.05 was considered statistically significant.
), and the data were analyzed using an independent sample t-test or one-way analysis of variance. The correlation between factors was evaluated using Spearman correlation analysis. P < 0.05 was considered statistically significant.
Results
Comparison of Clinical Data Between the Two Groups
Compared with the patients in the control group, the body mass index (BMI), anti-Müllerian hormone (AMH), luteinizing hormone (LH), testosterone (T), fasting insulin (FINS), homeostasis model assessment insulin resistance (HOMA-IR), triglyceride (TG), and low-density lipoprotein (LDL) in patients with PCOS were significantly higher, and high-density lipoprotein (HDL) was significantly lower (all P < 0.05). There were no significant differences between the two groups in age, follicle-stimulating hormone, estradiol, and fasting blood glucose levels (see Table 1).
 |
Table 1 Comparison of Clinical Data Between the PCOS Group and Control Group of Patients ( |
Comparison of TNF-α Levels Between the Two Groups
The TNF-α levels in the serum and uterine fluid of patients with PCOS were significantly higher than those of the patients in the control group (P < 0.01; see Table 2).
 |
Table 2 Comparison of TNF-α in Serum and Uterine Fluid Between the PCOS Group and Control Group ( |
Correlation Between TNF-α in the Uterine Fluid, the Clinical Features of PCOS, and Serum TNF-α Levels in Patients with PCOS
The TNF-α level in the uterine fluid of patients with PCOS was significantly correlated with BMI, T, FINS, HOMA-IR, serum TNF-α, TG, and LDL (all P < 0.05; see Tables 3 and 4).
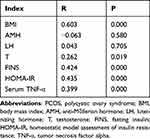 |
Table 3 Correlation Analysis of TNF-α Level in Uterine Fluid with Clinical Parameters of PCOS and Serum TNF-α Level in PCOS Patients |
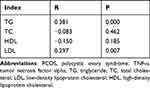 |
Table 4 Correlation Analysis of TNF-α in Uterine Fluid with Lipid Metabolism Indexes in PCOS Patients |
Discussion
Embryo implantation failure and clinical pre-pregnancy loss are important factors affecting the success rate of in vitro fertilization and embryo transfer (IVF-ET). This has also prompted researchers to seek an index to evaluate endometrial receptivity as an important breakthrough in improving pregnancy outcomes. Many candidate factors, such as integrins, interleukins, and glycoproteins have become important markers for evaluating endometrial receptivity, but there are still some limitations in their clinical application.
Uterine fluid is an important marker reflecting the microenvironment of embryo implantation, and the cytokines in this fluid play an important role in embryo implantation and development. The results of the present study revealed that, compared with the normal population, the levels of TNF-α in the serum and uterine fluid of patients with PCOS were elevated. A previous study conducted by our research team found that the expressions of TNF-α and NF-κBp65 in the proliferative endometrium of patients with PCOS were higher than those of normal controls, suggesting that increased expressions of inflammatory factors in patients with PCOS not only exist in the endometrium but also in the uterine fluid.14 By analyzing the levels of cytokines in the uterine fluid, clinicians can gain a greater understanding of the microenvironment of the uterine cavity in which the embryo is located. Compared with the invasive examination of endometrial biopsy, uterine fluid examination is a simple and feasible method that can be performed in the peri-implantation period. This provides a new idea for studying the microenvironment of the uterine cavity during embryo implantation.
At present, there are different conclusions about the relationship between the local level of TNF-α in the uterine cavity and pregnancy outcome. A previous study found that high levels of TNF-α were closely related to infertility and recurrent miscarriage,15 but another study found that high levels of TNF-α improved endometrial receptivity,16 and Khadem found that TNF-α in the uterine fluid was not associated with pregnancy outcome in patients undergoing IVF-ET.17 The reasons for these differences in findings may be related to the differences in the composition of subject populations and the time and manner of sampling used in the studies. The patients included in the present study were recruited from outpatient clinics, and there were many interference factors affecting pregnancy. Therefore, no correlation analysis of pregnancy outcomes was performed. Future research should further observe the correlation between TNF-α in uterine fluid and implantation rate, clinical pregnancy rate, and abortion rate in subjects undergoing IVT-ET. Furthermore, the correlation between TNF-α in the uterine fluid of patients with PCOS and pregnancy outcome remains to be further studied.
Insulin resistance, hyperandrogenism, obesity, and abnormal glucose and lipid metabolism are important features of PCOS. The present study found that, compared with the patients in the control group, the BMI, AMH, LH, T, FINS, and HOMA-IR in patients with PCOS were significantly higher (all P < 0.05); the patients with PCOS were also found to have lipid metabolism disorder, increased TG, increased LDL, and decreased HDL. Further correlation analysis revealed that the TNF-α level in the uterine fluid of patients with PCOS was significantly and positively correlated with BMI, T, HOMA-IR, and serum TNF-α (all P < 0.05). Previous studies found that obesity, hyperandrogenism, insulin resistance, etc.14,18,19 could all affect the expression of local inflammatory factors in the endometrium and that controlling body weight, reducing androgen levels, and improving insulin resistance could improve the chronic low-grade inflammatory state in patients with PCOS.
The results of the present study also revealed that the TNF-α level in uterine fluid was significantly positively correlated with TG and LDL (both P < 0.01). Using the lipidome analysis of endometrial fluid, a recent study confirmed that both phospholipid metabolites and lipid metabolites are involved in regulating endometrial receptivity.20 A large amount of non-free fatty acids can also activate the production of pro-inflammatory cytokines and promote the gene expressions of TNF-α, interleukin-8, and interleukin-6 in different cell types; it therefore plays a pro-inflammatory role in various tissues.21 Whether it can improve the local inflammatory state of the uterus by correcting the lipid metabolism disorder in patients with PCOS deserves further study.
Conclusion
The levels of TNF-α in the serum and uterine fluid of patients with PCOS are increased, suggesting that these patients have different degrees of inflammatory response both systemically and locally. Analyzing the expression of cytokines in endometrial secretions provides a relatively less invasive new method for studying the microenvironment of the uterine cavity in which embryos are located.
Funding
This study was supported by The National Key Research and Development Program of China(Grant No.2021YFC2700402),Ningxia Hui Autonomous Region Science and Technology Special Project to Benefit the People(Grant No.2022CMG03018) and the Natural Science Foundation of Ningxia (Grant No. 2019AAC03190).
Disclosure
The authors declare that they have no conflicts of interest in this work.
References
1. Lizneva D, Suturina L, Walker W, Brakta S, Gavrilova-Jordan L, Azziz R. Criteria, prevalence, and phenotypes of polycystic ovary syndrome. Fertil Steril. 2016;106(1):6–15. doi:10.1016/j.fertnstert.2016.05.003
2. Azziz R, Carmina E, Chen Z, et al. Polycystic ovary syndrome. Nat Rev Dis Primers. 2016;2:16057.
3. Rudnicka E, Suchta K, Grymowicz M, et al. Chronic low grade inflammation in pathogenesis of PCOS. Int J Mol Sci. 2021;22(7):3789. doi:10.3390/ijms22073789
4. Oróstica L, Poblete C, Romero C, Vega M. Pro-inflammatory markers negatively regulate IRS1 in endometrial cells and endometrium from women with obesity and PCOS. Reprod Sci. 2020;27(1):290–300. doi:10.1007/s43032-019-00026-3
5. Amato G, Conte M, Mazziotti G, et al. Serum and follicular fluid cytokines in polycystic ovary syndrome during stimulated cycles. Obstet Gynecol. 2003;101(6):1177–1182. doi:10.1016/s0029-7844(03)00233-3
6. Oróstica L, García P, Vera C, et al. Effect of TNF-α on molecules related to the insulin action in endometrial cells exposed to hyperandrogenic and hyperinsulinic conditions characteristics of polycystic ovary syndrome. Reprod Sci. 2018;25(7):1000–1009. doi:10.1177/1933719117732157
7. Lv J, Shan X, Yang H, et al. Single cell proteomics profiling reveals that embryo-secreted TNF-α plays a critical role during embryo implantation to the endometrium. Reprod Sci. 2022;29(5):1608–1617. doi:10.1007/s43032-021-00833-7
8. van der Gaast MH, Macklon NS, Beier-Hellwig K, et al. The feasibility of a less invasive method to assess endometrial maturation–comparison of simultaneously obtained uterine secretion and tissue biopsy. BJOG. 2009;116(2):304–312. doi:10.1111/j.1471-0528.2008.02039.x
9. Salama KM, Alloush MK, Al Hussini RM. Are the cytokines TNF alpha and IL 1Beta early predictors of embryo implantation? Cross sectional study. J Reprod Immunol. 2020;137:102618. doi:10.1016/j.jri.2019.102618
10. Boomsma CM, Kavelaars A, Eijkemans MJ, et al. Endometrial secretion analysis identifies a cytokine profile predictive of pregnancy in IVF. Hum Reprod. 2009;24(6):1427–1435. doi:10.1093/humrep/dep011
11. Pantos K, Grigoriadis S, Maziotis E, et al. The role of interleukins in recurrent implantation failure: a comprehensive review of the literature. Int J Mol Sci. 2022;23(4):2198. doi:10.3390/ijms23042198
12. Kniotek M, Zych M, Roszczyk A, Szafarowska M, Jerzak MM. Decreased production of TNF-α and IL-6 inflammatory cytokines in non-pregnant idiopathic RPL women immunomodulatory effect of sildenafil citrate on the cellular response of idiopathic RPL women. J Clin Med. 2021;10(14):3115. doi:10.3390/jcm10143115
13. Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod. 2004;19(1):41–47. doi:10.1093/humrep/deh098
14. Ha LX, Yin T, Wu YY, Li WX, Du YD. Correlation between insulin resistance and expressions of local inflammatory factors and glucose transporter protein type-4 in the endometrium of patients with polycystic ovary syndrome. J Shandong Univ. 2021;59(11):41–47.
15. Finan RR, Al-Irhayim Z, Mustafa FE, et al. Tumor necrosis factor-alpha polymorphisms in women with idiopathic recurrent miscarriage. J Reprod Immunol. 2010;84(2):186–192. doi:10.1016/j.jri.2009.12.005
16. You Y, Stelzl P, Joseph DN, et al. TNF-α regulated endometrial stroma secretome promotes trophoblast invasion. Front Immunol. 2021;12:737401. doi:10.3389/fimmu.2021.737401
17. Khadem N, Mansoori M, Attaran M, Attaranzadeh A, Zohdi E. Association of IL-1 and TNF-α levels in endometrial secretion and success of embryo transfer in IVF/ICSI cycles. Int J Fertil Steril. 2019;13(3):236–239. doi:10.22074/ijfs.2019.5668
18. Liu S, Hong L, Mo M, et al. Evaluation of endometrial immune status of polycystic ovary syndrome. J Reprod Immunol. 2021;144:103282. doi:10.1016/j.jri.2021.103282
19. Lv M, Xu ZJ, Sun RR, Mo ZC, Xie YJ. Research progress of hyperandrogen-related chronic inflammation and polycystic ovary syndrome. Progress Biochem Biophysics. 2022;49(04):767–774. doi:10.16476/j.pibb.2021.0339
20. Lin CC, Chen ZY, Wang CY, Xi YM. Research progress on biomarkers for endometriosis based on lipidomics. J Zhejiang Univ. 2020;49(06):779–784.
21. Gao F, Li H, Feng Y, Tian W, Cao R, Fu K. Aucubin ameliorates the LPS-induced inflammatory response in bovine endometrial epithelial cells by inhibiting NF-κB and activating the Keap1/Nrf2 signalling pathway. Reprod Domest Anim. 2021;56(7):972–982. doi:10.1111/rda.13939
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.