Back to Journals » Cancer Management and Research » Volume 12
Tumor Differentiation and EGFR Mutation Associated with Disease-Free Survival in Stage IA Lung Adenocarcinoma Patients with Curative Surgery
Authors Yang L, Pang C, Xu F, Yang G, Xu H , Wang C , Wang Y
Received 13 October 2020
Accepted for publication 20 November 2020
Published 7 December 2020 Volume 2020:12 Pages 12549—12556
DOI https://doi.org/10.2147/CMAR.S286503
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Kenan Onel
Lu Yang,1,* Chong Pang,2,* Fei Xu,1 Guangjian Yang,1 Haiyan Xu,3 Changli Wang,2 Yan Wang1
1Department of Medical Oncology, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, 100021, People’s Republic of China; 2Department of Lung Cancer, Tianjin Medical University Cancer Institute and Hospital, National Clinical Research Center for Cancer, Key Laboratory of Cancer Prevention and Therapy, Tianjin’s Clinical Research Center for Cancer, Tianjin Lung Cancer Center, Tianjin, 300060, People’s Republic of China; 3Department of Comprehensive Oncology, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, 100021, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yan Wang
Department of Medical Oncology, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing 100021, People’s Republic of China
Tel +86 139 1179 3771
Fax +86 106773 4107
Email [email protected]
Changli Wang
Department of Lung Cancer, Tianjin Medical University Cancer Institute and Hospital, National Clinical Research Center for Cancer, Key Laboratory of Cancer Prevention and Therapy, Tianjin’s Clinical Research Center for Cancer, Tianjin Lung Cancer Center, Tianjin 300060, People’s Republic of China
Email [email protected]
Background: Nearly 30% of stage IA non-small-cell lung cancer patients eventually die of recurrence or metastasis. This study aimed to predict stage IA lung adenocarcinoma (LADC) patients who underwent radical resection with a high risk of recurrence or metastasis.
Methods: Information on clinicopathological, genetic and therapeutic features and recurrence status was collected in this retrospective and two-center study. A nomogram based on multivariate analysis was established to predict disease-free survival. Further stratification was performed to identify populations with a high risk of relapse.
Results: A total of 1584 patients with pathological stage IA LADC who underwent radical surgery between 2011 and 2015 were enrolled from two medical institutions in this study. The nomogram including tumor differentiation and EGFR mutation had a higher C-index of 0.880 (95% CI 0.833– 0.926) compared to 0.598 (95% CI 0.486– 0.711) for the AJCC 8th TNM staging system. Furthermore, the C-index for the validation cohort was 0.798 (95% CI 0.738– 0.857). In addition, the 3-year cumulative nonrecurrence rate in the high-risk group stratified by this model was 21.8% compared to 98.1% in the low-risk group.
Conclusion: This study proposed a new nomogram including tumor differentiation and EGFR mutation to predict recurrence or metastatic probability in stage IA LADC patients who underwent radical surgery. This nomogram could identify patients in the high-risk group and help guide adjuvant treatment in the future.
Keywords: nomogram, stage IA, lung adenocarcinoma, disease-free survival
Introduction
Non-small-cell lung cancer (NSCLC) accounts for 80%-85% of lung cancer cases and remains the leading cause of cancer death worldwide.1,2 Currently, an increasing number of patients are diagnosed with early-stage NSCLC resulting from the prevalence of low-dose computed tomography (CT) for screening.3,4 However, the predicted 5-year survival rates of completely resected patients with stage IA NSCLC has reached a plateau, with only a 67–82% 5-year survival rate without adjuvant therapy.5,6 In other words, nearly 30% of patients eventually die of recurrence or metastasis, although they are predicted to have good outcomes.7
Although each centimeter from ≤1 cm to 3 cm has been verified as a significant prognostic factor in the 8th edition of the American Joint Committee on Cancer Tumor Node Metastasis (AJCC TNM) staging system, the predictive accuracy of this system has been shown to be limited.8 The treatment of stage IA patients with different prognoses has not changed because of the current TNM staging system.
The anatomic extent of the tumor is only a single snapshot of tumor burden, which is mainly described in TNM staging. Parameters beyond the TNM system that affect prognosis include clinicopathological information9 and surgical procedure.10 In addition, lung adenocarcinoma (LADC) carries significantly different clonal mutations than squamous lung carcinoma.11 Genetic characterizations should not be ignored as one of numerous factors to consider in adenocarcinoma.12–14 Therefore, a new prognostic evaluation algorithm should be created in terms of stage IA lung adenocarcinoma.
The visual format of nomograms, which represent a statistical prognostic model, is readily understood by physicians. The main goals of this study were to identify significant prognostic variables of disease-free survival (DFS), to develop a reliable prognostic nomogram for the estimation of outcomes and to validate its prognostic capacity in an independent cohort. In addition, this study also evaluated whether this model could provide a more accurate prediction of DFS compared to the conventional TNM staging system.
Methods
Patient Population and Data Collection
From September 2011 to December 2015, we retrospectively reviewed a total of 1502 patients diagnosed with stage IA LADC at the National Cancer Center/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College as a training cohort. The study inclusion criteria were as follows: (1) patients pathologically diagnosed with stage IA1 to IA3 LADC using the AJCC 8th TNM system; and (2) all patients underwent R0 tumor resection and had systemic lymph node dissection with at least 10 lymph nodes. The exclusion criteria of this study were as follows: (1) patients receiving neoadjuvant chemotherapy or radiotherapy; (2) patients receiving adjuvant chemotherapy, radiotherapy or targeted therapy before disease recurrence; and (3) a diagnosis with multiple primary carcinomas. In addition, the validation cohort included 82 patients with stage IA LADC from the Tianjin Medical University, Cancer Institute and Hospital, National Clinical Research Center for Cancer in 2012.
This study was approved by the institutional review board of Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College and Tianjin Medical University, Cancer Institute and Hospital, National Clinical Research Center for Cancer. All patients provided written informed consent, in accordance with the Declaration of Helsinki.
A total of 11 baseline characteristics, including sex, age, smoking history, tumor differentiation, lymphatic vessel invasion (LVI), tumor stage, operation methods, surgical intervention, surgical side, EGFR mutation and KRAS mutations, were documented. All these variables related to recurrence were selected based on their clinical importance and previous references. Smoking history was divided into never smokers and smokers. Tumor stage was classified according to the 8th edition of the AJCC Cancer Staging Manual. Tumor differentiation were divided into five grades as follows: grade 1: lepidic predominant; grade 2: two main components with grade 1 and grade 3; grade 3: acinar or papillary predominant; grade 4: two main components with grade 3 and grade 5; and grade 5: solid or micropapillary predominant; these grades correspond to well-, moderate-well-, moderately, moderate-poorly and poorly differentiated tumors, respectively. The surgical intervention included thoracotomy and video-assisted thoracoscopic surgery (VATS). Anatomic pulmonary resection was classified as sublobar resection, segmentectomy and wedge resection. EGFR mutations were mainly using surgical specimen with the polymerase chain reaction (PCR) technology. Unknown genetic status existed because some patients without recurrence had not undergone genetic testing due to the absence of a clinical need, and some tissue samples could not be tested because of long-term storage and gene degradation.
DFS was defined as the time from radical surgery for lung cancer to any kind of recurrence. Recurrence referred to regional and distant recurrences. Regional recurrence was recorded as recurrence on resection margins such as bronchial stumps or stapler lines or within ipsilateral subcarinal lymph nodes. Distant recurrence was defined as any recurrence occurring in the contralateral lung, brain, liver, adrenal gland, bone, and other locations. Disease recurrence was assessed by CT scan, magnetic resonance imaging (MRI), and bone scanning. PET-CT was also permitted for the screening or confirmation of recurrences.
Follow-up information was obtained through hospital visits or by telephone contact with patients or relatives. Surveillance with history and physical and chest CT (with or without contrast) was recommended according to NCCN guidelines during the first 2–5 years. April 2, 2018 was the last follow-up time. The median follow-up was 45.4 months (95% CI: 44.1–46.8).
Statistical Analysis
The chi-square test or Fisher’s exact probability method was used to determine differences between groups, and a univariate Cox proportional hazards model was used to analyze the association between factors and DFS. Multivariate analysis using a Cox proportional hazards regression model was based on the significant factors in univariate analysis. The nomogram was established mainly on the basis of the Cox model. The AJCC TNM stage for each patient was entered into the Cox regression model to determine whether traditional staging was an independent predictor of survival. Model performance was evaluated using discrimination and calibration with a bootstrap approach with 1000 resamples. The index of probability of concordance (C-index) between the predicted probability and DFS was calculated for the model to assess the discrimination of the nomogram. The discrimination of the AJCC TNM staging system was also analyzed. Statistical tests with two-sided P <0.05 were considered statistically significant. All statistical analyses were performed using SAS 9.4 software and R 3.1.1 software.
Results
Baseline Characteristics
There were a total of 1502 enrolled patients as a training cohort in the National Cancer Center/Cancer Hospital, Chinese Academy of Medical Sciences from September 2011 to December 2015. The cases were stage IA lung adenocarcinoma patients who underwent radical surgery, and recurrence was recorded in the database of cancer hospitals. The controls were selected among cohort members who had no confirmed recurrence prior to selection. We included at least three controls for each case. Finally, 52 cases were confirmed relapses cases and the corresponding recurrence rate was 3.5%. Then, 180 surgery-time-matched controls were analyzed in this study, as shown in Figure S1. The patients’ baseline characteristics, including patients in the training and validation cohorts, are shown in Table 1.
 |
Table 1 Demographics and Clinicopathologic Characteristics of the Training Set and Validation Set |
In addition, five patients were recorded relapse in the validation cohort from the Tianjin Medical University, Cancer Institute and Hospital, National Clinical Research Center for Cancer in 2012.
Predictors of DFS and the Nomogram Model
The Cox proportional hazards regression analysis identified that tumor differentiation and EGFR mutation were strongly associated with recurrence risk (Tables 2 and 3). We established a nomogram model based on the above variables, as shown in Figure 1, and the corresponding scoring system is shown in Table S1. This nomogram model had a higher C-index of 0.880 (95% CI 0.833–0.926) in the training cohort, while the 8th TNM staging system had a dramatically lower C-index of 0.598 (95% CI 0.486–0.711). The C-index for the validation cohort was 0.798 (95% CI 0.738–0.857). In addition, the integrated time-dependent area under the curve (AUC) of this nomogram was 0.915. The correspondence between the predicted and actual survival in the nomogram’s calibration plot was well calibrated in the development cohort and validation cohort (Figure 2).
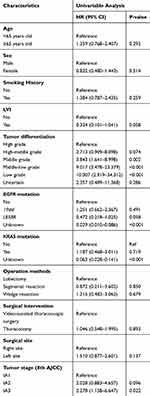 |
Table 2 Univariable Analysis |
 |
Table 3 Multivariable Analysis |
 |
Figure 1 Nomogram predicting DFS in stage IA LADC with radical resection. |
 |
Figure 2 Calibration plots in training set (A) and validation set (B). |
Different Risk Groups Classified by DFS Rate
A Kaplan-Meier curve of the nomogram model was constructed for the development cohort when patients were stratified into risk groups including high-, average- and low-risk groups. Groups with higher scores were associated with a higher risk of death, and each group was correlated with a markedly different DFS according to the log rank test (p<0.001).
Stage IA LADC patients with nomogram scores greater than 131 points had the highest risk, those with 13 to 131 points had an average risk, and those with less than 13 points had the lowest risk of relapse or metastasis. This nomogram well distinguished the three risk groups with significant differences both in the training cohort (p<0.0001) and in the validation cohort (p=0.0380), as shown in Figure 3A and B, respectively. However, the AJCC 8th TNM staging system was unable to clearly distinguish between the average-risk and high-risk groups in the training cohort (p=0.0610) and in the validation cohort (p=0.0720), as shown in Figure 3C and D. The 3-year DFS rates were 98.1%, 84.3% and 21.8% in the low-, average- and high-risk groups, respectively. The 5-year DFS rates were 98.1%, 78.2% and 10.9% in the low-, average- and high-risk groups, respectively (Table S2).
Discussion
Nearly 30% of stage IA NSCLC patients die of tumor recurrence or metastasis, despite the performance of regular follow-ups. Therefore, we developed a nomogram to determine which stage IA LADC patients with radical surgery and without adjuvant treatment were susceptible to relapse.
This study may have significant implications for our understanding of tumor heterogeneity and surveillance in stage IA LADC patients. This nomogram model consisted of two variables, tumor differentiation and EGFR mutation, which are well documented and easy to use in clinical practice. Different from TNM staging, the prognostic model reflected the tumor biology other than anatomic features.
Both factors presented in this study have been previously associated with the outcomes of stage IA NSCLC patients.
First, tumor differentiation is a well-known risk factor. Several studies have demonstrated that poor or moderate histology is related to recurrence in patients with stage I NSCLC.9,15,16 The finding in our study that tumor differentiation was a statistically significant prognostic variable was consistent with the finding of the previous study.
EGFR mutation was found to be another prognostic variable, and its efficacy was more important than tumor differentiation in this study. Recent studies have demonstrated that EGFR mutation is an early trunk mutation during the carcinogenesis of LADC.17–21 When tracking the evolution of NSCLC, EGFR mutation was demonstrated to be one of the most common clonal driver mutations in early-stage NSCLC tumors with radical resection and before systemic therapy.11 Therefore, EGFR mutations have a vital implication in resected lung carcinoma.
Izar et al previously reported that the presence of EGFR mutations (p=0.026) was identified as an independent prognostic marker for DFS in stage I NSCLC.12,13 However, the prognostic value of different subtypes in EGFR mutations remains controversial.22,23 A recent study conducted in 835 patients with stage IA to stage III LADC who underwent radical surgery and did not have EGFR TKIs as neoadjuvant or adjuvant therapy showed that patients with the L858R mutation had a significantly longer recurrence-free survival than patients without EGFR mutation and with the 19del mutation, which is consistent with the findings of our study. It has been demonstrated in this nomogram that the EGFR 19del group and WT group were associated with poor prognosis in stage IA LADC patients.24
The nomogram aimed to integrate the efficacy of these two different variables into one methodology and provide clinicians and patients with an individualized risk assessment. Most importantly, this nomogram is not only practical but also reliable. Our proposed nomogram demonstrated a higher C-index of 0.880 in the training cohort and 0.798 in the validation cohort and had better calibration than previous nomograms. The C-index of previous nomograms ranged from 0.612 to 0.838.16,25–27 Then, further risk stratifications in this study were classified, with the aim of accurately identifying high-risk patients with this methodology. The 3-year DFS rates of the high-risk and low-risk groups were 98.1% and 21.8%, respectively. The 5-year DFS rates of the high-risk and low-risk groups were 10.9% and 98.1%, respectively. Obviously, the prognosis of patients in the high-risk group was worse.
Currently, there are some stage II studies addressing neoadjuvant immunotherapy (NCT03634241) and targeted therapy (NCT00188617) in early-stage lung cancer, including stage IA, but there are few trials on adjuvant therapy in this high population to the best of our knowledge. The patients in the high-risk group with a relatively younger age might benefit from adjuvant therapy. However, this conclusion needs to be validated in a prospective study.
This study integrated the efficacy of two variables, EGFR mutation and tumor differentiation, into one methodology to provide clinicians and patients with an individualized risk assessment for stage IA LADC with radical surgery. When stratified into tertiles, the proposed nomogram was able to identify distinct groups with different risks of recurrence, which may contribute to guiding individual surveillance and help to guide adjuvant treatment in the future. Future studies are warranted to evaluate the efficacy of this nomogram in stage IA LADC patients with curative resection.
Data Sharing Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Acknowledgments
We would like to thank the participating patients, their families, and all the study co-investigators.
Funding
This research was supported by the CAMS Innovation Fund for Medical Sciences (CIFMS; No.2019-12M-2-003).
Disclosure
The authors report no conflicts of interest for this work.
References
1. Noone A-M, Cronin KA, Altekruse SF, et al. Cancer incidence and survival trends by subtype using data from the surveillance epidemiology and end results program, 1992–2013. Cancer Epidermiol Biomark Prev. 2017;26(4):632–641. doi:10.1158/1055-9965.EPI-16-0520
2. Fehrenbacher L, Spira A, Ballinger M, et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, Phase 2 randomised controlled trial. Lancet. 2016;387(10030):1837–1846. doi:10.1016/S0140-6736(16)00587-0
3. Abraham J. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365(5):395–409.
4. Wang X, Liu H, Shen Y, Li W, Chen Y, Wang H. Low-dose computed tomography (LDCT) versus other cancer screenings in early diagnosis of lung cancer. Medicine. 2018;97(27).
5. Goldstraw P, Crowley J, Chanksy K, et al. Lung cancer staging project: proposals for the revision of the TNM stage groupings in the forthcoming (Seventh) Edition of the TNM classification of malignant tumors. J Thorac Oncol. 2007;2(10):985. doi:10.1097/JTO.0b013e31812f3c1a
6. Goldstraw P, Chansky K, Crowley J, et al. The IASLC lung cancer staging project: proposals for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM classification for lung cancer. J Thorac Oncol. 2016;11(1):39–51. doi:10.1016/j.jtho.2015.09.009
7. Igai H, Matsuura N, Tarumi S, et al. Prognostic factors in patients after lobectomy for p-T1aN0M0 adenocarcinoma. Eur J Cardio-Thorac Surg. 2012;41(3):603–606. doi:10.1093/ejcts/ezr006
8. Rami‐Porta R, Asamura H, Travis WD, Rusch VW. Lung cancer — major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017;67(2):138–155.
9. Shimada Y, Saji H, Yoshida K, et al. Pathological vascular invasion and tumor differentiation predict cancer recurrence in stage ia non–small-cell lung cancer after complete surgical resection. J Thorac Oncol. 2012;7(8):1263–1270. doi:10.1097/JTO.0b013e31825cca6e
10. Aberle DR, DeMello S, Berg CD, et al. Results of the two incidence screenings in the national lung screening trial. N Engl J Med. 2013;369(10):920–931. doi:10.1056/NEJMoa1208962
11. Jamal-Hanjani M, Wilson GA, McGranahan N, et al. Tracking the evolution of non–small-cell lung cancer. N Engl J Med. 2017;376(22):2109–2121. doi:10.1056/NEJMoa1616288
12. Izar B, Sequist L, Lee M, et al. The impact of EGFR mutation status on outcomes in patients with resected stage I non-small cell lung cancers. Ann Thorac Surg. 2013;96(3):962–968. doi:10.1016/j.athoracsur.2013.05.091
13. Izar B, Zhou H, Heist RS, et al. The prognostic impact of KRAS, its codon and amino acid specific mutations, on survival in resected stage I lung adenocarcinoma. J Thorac Oncol. 2014;9(9):1363–1369. doi:10.1097/JTO.0000000000000266
14. Tsao M-S, Le Teuff G, Shepherd F, et al. PD-L1 protein expression assessed by immunohistochemistry is neither prognostic nor predictive of benefit from adjuvant chemotherapy in resected non-small cell lung cancer. Ann Oncol. 2017;28(4):882–889. doi:10.1093/annonc/mdx003
15. Kuo S-W, Chen J-S, Huang P-M, Hsu -H-H, Lai H-S, Lee J-M. Prognostic significance of histologic differentiation, carcinoembryonic antigen value, and lymphovascular invasion in stage I non–small cell lung cancer. J Thorac Cardiovasc Surg. 2014;148(4):1200–7.e3. doi:10.1016/j.jtcvs.2014.04.038
16. Zhang Y, Sun Y, Xiang J, Zhang Y, Hu H, Chen H. A clinicopathologic prediction model for postoperative recurrence in stage Ia non–small cell lung cancer. J Thorac Cardiovasc Surg. 2014;148(4):1193–1199. doi:10.1016/j.jtcvs.2014.02.064
17. Zhang C, Zhang J, Xu F-P, et al. Genomic landscape and immune microenvironment features of preinvasive and early invasive lung adenocarcinoma. J Thorac Oncol. 2019;14(11):1912–1923. doi:10.1016/j.jtho.2019.07.031
18. Izumchenko E, Chang X, Brait M, et al. Targeted sequencing reveals clonal genetic changes in the progression of early lung neoplasms and paired circulating DNA. Nat Commun. 2015;6:8258. doi:10.1038/ncomms9258
19. Hu X, Fujimoto J, Ying L, et al. Multi-region exome sequencing reveals genomic evolution from preneoplasia to lung adenocarcinoma. Nat Commun. 2019;10(1):2978. doi:10.1038/s41467-019-10877-8
20. Xu X, Li N, Zhao R, Zhu L, Shao J, Zhang J. Targeted next-generation sequencing for analyzing the genetic alterations in atypical adenomatous hyperplasia and adenocarcinoma in situ. J Cancer Res Clin Oncol. 2017;143(12):2447–2453. doi:10.1007/s00432-017-2500-9
21. Sakamoto H, Shimizu J, Horio Y, et al. Disproportionate representation of KRAS gene mutation in atypical adenomatous hyperplasia, but even distribution of EGFR gene mutation from preinvasive to invasive adenocarcinomas. J Pathol. 2007;212(3):287–294. doi:10.1002/path.2165
22. Okamoto T, Kitahara H, Shimamatsu S, et al. Prognostic impact of EGFR driver mutations on postoperative disease recurrence in lung adenocarcinoma. Anticancer Res. 2016;36(6):3057–3063.
23. Yotsukura M, Yasuda H, Shigenobu T, et al. Clinical and pathological characteristics of EGFR mutation in operable early-stage lung adenocarcinoma. Lung Cancer. 2017;109:45–51. doi:10.1016/j.lungcan.2017.04.014
24. Hayasaka K, Shiono S, Matsumura Y, et al. Epidermal growth factor receptor mutation as a risk factor for recurrence in lung adenocarcinoma. Ann Thorac Surg. 2018;105(6):1648–1654. doi:10.1016/j.athoracsur.2018.01.052
25. Zhou H, Zhang Y, Qiu Z, et al. Nomogram to predict cause-specific mortality in patients with surgically resected stage I non–small-cell lung cancer: a competing risk analysis. Clin Lung Cancer. 2018;19(2):e195–e203. doi:10.1016/j.cllc.2017.10.016
26. Yang HC, Kim HR, Jheon S, et al. Recurrence risk-scoring model for stage I adenocarcinoma of the lung. Ann Surg Oncol. 2015;22(12):4089–4097. doi:10.1245/s10434-015-4411-9
27. Chen H, Sui X, Yang F, Liu J, Wang J. Nomograms for predicting recurrence and survival of invasive pathological stage IA non-small cell lung cancer treated by video assisted thoracoscopic surgery lobectomy. J Thorac Dis. 2017;9(4):1046. doi:10.21037/jtd.2017.03.130
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

