Back to Journals » Infection and Drug Resistance » Volume 15
Tuberculous Meningitis in Children: Treatment Outcomes at Discharge and Its Associated Factors in Eastern Ethiopia: A Five Years Retrospective Study
Authors Abdella A , Deginet E , Weldegebreal F , Ketema I , Eshetu B, Desalew A
Received 10 March 2022
Accepted for publication 21 May 2022
Published 31 May 2022 Volume 2022:15 Pages 2743—2751
DOI https://doi.org/10.2147/IDR.S365753
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Ahmed Abdella,1 Endayen Deginet,1 Fitsum Weldegebreal,2 Indeshaw Ketema,3 Bajrond Eshetu,4 Assefa Desalew5
1Department of Pediatrics and Child Health, School of Medicine, College of Health and Medical Sciences, Haramaya University, Harar, Ethiopia; 2School of Medical Laboratory Sciences, College of Health and Medical Sciences, Haramaya University, Harar, Ethiopia; 3Department of Emergency and Critical Care Nursing, School of Nursing and Midwifery, College of Health and Medical Sciences, Haramaya University, Harar, Ethiopia; 4Department of Midwifery, School of Nursing and Midwifery, College of Health and Medical Sciences, Haramaya University, Harar, Ethiopia; 5Department of Pediatrics and Child Health Nursing, School of Nursing and Midwifery, College of Health and Medical Sciences, Haramaya University, Harar, Ethiopia
Correspondence: Assefa Desalew, Email [email protected]
Background: Tuberculous meningitis is a serious public health problem, particularly in low-income countries. It is associated with high rates of mortality and morbidity. The outcome of tuberculous meningitis in children is not well documented in Ethiopia, particularly in eastern Ethiopia. This study aimed to determine the treatment outcomes of tuberculous meningitis at discharge and its associated factors in eastern Ethiopia.
Methods: An institutional-based retrospective cross-sectional study was conducted on 121 children who were admitted and treated for tuberculous meningitis between January 2017 and December 2021. Data were collected using a pretested checklist, coded and entered into EpiData version 3.1, and analyzed using Statistical Package for the Social Sciences (SPSS) version 25. Factors associated with treatment outcomes were identified using multivariable logistic regression analyses. The association was described using the adjusted odds ratio (AOR) at a 95% confidence interval (CI). Finally, statistical significance was set at a p-value < 0.05.
Results: Of the 121 medical records of children, 33.9% (95% CI:25– 42%) died. Among the survivors, 28.1% were discharged with neurological sequelae and the remains (38.0%) were discharged with normal outcomes. In multivariable analyses, nutritional status (AOR=2.87; 95% CI:1.04– 7.94), duration of illness (AOR = 0.33; 95% CI: 0.15– 0.86), hydrocephalus (AOR=3.78; 95% CI:1.08– 13.34), and stage-III Tuberculous Meningitis (AOR = 5.29; 95% CI:1.88– 14.84) were identified as significantly associated factors with poor clinical outcomes.
Conclusion: The treatment outcomes for tuberculous meningitis in children are unfavorable. Two-thirds of children had poor treatment outcomes. Malnutrition, disease stage, hydrocephalus, and illness duration were associated with poor treatment outcomes at discharge. Health workers in primary health care should be aware of the importance of early screening, diagnosis, and treatment to improve clinical outcomes and reduce associated mortality and disability. In practice, more attention should be paid to children with malnutrition and hydrocephalus.
Keywords: tuberculous meningitis, pediatric, children, clinical outcome, Ethiopia
Background
Tuberculous meningitis (TBM) disproportionately affects young children.1 It is extra-pulmonary tuberculosis characterized by sub-acute inflammation of the meninges that covers the brain and spinal cord.2,3 TBM is the most severe form of tuberculosis and is associated with high mortality and morbidity rates in children.1,4 Even if diagnosed and treated, nearly 20% of children die, and of those surviving more than half will suffer from a neurological disability.5 In children, it develops shortly after primary pulmonary tuberculosis due to the hematogenous dissemination of Mycobacterium tuberculosis (MTB) bacilli. If children with TBM are not diagnosed and treated at an early stage, their treatment outcomes become unfavorable.2,3
The burden of TBM remains a challenge in low-income countries. At present, there are no clear estimates of the number of children affected by TBM or the outcomes of the interventions. Globally, the outcomes of TBM vary with the disease stage. Different studies have identified that children admitted with TBM had poor outcomes, ranging from 5% to 43% for death and 21 to 95% developed neurological sequelae. The most common neurological sequelae were blindness, hearing loss, motor deficits, cranial nerve palsy, hydrocephalus, and mental retardation.3,6,7 According to a hospital-based study in South Africa, TBM affects more than 10% of tuberculosis cases and about 8% and 50% had treatment outcomes of death and neurological disability among children, respectively.8,10 The disease burden increases up to one-fifth of the cases with a mortality rate of 15% and neurological sequelae of 21%.9
There is available evidence of the risk factors associated with TBM such as under-five children, contact history with known tuberculosis patients, lack of Bacille Calmette-Guerin (BCG) vaccine, HIV infection, and malnutrition.11–13 Moreover, advanced stage of TBM,4,5,12–15 coma at presentation, delay in diagnosis and treatment, and complications such as hydrocephalus are significantly associated factors.16–19 Few studies in central Ethiopia have revealed that TBM in children leads to poor treatment outcomes. For example, TBM-related death ranges from 14% to 43%, and neurological deficits range from 28% to 47%.20,21 Poor prognosis and difficulty of early diagnosis showed the importance of preventive interventions for a child in contact with patients with tuberculosis, need to be emphasized.22
Ethiopia has been implementing different tuberculosis preventive strategies to address the challenges related to tuberculosis infection; like BCG vaccination, isoniazid preventive therapy, and tuberculosis infection control in healthcare settings.23 In 2015, Ethiopia also adopted the “End Tuberculosis Strategy” to reduce 90% of national tuberculosis incidence and death by the year 2030.24,25 Despite the implementation of a tuberculosis preventive strategies, the burden of all types of tuberculosis is still high.7
Moreover, Ethiopia is among the countries with a high tuberculosis burden but the outcome of TBM in children is not well documented. Specifically, no study has described TBM among children in eastern Ethiopia. Therefore, this study aimed to determine the treatment outcomes of TBM at discharge and its associated factors among children admitted and treated at Hiwot Fana Comprehensive Specialized University Hospital (HFCSUH), eastern Ethiopia.
Methods
Study Area and Period
The study was conducted using the medical records of the pediatric population who were admitted and treated for TBM at HFCSUH from January 2017 to December 2021. Data extraction was performed in January 2022. The hospital was located in Harar town, 525 km from Addis Ababa, Ethiopia. It serves as a referral hospital for the entire eastern part of the country; including the Eastern Oromia Region, Dire Dawa Administration, Somali Regional State, and Harari Regional State. This hospital provides inpatient, outpatient, and emergency services. The hospital has four major departments (medical, surgery, pediatrics, and gynecology-obstetrics). The department of pediatrics has six units: the pediatric ward, intensive care unit, nutritional rehabilitation unit, Neonatal intensive care unit, outpatient Department, and chronic follow-up units.
Study Design and Population
This was an institutional-based retrospective study. All medical records of children; admitted and treated in the pediatric inpatient service who fulfilled the diagnostic criteria for TBM, during the study period were included. However, medical records with incomplete study variables and those aged <3 months were excluded.
Sample Size and Sampling Procedures
All medical records of the pediatric population admitted and treated for TBM at HFCSUH between January 2017 and December 2021 that fulfilled the inclusion criteria were included and studied.
Data Collection Methods
The data were collected by two trained pediatric residents and supervised by a Senior pediatrician. Data were collected using a semi-structured data extraction checklist prepared in English and validated by experts. Moreover, before the actual data collection; the checklist was pretested on 5% of the sample size, and necessary modifications were made. The clinical notes of the selected patients were reviewed and relevant clinical data related to the socio-demographic history, presentation, diagnosis, and discharge outcomes were extracted.
Operational Definitions
TBM case definition is defined as “Definite TBM” if AFB seen on CSF microscopy or MTB detected by Xpert MTB/RIF assay or culture from Cerebrospinal fluid (CSF), “Probable TBM” if the total score of ≥12 when neuroimaging is available or a total score of ≥10 when neuroimaging is not available, and “Possible TBM” if the total score of 6–11 when neuroimaging is available and a total score of 6–9 when neuroimaging is not available.26
The assessment of the clinic stages of TBM was performed according to the criteria of the Medical Research Council as follows: Stage I, patients with no focal neurological findings and a Glasgow Coma Scale (GCS) score of 15; Stage II, children with GCS score of 15 and who presented with focal neurological deficit or all patients with GCS score between 10 and 14, regardless of the presence of focal neurological deficit; Stage III, all children with GCS score < 10.17
Data on the nutritional status of children were extracted from the medical records on the following anthropometric indices; Height for age Z score (HAZ), weight for age Z score (WAZ), and body mass index for age Z score (BAZ). Children who fall below negative 2 (<−2SD) and negative 3 (−3SD) standard deviations from the median of the reference population were regarded as moderately and severely malnourished, respectively.27
Treatment Outcome at Discharge was defined as “good” in the case of children with mild neurological sequelae or normal neurological outcomes and defined as “poor” in the case of children with severe neurological sequelae or death.4
Children: If the age of the child is less than 15 years.
Data Processing and Analysis
Data were categorized, coded, and entered into EpiData version 3.1 and exported to SPSS version 25 for analysis. Descriptive data were expressed as frequency, percentages, means, and standard deviations to summarize the findings. A binary logistic regression model was used to identify factors associated with the outcome variable. All variables with a p-value <0.25 in the bivariable logistic regression were included in the multivariable logistic regression analysis. Multi-collinearity was checked using variance inflation factor (>10) and standard error (>2). The goodness-of-fit was checked using the Hosmer–Lemeshow test (>0.05). Finally, variables with a p-value less than 0.05 at an adjusted odds ratio (AOR) at a 95% confidence interval (CI) were considered statistically significant.
Quality Assurance
Before the actual data collection, one-day training was given to data collectors and the supervisor on how to collect and record data appropriately. Data were checked daily for completeness, accuracy, and consistency by data collectors, supervisors, and investigators.
Ethical Consideration
This study was conducted following the declaration of Helsinki. Ethical clearance was obtained from Haramaya University CHMS Institutional Review Board (Ref.No: IHRERC/187/2021). Permission to collect the data was obtained from the hospital. Following approval, an official written letter of cooperation was given to the administrative health bureau and facility. Written informed consent was obtained from the Hospital director. Confidentiality was ensured throughout the process.
Results
Sociodemographic Characteristics
A total of 131 pediatric patients were admitted and received treatment for TBM at HFCSUH between January 2017 and December 2021. Ten records were excluded from the analysis due to incomplete data and unknown clinical outcomes. Nearly, two-thirds of the children (62.8%) were male. Most (71.1%) of the children were younger than 5 years. Most children (78.5%) were rural residents. More than two-thirds (67.8%) of the patients had a contact history with a known tuberculosis patient. Out of the total admission with TBM, only 5 (6.6%) children were positive for HIV infection. All the children were examined for malnutrition and nearly half of them had malnutrition (47.1%). Almost one-fifth of patients 98 (81.1%) were referred from primary hospitals (Table 1).
 |
Table 1 Sociodemographic Characteristics of TBM Among Children Admitted and Treated in HFCSUH from 2017 to 2021 (N=121) |
Clinical Characteristics
Most children presented with advanced stage of TBM; stage III 58 (47.9%) and stage II 46 (38.1%). More than half (57.0%) of the children presented after one month of initial symptoms. On admission, the majority of the children had a history of fever 115 (95.0%), cough 109 (90.1%), poor weight gain 100 (82.6%), altered mental status 100 (82.6%), seizure 71 (58.7%), and headache 70 (57.8%). Meningeal irritation signs were presented in 45 (37.7%) children. Common complications of TBM were seizure 57 (58.7%), coma 43 (35.5%), increased intracranial pressure (ICP) 68 (56.2%), brain herniation 17 (14.0%), hydrocephalus 36 (29.8%), motor deficit 34 (28.1%), and cranial nerve palsy 18 (14.9%) (Table 2).
 |
Table 2 Clinical Features of TBM Among Children Admitted and Treated in HFCSUH from 2017 to 2021 (N=121) |
Diagnostic Characteristics
Among the 121 children, CSF analysis was performed for 48 (40%) children. Approximately, 41 (85%) of patients had a finding suggestive of TBM. Among the 117 patients sent for the Xpert MTB/RIF assay, 80 (68.4%) had concomitant pulmonary tuberculosis. Regarding the imaging study, brain CT scans were performed for 49 (40.5%) children and almost all of them had abnormal findings. The common CT findings were hydrocephalus 36 (73.5%) followed by brain abscess 13 (26.5%) and infarction 12 (24.5%). Almost all (119) patients were sent for chest X-ray examination and nearly 101 (85%) of the study findings were suggested pulmonary tuberculosis (Table 3).
 |
Table 3 Investigation Findings of TBM Among Children Admitted and Treated in HFCSUH from 2017 to 2021 (N=121) |
Clinical Outcome of TBM
Of the 121 children, 33.9% (95% CI:25–42%) of them died. Thirty-four (28.1%) were discharged with neurological sequelae and the remains 46 (38.0%) were discharged with a normal outcomes (Figure 1). The common neurological sequelae were motor deficits 18 (52.9%), cranial nerve abnormalities including blindness 3 (8.8%) and hearing loss 1 (2.9%), seizure 8 (23.5%), and hydrocephalus 4 (11.7%). All 121 children received antimycobacterial drugs, and 119 (98.3%) received corticosteroids. Intubation and mechanical ventilation were performed in 23 (19.0%) of the patients. The mean duration of hospital stay was 19 ± 10 days ranging from 5 to 60 days.
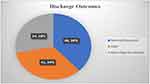 |
Figure 1 Tuberculous meningitis treatment outcome at discharge among children admitted and treated in HFCSUH from 2017 to 2021 (N=121). |
Factors Associated with Clinical Outcome of TBM
In the multivariable analyses, seven variables were eligible and included in the final model to identify factors associated with poor clinical outcomes. These include sex, place of residence, nutritional status, duration of illness, and complications such as hydrocephalus, and stage-III TBM. However, only nutritional status, illness duration, hydrocephalus, and stage-III TBM were statistically associated with poor clinical outcomes.
Malnourished children were nearly three times (AOR=2.87;95% CI:1.04–7.94) more likely to have worse outcomes than well-nourished children. Children who developed hydrocephalus were nearly 4 times (AOR=3.78; 95% CI:1.08–13.34) more likely to have a poorer clinical outcomes than their counterparts. In addition, children with a duration of illness less than one month were 77% times (AOR = 0.33; 95% CI: 0.15–0.86) less likely to have poor clinical outcomes than those with a duration of illness greater than one month. Furthermore, children who presented to the hospital with stage III TBM were 5 times (AOR = 5.29; 95% CI:1.88–14.84) more likely to encounter a poor treatment outcome compared with children who presented with stage I and II TBM (Table 4).
 |
Table 4 Factors associated with treatment outcomes of TBM at discharge among children who were admitted at HFCSUH from 2017 to 2021 (N=121) |
Discussions
Tuberculosis meningitis is an important cause of morbidity and mortality in low-income countries.28 Although symptoms are often non-specific and challenging, early identification, detection, and management of TBM play a key role in determining clinical outcomes.4,15 Evidence from low-income countries with high TBM burden regions is crucial for the implementation of appropriate interventions and control measures.15 Therefore, this study aimed to determine the treatment outcomes at discharge and factors associated with TBM among children admitted to the HFCSHU in eastern Ethiopia.
The World Health Organization estimated that more than 80% of tuberculosis cases in pediatrics under 15 are concentrated in 22 developing countries, mainly in Africa and Southeast Asia.29 The difficulty of diagnosis, lack of resources for contact tracing in many areas, and limited pediatric surveillance data in tuberculosis control programs are among the main reasons for the lack of knowledge on an accurate estimate of how many of these children will develop tuberculosis complications and poor treatment outcome.30 It seems young children due to immature immune systems and nonspecific signs and symptoms of pulmonary tuberculosis are at increased risk for disseminated tuberculosis and TBM with poor treatment outcomes and higher mortality.31–33
In the present study, two-thirds of the children had poor discharge outcomes. We found that 33.9% (95% CI:25–42%) of children died. Nearly, one-third (28.1%) were discharged with neurological sequelae and the remains (38.0%) were discharged with a normal outcomes. This is comparable to studies conducted in Indonesia (26.5%),3 Bangladesh (33%),6 and India18 However, it is higher than that reported in many studies such as Uganda (8%),10 Ethiopia (14.3%),21 South Africa (13%), (11.1%),4,34 and supported by systematic review and meta-analysis (19.3%).5 This may be due to children presenting to the hospital at an advanced stage of the disease and complications of TBM such as seizures, raised intracranial pressure, brain herniation, and hydrocephalus. This may also be related to low socioeconomic conditions and poor infrastructure to treat such children in the abovementioned facility.
In the multivariable analyses; nutritional status, advanced disease stage III TBM at presentation, duration of illness, and hydrocephalus were statistically associated with poor discharge treatment outcomes. Malnourished children were 9 times more likely to have unfavorable treatment outcomes at discharge compared with their counterparts. This is consistent with other findings and the WHO report.11,13 The high mortality rate in this study might be due to malnourished children being prone to infection because of poor immune function and response to treatment.13 Moreover, in the present study, the advanced stage of TBM at presentation strongly determined the discharge treatment outcomes of TBM in children, which is supported by many studies.4,5,12–15 In the current study, children with advanced stage-III TBM were 4 times more likely to have worse outcomes than those with stages I and II TBM.
Delays in diagnosis and treatment are the strongest risk factors for death.17 In this finding, children who presented with less than the one-month duration of illness were 77% less likely to have poor outcomes than those with a duration of illness greater than one month. Besides, Hydrocephalus was significantly associated with poor outcomes; since hydrocephalus is the most common and serious complication of TBM.18,19 This finding is supported by those of other studies.17 Hence, the results of this study agree with the findings of previous studies. The key fact for a pediatrician is believed that children with advanced stages of the disease and neurological complications have poor outcomes. The intervention needs to be rapid diagnosis and treatment without delay.
Limitations of the Study
It was a relatively small sample size. Besides, some variables have not been available in a retrospective medical record review. The time of treatment outcome was assessed at discharge from the hospital. So, this did not reflect the entire treatment outcome of TBM. Furthermore, multicenter large-scale prospective studies in eastern Ethiopia are required to guide clinical judgment regarding TBM.
Conclusion
Two-thirds of the children with TBM had poor treatment outcomes at discharge. Malnutrition, advanced stage of the disease, hydrocephalus, and illness duration were associated with poor treatment outcomes in children with TBM. Health workers in primary health care should be aware of the importance of early screening, diagnosis, and treatment to improve clinical outcomes and reduce associated disability. In practice, more attention needs to be paid to children with malnutrition and hydrocephalus.
Abbreviations
BCG, Bacillus Calmette-Guerin; CSF, Cerebrospinal Fluid; CT Scan, Computed Tomography Scan; HFCSUH, Hiwot Fana Comprehensive Specialized University Hospital; MTB, Mycobacterium Tuberculosis; TBM, Tuberculous Meningitis; WHO, World Health Organization.
Data Sharing Statement
All data of this study are available from the corresponding author upon reasonable request.
Acknowledgments
We thank Haramaya University for its financial and unreserved technical support. We would also like to extend our gratitude to Hiwot Fana Comprehensive Specialized University Hospital staff, data collectors, and supervisors for their collaboration to conduct the study.
Author Contributions
All authors made a significant contribution to the conception, study design, execution, acquisition of data, analysis, and interpretation. They took part also in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was financially supported by the Haramaya University, Ethiopia. The funder had no role in the study selection, data collection, analysis, conclusion, and interpretation.
Disclosure
The authors declare that they have no conflicts of interest in relation to this work.
References
1. Huynh J, Abo YN, du Preez K, Solomons R, Dooley KE, Seddon JA. Tuberculous meningitis in children: reducing the burden of death and disability. Pathogens. 2022;11(1):1–15.
2. Jullien S, Ryan H, Modi M, Bhatia R. Six months therapy for tuberculous meningitis. Cochrane Database Syst Rev. 2016;2016(9):CD012091.
3. Nataprawira HM, Ruslianti V, Solek P, et al. Outcome of tuberculous meningitis in children: the first comprehensive retrospective cohort study in Indonesia. Int J Tuberc Lung Dis. 2016;20(7):909–914. doi:10.5588/ijtld.15.0555
4. Van Well GTJ, Paes BF, Terwee CB, et al. Twenty years of pediatric tuberculous meningitis: a retrospective cohort study in the western cape of South Africa. Pediatrics. 2009;123(1):e1–e8. doi:10.1542/peds.2008-1353
5. Chiang SS, Khan FA, Milstein MB, et al. Treatment outcomes of childhood tuberculous meningitis: a systematic review and meta-analysis. Lancet Infect Dis. 2014;14(10):947–957. doi:10.1016/S1473-3099(14)70852-7
6. Fatema K, Rahman MM, Akhter S, et al. Clinicoradiologic profile and outcome of children with tubercular meningitis in a tertiary care hospital in Bangladesh. J Child Neurol. 2020;35(3):195–201. doi:10.1177/0883073819884169
7. Mohammed H, Oljira L, Roba KT, et al. Burden of tuberculosis and challenges related to screening and diagnosis in Ethiopia. J Clin Tuberc Other Mycobact Dis. 2020;19:100158. doi:10.1016/j.jctube.2020.100158
8. Seddon JA, Hesseling AC, Marais BJ, Jordaan A, Victor T, Schaaf HS. The evolving epidemic of drug-resistant tuberculosis among children in Cape Town, South Africa. Int J Tuberc Lung Dis. 2012;16(7):928–933. doi:10.5588/ijtld.11.0679
9. Cho YH, Ho TS, Wang SM, Shen CF, Chuang PK, Liu CC. Childhood tuberculosis in southern Taiwan, with emphasis on central nervous system complications. J Microbiol Immunol Infect. 2014;47(6):503–511. doi:10.1016/j.jmii.2013.06.008
10. Nabukeera-Barungi N, Wilmshurst J, Rudzani M, Nuttall J. Presentation and outcome of tuberculous meningitis among children: experiences from a tertiary children’s hospital. Afr Health Sci. 2014;14(1):143–149. doi:10.4314/ahs.v14i1.22
11. World Health Organization. Guideline: nutritional care and support for patients with tuberculosis. World Health Organization; 2013. Available from: http://apps.who.int/iris/bitstream/10665/94836/1/978924.
12. Israni AV, DiA D, Mandal A, et al. Tubercular meningitis in children: clinical, pathological, and radiological profile and factors associated with mortality. J Neurosci Rural Pract. 2016;7(03):400–404. doi:10.4103/0976-3147.181475
13. Jadaun P, Patil R, Ramteke S, Goel M. A study to assess the clinico-radiological presentation and outcome predictors in cases of tubercular meningitis. Indian J Tuberc. 2021;68(3):384–388. doi:10.1016/j.ijtb.2020.12.010
14. Dhawan SR, Gupta A, Singhi P, Sankhyan N, Malhi P, Khandelwal N. Predictors of the neurological outcome of tuberculous meningitis in childhood: a prospective cohort study from a developing country. J Child Neurol. 2016;31(14):1–6. doi:10.1177/0883073816668112
15. Devaleenal B, Daniel G, Angeline Grace MN. Tuberculous meningitis in children: clinical management & outcome. Indian J Med Res. 2019;150(2):117–130. doi:10.4103/ijmr.IJMR_786_17
16. Wang MS, Zhao M, Liu XJ. Risk factors for poor outcome in childhood tuberculous meningitis. Sci Rep. 2021;11(1):3–7. doi:10.1038/s41598-020-79552-z
17. Thwaites GE, van Toorn R, Schoeman J. Tuberculous meningitis: more questions, still too few answers. Lancet Neurol. 2013;12(10):999–1010. doi:10.1016/S1474-4422(13)70168-6
18. Raut T, Garg RK, Jain A, et al. Hydrocephalus in tuberculous meningitis: incidence, its predictive factors, and impact on the prognosis. J Infect. 2013;66(4):330–337. doi:10.1016/j.jinf.2012.12.009
19. Huang HJ, Ren ZZ, Dai YN, et al. Old age and hydrocephalus are associated with poor prognosis in patients with tuberculous meningitis: a retrospective study in a Chinese adult population. Medicine. 2017;96(26):2015–2018.
20. Tenase Tadesse GB. Prevalence and determinants of death from tuberculosis meningitis at ethio-Swedish children’s hospital. Ethiop J Pediatr Child Health. 2006;2(1):17–26.
21. Tola D. Clinical profile of children treated for Tuberculous meningitis at St. Paul’s and Yekatit 12 memorial hospitals in Addis Ababa: a three-year retrospective cross-sectional analysis. Ethiop J Pediatr Child Health. 2017;XIV(2):22–33.
22. World Health Organization. Guidance for National Tuberculosis Programmes on the management of tuberculosis in children. Geneva, Switzerland: World Health Organization; 2014. Available from: http://www.ncbi.nlm.nih.gov/pubmed/.
23. FMOH. Tuberculosis, Leprosy and TB/HIV Prevention and Control Programme.
24. FMOH. Ethiopia Launched Its National Childhood TB Prevention and Control Roadmap. Addis Ababa, Ethiopia: WHO Regional Office for Africa; 2015.
25. World Health Organization. End TB Strategy. Geneva, Switzerland: World Health Organization; Vol. 53, 2015:1689–1699.
26. Marais S, Thwaites G, Schoeman JF, et al. Tuberculous meningitis: a uniform case definition for use in clinical research. Lancet Infect Dis. 2010;10(11):803–812. doi:10.1016/S1473-3099(10)70138-9
27. World Health Organization. Growth reference data for 5–19 years. World Health Organization; 2007. Available from: https://www.who.int/tools/growth-reference-data-for-5.
28. Ramzan A, Nayil K, Asimi R, Wani A, Makhdoomi R, Jain A. Childhood tubercular meningitis: an institutional experience and analysis of predictors of outcome. Pediatr Neurol. 2013;48(1):30–35. doi:10.1016/j.pediatrneurol.2012.09.004
29. World Health Organization. Global tuberculosis report 2017. Geneva: World Health Organization; 2017. Available from: http://apps.who.int/iris.
30. Newton S, Brent A, Anderson S, Whittaker E, Kampmann B. Paediatric tuberculosis (UKPMC funders group). Lancet Infect Dis. 2008;8(8):498–510. doi:10.1016/S1473-3099(08)70182-8
31. Hailu D, Abegaz WE, Belay M. Childhood tuberculosis and its treatment outcomes in Addis Ababa: a 5-years retrospective study. BMC Pediatr. 2014;14(1):1–7. doi:10.1186/1471-2431-14-61
32. Wu XR, Yin QQ, Jiao AX, et al. Pediatric tuberculosis at Beijing Children’s Hospital: 2002–2010. Pediatrics. 2012;130(6):e1433–e1440. doi:10.1542/peds.2011-3742
33. Salari MH, Kalantari AB. Characteristics of tuberculosis patients in Yazd province, Islamic Republic of Iran, 1997–1999. East Mediterr Health J. 2004;10(1–2):175–179. doi:10.26719/2004.10.1-2.175
34. Hicks RM, Padayatchi N, Shah NS, et al. Malnutrition associated with unfavorable outcome and death among South African MDR-TB and HIV co-infected children. Int J Tuberc Lung Dis. 2014;18(9):1074–1083. doi:10.5588/ijtld.14.0231
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
