Back to Journals » Infection and Drug Resistance » Volume 13
Tuberculosis Peritonitis During Treatment of Polycythemia Vera with Ruxolitinib
Authors Sakiyama E , Chinen Y, Tsukamoto T, Takimoto-Shimomura T, Kuwahara-Ota S, Matsumura-Kimoto Y, Shimura Y , Kobayashi T , Horiike S, Kuroda J
Received 9 February 2020
Accepted for publication 31 March 2020
Published 8 April 2020 Volume 2020:13 Pages 1017—1021
DOI https://doi.org/10.2147/IDR.S249030
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Emiko Sakiyama, Yoshiaki Chinen, Taku Tsukamoto, Tomoko Takimoto-Shimomura, Saeko Kuwahara-Ota, Yayoi Matsumura-Kimoto, Yuji Shimura, Tsutomu Kobayashi, Shigeo Horiike, Junya Kuroda
Division of Hematology and Oncology, Department of Medicine, Kyoto Prefectural University of Medicine, Kyoto, Japan
Correspondence: Junya Kuroda Email [email protected]
Abstract: Ruxolitinib is a selective JAK1/2 inhibitor that is widely used for the treatment of myeloproliferative neoplasms (MPNs), including myelofibrosis and polycythemia vera (PV). Despite its clinical efficacy for MPNs, ruxolitinib possesses immunosuppressive properties that potentially increase the risks for opportunistic infection, such as mycobacterium tuberculosis (MTB) infection, and reactivation of occult viral infection. Herein, we report the case of a 76-year-old male with PV who developed tuberculosis peritonitis under ruxolitinib therapy for 28 weeks. While previous studies and case reports have suggested an increased risk of MTB infection of various organs during ruxolitinib treatment of MPNs, this case is apparently the first of tuberculosis peritonitis in a patient with MPN treated with ruxolitinib. A review of previous case reports suggests the need for careful observation for MTB from the relatively early phase of ruxolitinib treatment, given that the median duration from the start of ruxolitinib treatment to the emergence of MTB was 20 weeks (range: 3– 88 weeks). Clinicians should consider tuberculosis peritonitis as a differential diagnosis when patients with MPN treated with ruxolitinib develop infectious abdominal symptoms.
Keywords: ruxolitinib, tuberculosis peritonitis, polycythemia vera
Introduction
The Janus activating kinase (JAK)-signal transducer and activator of transcription (STAT) pathway is one of the critical cell signaling pathways for hematopoietic homeostasis and immune regulation.1–3 Constitutive activation of this pathway by pathologic mutations of JAK2, c-MPL or CALR also plays a central role in the development of myeloproliferative neoplasms (MPNs), such as primary myelofibrosis (PMF), polycythemia vera (PV), and essential thrombocythemia (ET).3 Ruxolitinib is a potent and selective inhibitor of JAK1/2 and is a current standard treatment for PMF and PV. Ruxolitinib ameliorates disease-associated splenomegaly and symptoms, such as fever, fatigue and body weight loss, and prolongs survival of patients with PMF.4,5 It also enables good hematocrit control and relief of symptoms in patients with PV, even in cases that are resistant or intolerant to treatment by hydroxyurea.6
Despite such clinical benefits in MPNs, increasing evidence suggests that ruxolitinib possesses immunosuppressive activity that may be associated with an increased risk of opportunistic infections. These include varicella-zoster virus (VZV) reactivation and mycobacterium infection with both typical mycobacterium tuberculosis (MTB) and atypical mycobacterial infections (AMI) in patients with MPNs.6–8 A search of the English-written literature on MTB infection during ruxolitinib treatment for MPNs revealed cases with disseminated tuberculosis, pulmonary tuberculosis, and tuberculosis lymphadenitis,9–17 but no case of tuberculosis peritonitis. Here, we report a rare case of PV complicated by tuberculosis peritonitis while under treatment with ruxolitinib.
Case Report
A 76-year-old male was admitted to our hospital for treatment of cerebral infarction in January 2018. The patient had a history of bladder cancer that was successfully treated by transurethral resection, and had also been treated for hypertension and dyslipidemia for several years. In addition, the patient had subclinical nonspecific interstitial pneumonia (NSIP). At admission, a complete blood count revealed the presence of polycythemia with an elevation of hematocrit to 64.7% (normal range: 40–50%) and hemoglobin to 20.9 g/dL (normal range: 13.7–16.8 g/dL). The peripheral leukocyte cell count and platelet count were 12.3×109/L (normal range: 3.3–8.6×109/L) and 274.0×109/L (158.0–348.0×109/L), respectively. A routine polycythemia work-up, including cytologic, histologic and chromosomal analyses of bone marrow hematopoietic cells and genetic analysis of peripheral blood mononuclear cells, showed that the patient fulfilled the diagnostic criteria for JAK2V617F mutation-positive PV.18 Ruxolitinib treatment at 20 mg/day was initiated in March 2018, while hydroxyurea was avoided as the first-line treatment due to NSIP.
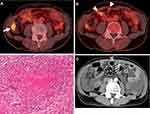
Three months later, a solitary tumor at the ascending colon and several peritoneal nodules were identified by colonoscopy and 18F-fluorodeoxyglucose-positron emission tomography combined with computed tomography (Figure 1A and B). Laparoscopic surgical resection of the colon tumor and peritoneal nodules resulted in a histologic diagnosis of adenocarcinoma for the ascending colon tumor and epithelioid cell granulomas with partial necrosis for the peritoneal nodules (Figure 1C). The peritoneal nodules were suspected to involve mycobacterial infection, but histologic examination with Ziehl-Neelsen staining, mycobacteria culture, and polymerase chain reactions (PCRs) for MTB and AMI of these nodules were all negative.
During this period, ruxolitinib was continued for the treatment of PV. However, in September 2018, the patient presented with pyrexia, fatigue, and abdominal distension. Blood tests revealed elevation of the peripheral leukocyte count to 10.2×109/L, including 83% neutrophils, 8% lymphocytes, 7% monocytes, 1% basophils and 1% eosinophils, thrombocytosis of 789.0×109/L, and modest anemia with a hemoglobin level of 11.5 g/dL. Further laboratory tests showed slight elevation of serum lactate dehydrogenase to 296 U/L (124–222 U/L) and elevation of C-reactive protein to 15.0 mg/dL (0.00–0.14 mg/dL). An interferon-gamma (IFN-γ) release assay (IGRA) was negative. A CT scan showed the emergence of an abnormally thickened peritoneum and massive ascites (Figure 1D). Abdominal paracentesis revealed the presence of exudative ascites containing 1.21×109 cells/L of mononuclear cells and 0.71×109 cells/L of lobulated neutrophils without neoplastic cells. Further work-up of ascites disclosed an elevated level of adenosine deaminase (ADA) to 57.5 U/L (−36.0 U/L), while PCR for MTB or atypical mycobacteria was negative.
The patient reported no past MTB infection or history of exposure to MTB, but we tentatively diagnosed peritoneal MTB based on the high ADA level in ascites and the histologic finding of epithelioid cell granuloma in peritoneal nodules resected in surgery. Treatment for PV was changed from ruxolitinib to hydroxyurea, and four-drug anti-MTB therapy of isoniazid, pyrazinamide, rifampicin, and streptomycin was initiated. Finally, a mycobacterial culture of ascites yielded MTB colonies after 50 days of culture, which led to the definitive diagnosis of tuberculosis peritonitis. The four-drug anti-MTB treatment successfully resolved the symptoms and was terminated after 6 months of treatment. The patient has continued hydroxyurea treatment with no signs of recurrence of tuberculosis peritonitis or worsening of NSIP.
Discussion
While the JAK-STAT pathway is essential for hematopoietic homeostasis, it also plays critical roles in mediating cellular signals by various cytokines and growth factors, including IL-6 and tumor necrosis factor (TNF-α), for transcription of numerous genes associated with immune regulation in immune cells, such as dendric cells, natural killer cells, and regulatory T cells.1–3,19–21 Therefore, through inhibition of the JAK/STAT pathway, ruxolitinib potentially impairs cellular functions for host defense. Indeed, an increased risk of VZV reactivation during ruxolitinib treatment has been reported in clinical trials for MPN,4–6 and a more recent retrospective pharmacovigilance review did find an increased risk of MTB and AMI that were sometimes fatal in MPN patients receiving ruxolitinib.7,8 During ruxolitinib treatment, MPN cases may be complicated by opportunistic infections such as MTB and AMI,9–17 pneumocystis jiroveci pneumonitis,22 toxoplasma retinitis,23 Cryptococcus neoformans pneumonitis,24 and progressive multifocal leukoencephalopathy,25 and by reactivation of latent viral infection with hepatitis B virus26 and cytomegalovirus.27 Thus, optimal anti-infective prophylaxis or monitoring for occult viral infection is needed with the use of ruxolitinib.28
Our search of the English-written literature identified nine reports of MPN cases affected by MTB infection (Table 1).9–17 Including the present case, the reported cases include 7 of PMF and 3 of PV, including two that progressed to secondary myelofibrosis. Among the 10 patients, 7 had pulmonary tuberculosis and 5 of these 7 cases also had extrapulmonary lesions in lymph nodes, bone, or brain. Although tuberculosis peritonitis is one of the most common extrapulmonary sites of MTB infection, this is the first report of tuberculosis peritonitis during the treatment of MPN with ruxolitinib. Although the gold standard for diagnosis of tuberculosis peritonitis is histologic confirmation of caseous granulomas or bacteriologic confirmation by acid-fast smears or mycobacterium cultures, it is often difficult to diagnose the disease by these modalities.29–33 Tuberculosis peritonitis may be fatal, as in two cases in Table 1 and also reported previously.7,16,17 Therefore, it is important to suspect tuberculosis peritonitis, measure the ADA level in ascites as an alternative for differential diagnosis of the disease, and make a decision on empirical treatment, even without histologic or bacteriologic confirmation of mycobacterial infection.32,33
 |
Table 1 List of Reported MPN Patients Complicated by Mycobacterium Tuberculosis (MTB) Infection During Treatment with Ruxolitinib |
An IGRA is a widely used in vitro blood test for the diagnosis of MTB infection. The assay measures the level of IFN-γ released from T cells induced by exposure to tuberculosis-specific antigen. However, as shown in Table 1, an IGRA gave a false negative result in 2 of 6 patients who developed MTB during ruxolitinib treatment. It is conceivable that T cell inactivation by ruxolitinib may have reduced reactive IFN-γ release in these IGRA-false negative cases.34 Therefore, the result of an IGRA should be carefully interpreted in patients under treatment with ruxolitinib.
Our findings may support the product label of ruxolitinib which recommends the screening for latent tuberculosis before initiating treatment with the drug in MPN cases. In previous cases (Table 1), MTB infection emerged at a median period of 20 weeks (range: 3–88 weeks) after initiation of ruxolitinib in 10 patients who developed MTB, suggesting the need for careful observation for MTB from a relatively early phase of ruxolitinib treatment. Ruxolitinib treatment was interrupted in all but one patient, with rechallenge in 3 of 8 patients. It is still unclear if rechallenge with ruxolitinib treatment is safe in patients who develop MTB, as the treatment for MPN general continues for a lifetime. A future study of the long-term safety of rechallenge with ruxolitinib is needed in patients with MPNs who develop an opportunistic infection, including mycobacterium infection, or reactivation of occult viral infection.
Conclusion
In conclusion, ruxolitinib may trigger unusual opportunistic infection at uncommon sites, as seen in our case with tuberculosis peritonitis. Clinicians should consider tuberculosis peritonitis as a differential diagnosis in patients treated with ruxolitinib who develop infectious abdominal symptoms.
Ethics and Consent Statement
Written informed consent has been provided by the patient for the publication of the case report and accompanying images. The institutional approval was not required for the publication of the case details.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Morris R, Kershaw NJ, Babon JJ. The molecular details of cytokine signaling via the JAK/STAT pathway. Protein Sci. 2018;27(12):1984–2009. doi:10.1002/pro.3519
2. O’Shea JJ, Holland SM, Staudt LM. JAKs and STATs in immunity, immunodeficiency, and cancer. N Engl J Med. 2013;368(2):161–170. doi:10.1056/NEJMra1202117
3. Quintás-Cardama A, Kantarjian H, Cortes J, Verstovsek S. Janus kinase inhibitors for the treatment of myeloproliferative neoplasias and beyond. Nat Rev Drug Discov. 2011;10(2):127–140. doi:10.1038/nrd3264
4. Vannucchi AM, Kantarjian HM, Kiladjian JJ, et al. A pooled analysis of overall survival in COMFORT-I and COMFORT-II, 2 randomized Phase III trials of ruxolitinib for the treatment of myelofibrosis. Haematologica. 2015;100(9):1139–1145. doi:10.3324/haematol.2014.119545
5. Mesa RA, Kiladjian JJ, Verstovsek S, et al. Comparison of placebo and best available therapy for the treatment of myelofibrosis in the Phase 3 COMFORT studies. Haematologica. 2014;99(2):292–298. doi:10.3324/haematol.2013.087650
6. Vannucchi AM, Kiladjian JJ, Griesshammer M, et al. Ruxolitinib versus standard therapy for the treatment of polycythemia vera. N Engl J Med. 2015;372(5):426–435. doi:10.1056/NEJMoa1409002
7. Anand K, Burns EA, Ensor J, Rice L, Pingali SR. Mycobacterial infections with ruxolitinib: a retrospective pharmacovigilance review. Clin Lymphoma Myeloma Leuk. 2020;20(1):18–23. doi:10.1016/j.clml.2019.08.008
8. Lussana F, Cattaneo M, Rambaldi A, Squizzato A. Ruxolitinib-associated infections: a systematic review and meta-analysis. Am J Hematol. 2018;93(3):339–347. doi:10.1002/ajh.24976
9. Branco B, Metsu D, Dutertre M, et al. Use of rifampin for treatment of disseminated tuberculosis in a patient with primary myelofibrosis on ruxolitinib. Ann Hematol. 2016;95(7):1207–1209. doi:10.1007/s00277-016-2684-0
10. Chen YH, Lee CH, Pei SN. Pulmonary tuberculosis reactivation following ruxolitinib treatment in a patient with primary myelofibrosis. Leuk Lymphoma. 2015;56(5):1528–1529. doi:10.3109/10428194.2014.963082
11. Shamil E, Cunningham D, Wong BL, Jani P. Ruxolitinib associated tuberculosis presenting as a neck lump. Case Rep Infect Dis. 2015;2015:284168.
12. Colomba C, Rubino R, Siracusa L, et al. Disseminated tuberculosis in a patient treated with a JAK2 selective inhibitor: a case report. BMC Res Notes. 2012;5(1):552. doi:10.1186/1756-0500-5-552
13. Palandri F, Polverelli N, Catani L, Vianelli N. Ruxolitinib-associated tuberculosis: a case of successful ruxolitinib rechallenge. Ann Hematol. 2015;94(3):519–520. doi:10.1007/s00277-014-2183-0
14. Hopman RK, Lawrence SJ, Oh ST. Disseminated tuberculosis associated with ruxolitinib. Leukemia. 2014;28(8):1750–1751. doi:10.1038/leu.2014.104
15. Abidi MZ, Haque J, Varma P, et al. Reactivation of pulmonary tuberculosis following treatment of myelofibrosis with ruxolitinib. Case Rep Hematol. 2016;2016:2389038.
16. Tsukamoto Y, Kiyasu J, Tsuda M, et al. Fatal disseminated tuberculosis during treatment with ruxolitinib plus prednisolone in a patient with primary myelofibrosis: a case report and review of the literature. Intern Med. 2018;57(9):1297–1300. doi:10.2169/internalmedicine.9165-17
17. Lescuyer S, Ledoux MP, Gravier S, et al. Tuberculosis and atypical mycobacterial infections in ruxolitinib-treated patients with primary or secondary myelofibrosis or polycythemia vera. Int J Infect Dis. 2019;80:134–136. doi:10.1016/j.ijid.2019.01.002
18. Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391–2405. doi:10.1182/blood-2016-03-643544
19. Schönberg K, Rudolph J, Vonnahme M, et al. JAK inhibition impairs NK cell function in myeloproliferative neoplasms. Cancer Res. 2015;75(11):2187–2199. doi:10.1158/0008-5472.CAN-14-3198
20. Heine A, Held SA, Daecke SN, et al. The JAK-inhibitor ruxolitinib impairs dendritic cell function in vitro and in vivo. Blood. 2013;122(7):1192–1202. doi:10.1182/blood-2013-03-484642
21. Elli EM, Baratè C, Mendicino F, Palandri F, Palumbo GA. Mechanisms underlying the anti-inflammatory and Immunosuppressive activity of ruxolitinib. Front Oncol. 2019;9:1186. doi:10.3389/fonc.2019.01186
22. Lee SC, Feenstra J, Georghiou PR. Pneumocystis jiroveci pneumonitis complicating ruxolitinib therapy. BMJ Case Rep. 2014;2014(jun02 1):bcr2014204950. doi:10.1136/bcr-2014-204950
23. Goldberg RA, Reichel E, Oshry LJ. Bilateral toxoplasmosis retinitis associated with ruxolitinib. N Engl J Med. 2013;369(7):681–683. doi:10.1056/NEJMc1302895
24. Wysham NG, Sullivan DR, Allada G. An opportunistic infection associated with ruxolitinib, a novel janus kinase 1,2 inhibitor. Chest. 2013;143(5):1478–1479. doi:10.1378/chest.12-1604
25. Wathes R, Moule S, Milojkovic D. Progressive multifocal leukoencephalopathy associated with ruxolitinib. N Engl J Med. 2013;369(2):197–198. doi:10.1056/NEJMc1302135
26. Caocci G, Murgia F, Podda L, Solinas A, Atzeni S, La Nasa G. Reactivation of hepatitis B virus infection following ruxolitinib treatment in a patient with myelofibrosis. Leukemia. 2014;28(1):225–227. doi:10.1038/leu.2013.235
27. Abedin S, McKenna E, Chhabra S, et al. Efficacy, Toxicity, and infectious complications in ruxolitinib-treated patients with corticosteroid-refractory graft-versus-host disease after hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2019;25(8):1689–1694. doi:10.1016/j.bbmt.2019.04.003
28. Maschmeyer G, De Greef J, Mellinghoff SC, et al. Infections associated with immunotherapeutic and molecular targeted agents in hematology and oncology. A position paper by the European Conference on Infections in Leukemia (ECIL). Leukemia. 2019;33(4):844–862. doi:10.1038/s41375-019-0388-x
29. Sanai FM, Bzeizi KI. Systematic review: tuberculous peritonitis–presenting features, diagnostic strategies and treatment. Aliment Pharmacol Ther. 2005;22(8):685–700. doi:10.1111/j.1365-2036.2005.02645.x
30. Wu DC, Averbukh LD, Wu GY. Diagnostic and therapeutic strategies for peritoneal tuberculosis: a review. J Clin Transl Hepatol. 2019;7(2):140–148. doi:10.14218/JCTH.2018.00062
31. Inadomi JM, Kapur S, Kinkhabwala M, Cello JP. The laparoscopic evaluation of ascites. Gastrointest Endosc Clin N Am. 2001;11(1):79–91. doi:10.1016/S1052-5157(18)30088-6
32. Bhargava DK, Gupta M, Nijhawan S, Dasarathy S, Kushwaha AK. Adenosine deaminase (ADA) in peritoneal tuberculosis: diagnostic value in ascitic fluid and serum. Tubercle. 1990;71(2):121–126. doi:10.1016/0041-3879(90)90007-U
33. Riquelme A, Calvo M, Salech F, et al. Value of adenosine deaminase (ADA) in ascitic fluid for the diagnosis of tuberculous peritonitis: a meta-analysis. J Clin Gastroenterol. 2006;40(8):705–710. doi:10.1097/00004836-200609000-00009
34. Lee YM, Park KH, Kim SM, et al. Risk factors for false-negative results of T-SPOT.TB and tuberculin skin test in extrapulmonary tuberculosis. Infection. 2013;41(6):1089–1095. doi:10.1007/s15010-013-0478-z
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.