Back to Journals » OncoTargets and Therapy » Volume 8
Tubeimoside-1 induces glioma apoptosis through regulation of Bax/Bcl-2 and the ROS/Cytochrome C/Caspase-3 pathway
Authors Jia G, Wang Q, Wang R, Deng D, Xue L, Shao N, Zhang Y, Xia X, Zhi F, Yang Y
Received 19 October 2014
Accepted for publication 18 December 2014
Published 30 January 2015 Volume 2015:8 Pages 303—311
DOI https://doi.org/10.2147/OTT.S76063
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Jianmin Xu
Geng Jia,1,* Qiang Wang,2,* Rong Wang,2,* Danni Deng,2 Lian Xue,2 Naiyuan Shao,1 Yi Zhang,1 Xiwei Xia,1 Feng Zhi,2 Yilin Yang1,2
1Department of Neurosurgery, Third Affiliated Hospital of Soochow University, Jiangsu, People’s Republic of China; 2Modern Medical Research Center, Third Affiliated Hospital of Soochow University, Jiangsu, People’s Republic of China
* These authors contributed equally to this work
Background: Tubeimoside-1 (TBMS1) is a natural compound isolated from tubeimoside, which has been widely used as a traditional Chinese herbal medicine. The purpose of the present study is to investigate the anti-tumor effect and the underling mechanism of TBMS1 on glioma cancer cells.
Methods: The MTT assay was performed to evaluate the effect of TBMS1 on glioma cell proliferation. The fluorescent microscopy and flow cytometry analysis were performed to evaluate the effect of TBMS1 on glioma cell apoptosis. The Western blot analysis was used to evaluate the protein change.
Results: TBMS1 inhibited glioma cancer cell proliferation in a dose- and time-dependent manner. Fluorescent microscopy and flow cytometry analysis demonstrated that TBMS1 induced glioma cell apoptosis in a concentration-dependent manner. Western blotting showed that TBMS1 induced apoptosis by increasing the expression of Bax and downregulating the level of Bcl-2. Furthermore, we found that TBMS1 induced apoptosis by increasing the concentration of reactive oxygen species through the release of Cytochrome C and activation of Caspase-3.
Conclusion: These findings indicate that TBMS1 may be developed as a possible therapeutic agent for the management of glioma.
Keywords: Tubeimoside-1, glioma, proliferation, apoptosis
Introduction
Gliomas are the most common type of intracranial tumors, accounting for approximately 40% of intracranial tumors.1 Gliomas have a high recurrence rate, a high mortality rate, and a low cure rate. The prognosis of patients with glioma is closely related to the World Health Organization (WHO) tumor grade, and patients with glioblastoma, the most common histological type, have a poor prognosis. In spite of significant improvements in neurosurgery, radiotherapy and chemotherapy, the median survival time of high-grade glioma patients has remained at 12–15 months over the past decade, and the cumulative 1 year survival rate remains lower than 30%.2 The discovery of more effective agents to treat glioma is becoming increasingly urgent.
In the last few decades, many traditional Chinese herbs with anti-tumor effects have drawn a great amount of attention due to their efficiency, lack of drug resistance, and low levels of toxicity and side effects.3 Tubeimoside is the tuber of Bolbostemma paniculatum (Maxim) Franquet (Cucurbitaceae). Because of its extensive antiviral, anti-inflammatory, anti-cancer and immunosuppressive effects, it has long been used as a traditional Chinese medicine.4 Previous studies have shown that Tubeimoside-1 (TBMS1) exhibits potent anticancer effects in several cancer cell lines. TBMS1 inhibited squamous esophageal carcinoma cell proliferation through mitochondria-induced intrinsic apoptosis and P21-cyclin B1/cdc2 complex-related G2/M cell cycle arrest.5 TBMS1 induced apoptosis in gastric cancer cells through regulation of the Bcl-2 gene family.6 TBMS1 induced apoptosis in hepatoma cancer cells through oxidative stress and G2/M cell cycle arrest by regulating the NF-κB, JNK, and p53 pathways.7,8 TBMS1 induces G2/M phase arrest and apoptosis in ovarian cancer cells through an increase in intracellular Ca2+ levels and caspase-dependent signaling.9 TBMS1 inhibited human choriocarcinoma cancer cell proliferation by inducing Cytochrome C release and apoptosis via the mitochondrial-related signaling pathway.10 TBMS1 inhibits proliferation and induces apoptosis by increasing the Bax to Bcl-2 ratio and decreasing COX-2 expression in lung cancer cells.11 TBMS1 is cytotoxic in cervical carcinoma cells through the mitochondrial dysfunction and endoplasmic reticulum stress pathways.12,13 However, the effects of TBMS1 on human glioma cancer cells remain unknown.
In the present study, the effects of TBMS1 on the growth of glioma cancer cells and the cellular mechanism involved in TBMS1-induced apoptosis were investigated in vitro.
Materials and methods
Materials
TBMS1 was bought from Zelang Medical Technology Co. Ltd (Nanjing, People’s Republic of China). The powder was dissolved in Dulbecco’s Modified Eagle’s Medium (DMEM) to obtain a stock solution of 1,000 μg/mL and stored at −20°C. Trypsin-ethylenediaminetetraacetic acid (EDTA) (1×) (0.25%), fetal bovine serum (FBS) and DMEM were from Thermo Fisher Scientific, Waltham, MA, USA. Penicillin and streptomycin (100×) and polyvinylidene fluoride membranes were from EMD Millipore (Billerica, MA, USA). MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) was bought from Sigma-Aldrich Co. (St Louis, MO, USA). Dimethyl sulfoxide (DMSO) was from MP Biomedicals LLC (Santa Ana, CA, USA). Hoechst 33258 was from the Beyotime Institute of Biotechnology (Haimen, People’s Republic of China). Antibodies against Bax, Bcl-2, Cytochrome C, Caspase-3 and β-actin were from Cell Signaling Technology (Beverly, MA, USA). Horseradish peroxidase-conjugated secondary antibody was from Jackson ImmunoResearch Laboratories, Inc. (West Grove, PA, USA). Electrochemiluminescence was from Thermo Fisher Scientific. All other reagents were of the highest available quality.
Cell culture
The human glioma cell lines U251 and U87 were obtained from the Type Culture Collection of the Chinese Academy of Sciences, Shanghai, People’s Republic of China. U251 and U87 cells were cultured in Thermo Fisher Scientific’s DMEM containing 10% FBS, 100 u/L penicillin and 100 mg/L streptomycin. All cells were maintained at 37°C with 5% CO2 in a humidified atmosphere by routine passage every 1–2 days.
MTT measurement of cell viability
Cell viability was measured by MTT assay. U251 cells in the logarithmic growth phase were digested with trypsin and plated in a 96-well plate with 6×103 cells per well. U87 cells were plated in a 96-well plate with 8×103 cells per well. Cells were incubated for 24 hours to allow adherence and growth. TBMS1 at different concentrations (10, 15, 20, 25, 30, 35, 40, 45, and 50 μg/mL) was incubated with the U251 cells for 24 hours, 48 hours and 72 hours. TBMS1 at different concentrations (10, 20, 30, 40, 50, 60, 70, and 80 μg/mL) was incubated with the U87 cells for 24 hours. MTT solution (20 μL, 5 mg/mL in phosphate buffered saline [PBS]) was added to each well, and cells were cultured for 4 hours at 37°C. The medium was removed and 150 μL DMSO was added to dissolve the MTT formazan crystals. After shocking for 10 minutes, the optical density was measured at 490 nm using an Absorbance Microplate Reader (ELx800; BioTek, Winooski, VT, USA).
Hoechst 33258 staining
Hoechst 33258 staining was used to observe the changes in nuclei morphology of U251 and U87 cells after TBMS1 treatment. Cells (2×105) in 2 mL media were seeded in each well of a 6-well plate and grown overnight. The cells were treated with TBMS1 at different concentrations (15, 20, and 25 μg/mL for U251 cells and 15, 25, and 35 μg/mL for U87 cells) for 24 hours and then washed with PBS twice. Cells were fixed with 500 μL stationary liquid for 10 minutes. After pouring out the fixing solution, cells were washed with PBS twice and stained with Hoechst 33258 (500 μL) for 10 minutes in the dark. The stained cells were then rinsed twice with PBS and observed under a fluorescence microscope (×400 magnification, IX71; Olympus Corporation, Japan).
Analysis of cell apoptosis
Flow cytometry was used to detect apoptosis. U251 cells were exposed to different concentrations of TBMS1 (15, 20, and 25 μg/mL) for 24 hours and then collected and washed twice with PBS. Then, 1×105 cells from each sample were suspended in a mixture of 185 μL binding buffer, 5 μL of Annexin V, and 10 μL propidium iodide (PI) and incubated on ice for 15 minutes in the dark. Next, binding buffer was added to bring the volume of liquid in each of the test tubes to 500 μL. The cells were immediately analyzed using a BD FACSCanto II flow cytometer (BD Biosciences, San Jose, CA, USA).
Measurement of reactive oxygen species (ROS) generation
Cellular ROS production was detected using dichlorofluorescein diacetate (DCFH-DA) staining. U251 cells incubated with TBMS1 for 24 hours were washed twice with DMEM without FBS and incubated with 10 μM DCFH-DA in DMEM without FBS for 20 minutes at 37°C in the dark. Cells were washed three times with DMEM without FBS, harvested, and then analyzed using fluorescein isothiocyanate (FITC) on a BD FACSCanto II Flow cytometer. Data were analyzed with BD FACSDiva 7.0 software.
Mitochondrial isolation
For mitochondria isolation, cells were harvested and then fractionated using a Cytosol/Mitochondria Fractionation Kit (EMD Millipore, Billerica, MA, USA) according to the manufacturer’s recommendations. The cytosol extract was then subjected to Western blotting analysis for Cytochrome C.
Western blot analysis
After treatment with TBMS1 for 24 hours, cells were harvested and washed three times with PBS. Cells were incubated with lysis buffer on ice for 30 minutes and then centrifuged at 12,000 rpm for 2 minutes at 4°C. The protein extract was detected using a bicinchoninic acid protein assay. After boiling with loading buffer for 5 minutes, protein samples were separated on 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) gels, transferred onto polyvinylidene fluoride membranes and blocked with 5% skimmed milk powder for 1 hour. The primary antibodies against Bax (1:1,000), Bcl-2 (1:1,000), Caspase-3 (1:1,000), Cytochrome C (1:2,000), and β-actin (1:1,000) were diluted according to the manufacturer’s instructions and incubated with the membrane overnight at 4°C. After washing three times, the horseradish peroxidase-conjugated secondary biotinylated antibodies were added (1:2,000) and incubated with the membranes for 1 hour. After washing three times with PBS, the blots were developed using an electrochemiluminescence Western blotting kit (Thermo Fisher Scientific) according to the manufacturer’s instructions. The films were processed using a Medical X-Ray Film Processor (SX435-T; Suxing, People’s Republic of China). The optical density of the protein was analyzed using a ChemiDoc XRS Imaging system (Bio-Rad Laboratories Inc., Hercules, CA, USA).
Statistical analysis
All assays were repeated at least three independent times. Data are expressed as the mean ± standard deviation. GraphPad Prism 5.0 software was used to perform the analyses. The statistical significance of correlations was determined using one-way analysis of variance (ANOVA) and two tailed Student’s t-test. Statistical significance was defined as P<0.05.
Results
TBMS1 inhibits cell proliferation
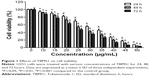
U251 cells were treated with different concentrations of TBMS1 for 24, 48 or 72 hours, and then the survival rates were determined via MTT assay. As shown in Figure 1, TBMS1 inhibited the proliferation of U251 cells in a concentration- and time-dependent manner. Based on our data and the MTT results, we chose to use concentrations of 15, 20, and 25 μg/mL to investigate the mechanism of TBMS1 induced apoptosis in glioma cells. Furthermore, U87 cells were treated with different concentrations of TBMS1 for 24 hours, and then the survival rates were determined. As shown in Figure S1, TBMS1 inhibited the proliferation of U87 cells in a concentration-dependent manner which was similar to U251. However, the IC50 value of U87 cells was slightly higher than that of U251 cells, indicating that U87 cells were more resistant to TBMS1 treatment.
TBMS1 induced cellular apoptosis
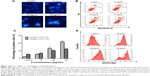
To investigate the apoptosis-inducing effect of TBMS1, U251 cells were treated with various concentrations of TBMS1. After treatment with TBMS1 for 24 hours, the cells were examined via fluorescent microscopy using Hoechst 33258 staining. As shown in Figure 2A, chromatin condensation, nuclear fragmentation and apoptotic bodies were clearly observed in the treated cells. The results revealed that when exposed to TBMS1, U251 cells underwent the morphological changes typical of apoptosis. The result was similar in U87 cells after TBMS1 treatment, as shown in Figure S2. Cellular apoptosis induced by TBMS1 was then detected via flow cytometry. After exposure to TBMS1 for 24 hours, the apoptosis rates were measured using Annexin V-FITC and PI staining. As shown in Figure 2B, when the drug concentration increased from 0 μg/mL to 25 μg/mL, the percentages of early apoptotic cells were 2.4, 5.6, 13.2, and 21.6% and the percentages of late apoptotic cells were 5.8, 7.1, 10.5, and 12.5%, respectively. The statistical analysis was shown in Figure 2C. These results show that treatment with TBMS1 for 24 hours significantly increased the percentage of apoptotic cells in a concentration-dependent manner.
TBMS1 increases ROS generation
U251 cells were treated with 15, 20, and 25 μg/mL TBMS1 for 24 hours and then analyzed using flow cytometry to measure the concentration of intracellular ROS. Compared to the control group, the intracellular level of ROS was much higher in the 15, 20, and 25 μg/mL TBMS1 treatment groups (Figure 2D). In addition, the levels in the 20 and 25 μg/mL TBMS1 treatment groups were higher than in the 15 μg/mL TBMS1 treatment group. These results were consistent with those observed by fluorescent microscopy.
Effect of TBMS1 on expression levels of the Bcl-2 gene family
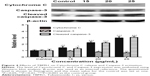
The expression of apoptosis-related proteins was evaluated using Western blot analysis. As shown in Figure 3, with increasing concentrations of TBMS1, expression of Bax was upregulated compared to that in the control group. In contrast, the level of Bcl-2 was significantly downregulated compared to that in the control group, resulting in an obvious increase in the ratio of Bax to Bcl-2. The ratio of Bax to Bcl-2 increased in a concentration-dependent manner.
Effect of TBMS1 on Cytochrome C release and Caspase-3 activation
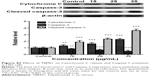
We extracted total protein from cancer cells incubated with 15, 20, and 25 μg/mL TBMS1 for 24 hours and analyzed the release of intracellular Cytochrome C and activation of Caspase-3 by Western blotting. As shown in Figure 4, compared with the control group, there was an obvious release of intracellular Cytochrome C and activation of Caspase-3 in the TBMS1 treated groups with a trend of TBMS1 concentration dependence. The result was similar in U87 cells after TBMS1 treatment, as shown in Figure S3.
Discussion
As a popular folk medicine, tubeimoside has a long history of being widely used. TBMS1, an anti-tumor candidate, is an active ingredient of tubeimoside. In this study, we investigated the anti-tumor mechanism of TBMS1 in glioma cells.
In the present study, TBMS1 inhibited glioma cancer cell proliferation in a concentration- and time-dependent manner. Chromosome condensation, nuclear fragmentation and apoptotic bodies were observed by fluorescent microscopy. Flow cytometric analysis revealed that TBMS1 induced glioma cell apoptosis in a concentration-dependent manner. The results from the Western blot analysis showed that the molecular basis of the TBMS1-induced apoptosis in the glioma cells was the downregulation of Bcl-2 protein levels and the upregulation of Bax protein expression. Furthermore, we found that TBMS1 could induce Cytochrome C release and Caspase-3 activation, showing that TBMS1 could also induce the release of apoptotic proteins by the induction of intracellular ROS.
Apoptosis is a common process of multicellular organisms.14 Defects in apoptosis are directly related to cancer.15 The Bcl-2 family has been shown to be involved in the regulation of apoptosis through the mitochondrial pathway. Bcl-2 is an anti-apoptotic member of this family, but Bax is a representative of the pro-apoptotic members.16 The ratio of Bax to Bcl-2 is a decisive factor in the induction of apoptosis and the balance between the expression levels of the proteins Bax and Bcl-2 is critical for cell survival or death. Zhang et al found that TBMS1 promoted apoptosis in BGC823 cells by regulating the expression of Bcl-2 family proteins.6 Yin et al suggested that TBMS1 altered expression of Bcl-2 family proteins to activate Caspase-3 and induce cell apoptosis in HepG2 cells.7 Zhang et al reported that TBMS1 increased the ratio of Bax to Bcl-2 in A549 cells to advance apoptosis.11 Tubeimoside V (1) is a new minor constituent from the ethanol extracts of tubers of Bolbostemma paniculatum (Maxim) Franquet (Cucurbitaceae). Cheng et al found that Tubeimoside V (1) induced apoptosis by decreasing the expression levels of Bcl-2 protein and increasing the expression levels of Bax protein in human glioblastoma U87MG cells.17 Similarly, the present results showed that the expression of Bax was upregulated by TBMS1, whereas the expression of Bcl-2 was downregulated, leading to an increase in the ratio of Bax to Bcl-2. This demonstrates the involvement of the Bcl-2 gene family in the regulation of TBMS1-induced glioma cell apoptosis.
ROS function as important chemical messengers, playing an essential role in cell homeostasis, growth and proliferation. When the production of ROS is out of control, ROS is capable of triggering apoptosis.18 The mitochondria play a significant role in the process of apoptosis.19 It was reported that an increase in ROS has been implicated in the induction of apoptosis through its ability to promote the release of Cytochrome C to facilitate caspase activation.20 Once released into the cytoplasm, Cytochrome C can bind to Apaf-1 and trigger the formation of an apoptosome, which is capable of recruiting and activating the initiator caspase-9.21 The caspase-9 effectively activates downstream targets including caspase-3, activating a signaling cascade and leading to apoptosis.22 Our data showed that TBMS1 treatment stimulated ROS generation in U251 cells, reflecting the participation of an ROS-mediated pathway, which was further demonstrated through the increase in Cytochrome C and the activation of Caspase-3. This implies that apoptosis induced by TBMS1 in the U251 cell line is mediated by the ROS/Cytochrome C/Caspase-3 pathway as well.
Conclusion
The present study demonstrated that TBMS1 inhibited proliferation and promoted apoptosis in U251 glioma cancer cells. This apoptotic response is associated with the regulation of the expression of the Bcl-2 gene family and the mitochondrial pathway of apoptosis by inducing intracellular ROS. These findings indicate that TBMS1 may be developed as a possible therapeutic agent for the management of glioma. It should be noted that this study has only examined the U251 and U87 cell lines in vitro. Further investigations are necessary to study the anti-tumor effect of TBMS1 by using animal models in vivo.
Acknowledgments
This work was supported by National Natural Science Foundation of China: 31071046, 81302197; Changzhou Social Development Project: CS20092015, CS20102010; Changzhou Health Bureau Project: ZD200903, ZD201007; Changzhou Science Technology Bureau Guiding Project: CY20119004.
Disclosure
The authors report no conflicts of interest in this work.
References
Grier JT, Batchelor T. Low-grade gliomas in adults. Oncologist. 2006;11(6):681–693. | ||
Gabayan AJ, Green SB, Sanan A, et al. GliaSite brachytherapy for treatment of recurrent malignant gliomas: a retrospective multi-institutional analysis. Neurosurgery. 2006;58(4):701–709. | ||
Zhou XJ, Liu M, Yan JJ, Cao Y, Liu P. Antidepressant-like effect of the extracted of Kai Xin San, a traditional Chinese herbal prescription, is explained by modulation of the central monoaminergic neurotransmitter system in mouse. J Ethnopharmacol. 2012;139(2):422–428. | ||
Yu TX, Ma RD, Yu LJ. Structure-activity relationship of tubeimosides in anti-inflammatory, antitumor, and antitumor-promoting effects. Acta Pharmacol Sin. 2001;22(5):463–468. | ||
Xu Y, Wang G, Chen Q, et al. Intrinsic apoptotic pathway and G2/M cell cycle arrest involved in tubeimoside I-induced EC109 cell death. Chin J Cancer Res. 2013;25(3):312–321. | ||
Zhang Y, Xu XM, Zhang M, et al. Effects of tubeimoside-1 on the proliferation and apoptosis of BGC823 gastric cancer cells in vitro. Oncology Lett. 2013;5(3):801–804. | ||
Yin Y, Chen W, Tang C, et al. NF-kappaB, JNK and p53 pathways are involved in tubeimoside-1-induced apoptosis in HepG2 cells with oxidative stress and G(2)/M cell cycle arrest. Food Chem Toxicol. 2011;49(12):3046–3054. | ||
Wang Y, Deng L, Zhong H, Jiang X, Chen J. Natural plant extract tubeimoside I promotes apoptosis-mediated cell death in cultured human hepatoma (HepG2) cells. Biol Pharm Bull. 2011;34(6):831–838. | ||
Chen WJ, Yu C, Yang Z, et al. Tubeimoside-1 induces G2/M phase arrest and apoptosis in SKOV-3 cells through increase of intracellular Ca(2)(+) and caspase-dependent signaling pathways. Int J Oncol. 2012;40(2):535–543. | ||
Huang P, Yu C, Liu XQ, Ding YB, Wang YX, He JL. Cytotoxicity of tubeimoside I in human choriocarcinoma JEG-3 cells by induction of cytochrome c release and apoptosis via the mitochondrial-related signaling pathway. Int J Mol Med. 2011;28(4):579–587. | ||
Zhang Y, Xu X, He P. Tubeimoside-1 inhibits proliferation and induces apoptosis by increasing the Bax to Bcl-2 ratio and decreasing COX-2 expression in lung cancer A549 cells. Mol Med Rep. 2011;4(1):25–29. | ||
Xu Y, Chiu JF, He QY, Chen F. Tubeimoside-1 exerts cytotoxicity in HeLa cells through mitochondrial dysfunction and endoplasmic reticulum stress pathways. J Proteome Res. 2009;8(3):1585–1593. | ||
Wang F, Ma R, Yu L. Role of mitochondria and mitochondrial cytochrome c in tubeimoside I-mediated apoptosis of human cervical carcinoma HeLa cell line. Cancer Chemother Pharmacol. 2006;57(3):389–399. | ||
Danial NN, Korsmeyer SJ. Cell death: critical control points. Cell. 2004;116(2):205–219. | ||
Krammer PH, Arnold R, Lavrik IN. Life and death in peripheral T cells. Nat Rev Immunol. 2007;7(7):532–542. | ||
Robertson JD, Orrenius S. Molecular mechanisms of apoptosis induced by cytotoxic chemicals. Crit Rev Toxicol. 2000;30(5):609–627. | ||
Cheng G, Zhang Y, Zhang X, et al. Tubeimoside V (1), a new cyclic bisdesmoside from tubers of Bolbostemma paniculatum, functions by inducing apoptosis in human glioblastoma U87MG cells. Bioorg Med Chem Lett. 2006;16(17):4575–4580. | ||
Su YT, Chang HL, Shyue SK, Hsu SL. Emodin induces apoptosis in human lung adenocarcinoma cells through a reactive oxygen species-dependent mitochondrial signaling pathway. Biochem Pharmacol. 2005;70(2):229–241. | ||
Dewson G, Kluck RM. Mechanisms by which Bak and Bax permeabilise mitochondria during apoptosis. J Cell Sci. 2009;122(Pt 16):2801–2808. | ||
Kagan VE, Tyurin VA, Jiang J, et al. Cytochrome c acts as a cardiolipin oxygenase required for release of proapoptotic factors. Nat Chem Biol. 2005;1(4):223–232. | ||
Zou H, Li Y, Liu X, Wang X. An APAF-1cytochrome c multimeric complex is a functional apoptosome that activates procaspase-9. J Biol Chem. 1999;274(17):11549–11556. | ||
Hastak K, Gupta S, Ahmad N, Agarwal MK, Agarwal ML, Mukhtar H. Role of p53 and NF-kappaB in epigallocatechin-3-gallate-induced apoptosis of LNCaP cells. Oncogene. 2003;22(31):4851–4859. |
Supplementary materials
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.