Back to Journals » OncoTargets and Therapy » Volume 11
TSPAN12 is overexpressed in NSCLC via p53 inhibition and promotes NSCLC cell growth in vitro and in vivo
Authors Hu Z, Hou D, Wang X, You Z, Cao X
Received 31 October 2017
Accepted for publication 16 January 2018
Published 1 March 2018 Volume 2018:11 Pages 1095—1103
DOI https://doi.org/10.2147/OTT.S155620
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Ingrid Espinoza
Zhongwu Hu,1 Daorong Hou,2 Xiaowei Wang,3 Zhenbing You,1 Xiufeng Cao4
1Department of Thoracic Surgery, Huai’an First People’s Hospital, Nanjing Medical University, Huai’an, Jiangsu, China; 2Key Laboratory of Model Animal Research, Animal Core Facility of Nanjing Medical University, Nanjing, China; 3Department of Medical Oncology, Huai’an First People’s Hospital, Nanjing Medical University, Huai’an, China; 4Department of Surgical Oncology, Nanjing First Hospital, Nanjing Medical University, Nanjing, China
Background: Tetraspanin 12 (TSPAN12), a member of the phylogenetically ancient tetraspanin family, is linked to impaired vascularization of the eye called familial exudative vitreoretinopathy, while the functional role of TSPAN12 in lung cancer has not been well characterized.
Results: In our study, TSPAN12 is able to regulate the growth of non-small-cell lung carcinoma (NSCLC) cells both in vitro and in vivo. TSPAN12 mRNA level was significantly increased in human NSCLC samples compared with their corresponding paracancerous histologic normal tissues. In addition, TSPAN12 expression, which is frequently upregulated in NSCLC, is inversely correlated with p53 expression. Furthermore, the expression levels of TSPAN12 were also increased in three human NSCLC cell lines compared to human bronchial epithelial (16HBE) cells. Then, we studied the effects of TSPAN12 silencing by short hairpin ribonucleic acid on NSCLC cell growth in vitro and tumorigenesis in vivo, along with the effect on the p53 pathway. Knockdown of TSPAN12 in NSCLC cells inhibited cell proliferation and colony formation. In addition, knockdown of TSPAN12 increased apoptosis in NSCLC cells. Mechanistically, TSPAN12 could modulate the expression of p53, p21, and p27 in NSCLC cells. In a tumor xenograft model, TSPAN12 silencing inhibits the tumor growth of H1299 cells.
Conclusion: Taken together, our results reveal that TSPAN12 plays an important role in NSCLC and is a potential biomarker and a promising target in the treatment of NSCLC.
Keywords: NSCLC, TSPAN12, p53, survival, cell proliferation, apoptosis
Introduction
Non-small-cell lung carcinoma (NSCLC) is one of the leading causes of cancer-related mortality worldwide and accounts for about 85% of all lung cancers.1,2 The most common types of NSCLC are squamous cell carcinoma, large cell carcinoma, and adenocarcinoma, but there are several other types that occur less frequently.2 Aberrant expressions of proto-oncogenes and tumor-suppressive genes are involved in the carcinogenesis and development of NSCLC, which lead to tumor cell growth and tumor metastasis.3–6 Compared to small-cell lung carcinoma, NSCLC is relatively insensitive to chemotherapy. Presently, chemotherapy is increasingly being used to treat NSCLC both preoperatively (neoadjuvant chemotherapy) and postoperatively (adjuvant chemotherapy), and surgical resection is still the primary therapy, but the 5-year overall survival rate is just 16% for all stages.7 Considering the complex nature of NSCLC, it is of great importance to discover molecular mechanisms involved in initiation and progression of NSCLC.
Tetraspanin 12 (TSPAN12) is a member of tetraspanin family, characterized by four transmembrane domains and two extracellular loops.8 Some of tetraspanin family proteins are shown to be involved in the suppression of metastasis (eg, TSPAN27 and TSPAN29) and tumor progression (eg, TSPAN8 and TSPAN24).9–12 Most studies on TSPAN12 have been performed on familial exudative vitreoretinopathy, an inherited blinding disorder of the retinal vascular system.13,14 In vivo functional analyses using TSPAN12-deficient mice revealed that TSAPN12 contributed to retinal vascular development by cooperating with FZD4 and LRP5, and regulated Norrin-induced β-catenin signaling.14 Furthermore, TSPAN12 has been shown to promote the maturation of tumor-facilitating sheddase ADAM10 and supports human breast cancer growth.15,16 TSPAN12 knockdown in p53-depleted fibroblasts inhibited cancer cell proliferation and invasion in vitro, and inhibited tumor growth in vivo.17 Thus, TSPAN12 has been suggested to play a role in cancer progression.
Approximately half of the human cancers result from p53 inactivation by mutations and deletations.17 p53 is known as a transcription factor that regulates the expression of genes associated with cell-cycle arrest, apoptosis, and senescence to prevent tumorigenesis.18,19 The tumor suppressor p53 plays a key role in cancer biology and represents an attractive target for the development of anticancer therapies.20
Materials and methods
Patients and samples
This study was approved by the Ethics Committee of Huai’an First People’s Hospital, Nanjing Medical University, Huai’an, Jiangsu, China. Paracarcinoma lung tissues and NSCLC tissues (n=16) were collected from NSCLC patients enrolled in Huai’an First People’s Hospital, Nanjing Medical University. Written informed consent was obtained from all patients and patients underwent curative surgery without prior treatments. Tissue samples were snap-frozen in liquid nitrogen and stored at −80°C until analysis.
Immunohistochemistry (IHC) analysis
IHC was performed to detect the TSPAN12 expression level in NSCLC tissues and normal adjacent tissues by the avidin–biotin–peroxidase complex method. All sections were deparaffinized in xylene and dehydrated through a gradient concentration of alcohol. Then endogenous peroxidase activity was blocked using 0.5% H2O2 in methanol for 10 min. After nonspecific binding was blocked, the slides were incubated with rabbit polyclonal antibodies for TSPAN12 (1:100) in 1× PBS at 4°C overnight in a humidified container. Next day, biotinylated goat anti-rabbit immunoglobulin G (IgG) (1:400; Sigma-Aldrich Co.) was incubated with the sections for 1 h at room temperature and detected using a streptavidin peroxidase complex. The brown color indicative of peroxidase activity was developed by incubation with 0.1% 3, 3′-diaminobenzidine (Sigma-Aldrich Co.) in 1× PBS with 0.05% H2O2 for 5 min at room temperature.
Cell lines
Human lung carcinoma cell lines (A549, H1299, H1650) were purchased from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China), and human bronchial epithelial (16HBE) cells were from Bogoo Biotechnology (Shanghai, China). These cells were seeded and cultured in RPMI 1640 medium (HyClone) with 10% fetal bovine serum (FBS; Thermo Fisher Scientific). HEK293T cells were obtained from the Institute of Cytobiology, Chinese Academy of Sciences, and maintained in Dulbecco’s Modified Eagle’s Medium (DMEM; Thermo Fisher Scientific) with 10% FBS. All cells were cultured in a 37°C cell incubator with 5% CO2. The authentication of all cell lines had been confirmed.
Lentivirus transduction and infection
For lentivirus packaging, HEK293T cells (7×106) were plated in a 15 cm dish with DMEM containing 10% FBS for 24 h, and then transfected with 15 μg of lentivirus plasmids. The virus containing medium was harvested after transfection for 48 and 72 h. The virus was filtered through a 0.45 μm filter (EMD Millipore) and centrifuged at 20,000× g for 2 h. The precipitate was suspended in 100 μL DMEM. For infections, gradient amount of lentivirus was added into the culture medium of H1299 cells with polybrene (4 μg/mL; Sigma-Aldrich, Co.). After incubation at 37°C for 36–48 h, the infected cell populations were confirmed by fluorescence microscope for green fluorescent protein expression to evaluate the virus titer.
RNA extraction, cDNA synthesis, and qRT-PCR
Cells and tissues were lysed by Trizol reagent (Thermo Fisher Scientific). Total RNA was purified and reverse transcribed to cDNA using avian myeloblastosis virus reverse transcriptase (Takara) according to the manufacturer’s instructions. The quantification of target gene transcripts was performed using SYBR Green Realtime PCR Master Mix (Toyobo) on an ABI Prism 7900 sequence detection system (Applied Biosystems). The primer sequences for quantitative reverse transcriptase polymerase chain reaction (qRT-PCR) of TSPAN12 and β-actin were as follows: 5′-TGGCCAGAGAAGATTCCGTGA-3′ and 5′-GACTGCTTCCTCTACCCTCGTT′ for TSPAN12, 5′-GATCATTGCTCCTCCTGAGC-3′ and 5′-ACTCCTGCTTGCTGATCCAC-3′ for β-actin. mRNA expression values of TSPAN12 were normalized to the internal control β-actin. Relative expression was calculated using the cycle threshold (Ct) method.
MTT and colony formation assays
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay was performed to detect cell growth. Cells were seeded into a 96-well plate (5×103 cells/well) and grew for 24, 48, and 72 h. Cell viability was measured by MTT (Sigma-Aldrich Co.) assay as follows: 100 μL MTT-medium mixed solution (0.5 mg/mL) was added to each well and incubated with cells for 4 h. The formazan was dissolved in 200 μL dimethyl sulfoxide following removal of the MTT solutions. Finally, the optical density was measured using a spectrophotometer at an absorption wavelength of 570 nm.
For colony formation assay, the cells were seeded in to a six-well plate (400 cells/well), and cultured for 5 days. Then the clones were washed with 1× PBS and stained with crystal violet for ~20 min. Finally, the clones were imaged and quantified.
Antibodies
The antibodies purchased were as follows: anti-p53, anti-p21, and anti-p27 from Santa Cruz Biotechnology; anti-β-actin from Cell Signaling Technology; Alexa Fluor 488 goat anti-mouse IgG from Abcam. An anti-TSPAN12 antibody was generated by immunizing the rabbits with C-terminal peptides of TSPAN12 (EHTSMANSFNTHFEMEEL). The antibodies were affinity-purified against recombinant GST–TSPAN12 fusion protein coupled to Glutathione Sepharose 4B according to the manufacturer’s instructions (GE Healthcare). The cells were lysed in a buffer containing 20 mM Tris·HCl (pH 7.5), 150 mM NaCl, 5 mM EGTA, and 1% Nonidet P-40 with a mixture of protease inhibitors and phosphotase inhibitor (Sigma-Aldrich Co.) before immunoprecipitation and immunoblotting assays.
Immunoblotting studies
RIPA lysis buffer (Thermo Fisher Scientific) with protease inhibitor (Sigma-Aldrich Co.) was used to extract the total protein. The same amounts of total proteins were separated via sodium dodecyl sulfate-polyacrylamide gel electrophoresis gel and then transferred to polyvinylidene difluoride membranes (EMD Millipore). After blocking with 5% milk, the membranes were incubated with the primary antibodies overnight at 4°C and then with the horseradish peroxidase-conjugated secondary antibody (Santa Cruz Biotechnology). Signals were detected with enhanced chemiluminescence system (Bio-Rad).
Immunofluorescence studies
A549 and H1299 cells were cultured on glass slides in a 12-well plate and transfected with different plasmids. At 24 h post-transfection, the cells were fixed with 4% paraformaldehyde, permeabilized by 0.1% Triton X-100 in 1× PBS, and blocked in 3% bovine serum albumin in 1× PBS at 4°C overnight and then incubated with the antibody against p53, followed by incubation with secondary antibodies including Alexa Fluor 488 goat anti-mouse IgG. Finally, 4′,6-diamidino-2-phenylindole was used to stain the nuclei for 5 min at room temperature. Fluorescence images were acquired with a Nikon microscope (E600).
Cell apoptosis assays
Four cell lines were constructed, including TSPAN12 knockdown A549 cells, TSPAN12 knockdown H1299 cells, and control cells for them, respectively. Cells were harvested, washed, and resuspended in the binding buffer containing Annexin V/fluorescein isothiocyanate (FITC) and propidium iodide (PI) (Beyotime). Cells were stained with Annexin V-FITC to monitor apoptosis cells and PI to detect dead cells, and then detected by flow cytometry (BD Biosciences, San Diego, CA, USA).
Nude mice xenograft model
All animals were maintained and used in accordance with the guidelines of the Institutional Animal Care and Use Committee of the Nanjing Medical University. All of the experimental procedures were approved by the Nanjing Medical University ethics commission. TSPAN12 knockdown H1299 cells and control cells in the logarithmic phase of growth were trypsinized, rinsed with 1× PBS for three times, and then resuspended in 100 μL 1× PBS. Every six nude mice (6 weeks old, male) were inoculated subcutaneously with a clonal population of 5×106 cells in the upper right shoulders. Xenograft tumor sizes were measured by measuring two perpendicular diameters with digital calibers every 5 days after appearance of tumors and calculated according to the formula: 0.5 × length × width2. All of the mice were killed 1 month after inoculation and the tumors were removed instantly.
Statistical analyses
Data were presented as the mean ± SD from three independent experiments. Two-tailed Student’s t-tests were used to evaluate the data. The difference was considered to be statistically significant at p<0.05 and p<0.01.
Results
TSPAN12 is upregulated in primary lung cancer tissues
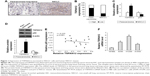
To detect the expression of TSPAN12 in lung cancer, we examined TSPAN12 expression in paracarcinoma lung tissue and lung cancer tissue by IHC. TSPAN12 was obviously upregulated in lung cancer tissues compared with normal tissues (Figure 1A). Most of the NSCLC tissues (68.8%, 11/16 cases) were found to exhibit high TSPAN12 expression. By contrast, most of the normal tissues (76.7%, 23/30 cases) expressed low TSPAN12 (Figure 1B). The qRT-PCR and Western blot analysis also indicated that mRNA and protein expression level of TSPAN12 was increased in NSCLC tissues, while the p53 level was lower in NSCLC tissues (Figure 1C and D). At the same time, TSPAN12 expression in 16 tumors and paired normal tissues from the patients was examined, and the results are presented in Table 1. Moreover, there was a negative correlation between the protein levels of TSPAN12 and p53 in NSCLC tissues (r=−0.225, p<0.0001; Figure 1E).
TSPAN12 is upregulated in human NSCLC cells
To study the role of TSPAN12 in NSCLC, we used one normal human bronchial epithelial [16HBE] cells and three different NSCLC cell lines, H1299, A549, and A1650. We analyzed the TSPAN12 expression levels of these cell lines. We found that TSPAN12 mRNA levels were significantly increased in NSCLC cell lines compared with 16HBE cells (Figure 1F).
Knockdown of TSPAN12 inhibits cell proliferation and colony formation of NSCLC cell lines
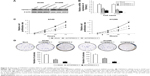
We also established A549 and H1299 cell lines with downregulated TSPAN12 expression by lentivirus infection. Western blot analysis and qRT-PCR analysis were conducted to determine the expression level of TSPAN12 in these cells (Figure 2A and B). The cell proliferation rate of these cells was investigated by MTT assay, and the results showed that the cell growth rate of NSCLC cells was significantly decreased by TSPAN12 downregulation (Figure 2C).
Next, colony formation was used to investigate the tumorigenesis potential of these cells. We found that the capacity of colony formation was reduced by TSPAN12 knockdown in NSCLC cells (Figure 2D). Therefore, these data suggest that TSPAN12 promotes the growth of NSCLC cells.
TSPAN12 regulates cell apoptosis in NSCLC cells
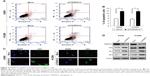
To determine how TSPAN12 promotes cell proliferation and colony formation in NSCLC cells, we examined cell apoptosis in A549 and H1299 cells with TSPAN12 knockdown. Downregulation of TSPAN12 in NSCLC cells greatly promotes cell apoptosis (Figure 3A and B). Collectively, these results suggested that TSPAN12 regulates cell proliferation in NSCLC via cell apoptosis.
TSPAN12 silencing upregulates p53, p21, and p27 in NSCLC cells
To determine whether p53 is a downstream target of TSPAN12 in NSCLC cells, expression of p53 protein in the cells with altered TSPAN12 expression was analyzed by immunofluorescence. The results showed that silencing of TSPAN12 expression in NSCLC cells dramatically increased p53 protein expression in A549 cells and wild-type p53 expression plasmid (pC53-SN3) in p53-null lung carcinoma H1299 cells. Furthermore, protein levels of p53 downstream genes like p21 and p27 were also changed after altering TSPAN12 expression in A549 and H1299 cells (Figure 3C and D). These results indicate that TSPAN12 may regulate NSCLC cell proliferation and apoptosis through affecting p53 pathway.
TSPAN12 promotes the growth of NSCLC cells in vivo
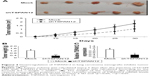
Next, we used a xenograft model to further elucidate the effect of TSPAN12 on tumor growth of NSCLC in vivo. H1299 cells with TSPAN12 knockdown were implanted into the nude mice. These mice were sacrificed in 1 month and tumors were removed. The growth of the NSCLC cells in these mice was measured by tumor volume and tumor weight. Consistent with our in vitro findings, tumors derived from TSPAN12 silenced H1299 cells grew at a much slower rate than those derived from control cells, as reflected in the significantly smaller tumor size, tumor volume (Figure 4A), and tumor weight (Figure 4B). Also, the mRNA level of TSPAN12 in these tumors was confirmed by qRT-PCR (Figure 4C). These observations, therefore, clearly indicated that TSPAN12 silencing can effectively suppress tumorigenicity of NSCLC cells in vivo.
Discussion
In this study, we have provided convincing evidence that TSPAN12 functions as a tumor promoter in human NSCLC. In the clinical NSCLC samples, TSPAN12 expression level was robustly upregulated as compared to the adjacent normal tissues. At the cellular level, knockdown of TSPAN12 in A549 and H1299 cells significantly inhibited cell growth and colony formation. At the animal level, TSPAN12 silencing strongly inhibited the growth of NSCLC in nude mice. Mechanistically, TSPAN12 could modulate apoptosis and the expression of p21, p27, and p53 in NSCLC cells and such factors likely underlie its tumor-promoting activity in these cells.
p53 is one of the most important tumor suppressor genes and plays a major role in inhibition of angiogenesis. p53 can activate DNA repair proteins, initiate apoptosis, or induce growth arrest by holding the cell cycle at the G1/S regulation point via transcriptional regulation. The activation of p53-mediated promotion of apoptosis in tumor cells is an important mechanism of antitumor drugs. Correlation analysis revealed a significantly inverse correlation between p53 and TSPAN12 protein levels in NSCLC specimens. To determine whether p53 is a downstream target of TSPAN12 in NSCLC, expression of p53 in the cells with altered TSPAN12 expression was evaluated by Western blot and immunofluorescence analysis. Furthermore, endogenous protein levels of p53 downstream genes like p21 and p27 were also changed after altering TSPAN12 expression in A549 and H1299 cells. However, molecular mechanisms underlying the link between TSPAN12 and p53 need further investigations.
Taken together, our study shows, for the first time, that TSPAN12 is overexpressed in human NSCLC tissues and NSCLC cell lines. Mechanistically, we have demonstrated that TSPAN12 silencing inhibits NSCLC cell proliferation and colony formation both in vitro and in vivo by affecting p53 pathway. High expression levels of TSPAN12 may serve as a novel molecular marker for human NSCLC and a promising target for drug development.
Acknowledgment
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work. Zhongwu Hu and Daorong Hou share first authorship.
Disclosure
The authors report no conflicts of interest in this work.
References
Goldstraw P, Ball D, Jett JR, et al. Non-small-cell lung cancer. Lancet. 2011;378:1727–1740. | ||
Ellis PG. Actionable biomarkers in a non-small cell lung cancer (NSCLC) clinical pathway (CP). Am Soc Clin Oncol. 2016;34:155. | ||
Joerger M, Baty F, Früh M, et al. Circulating microRNA profiling in patients with advanced non-squamous NSCLC receiving bevacizumab/erlotinib followed by platinum-based chemotherapy at progression (SAKK 19/05). Lung Cancer. 2014;85:306–313. | ||
Joshi P, Jeon YJ, Laganà A, et al. MicroRNA-148a reduces tumorigenesis and increases TRAIL-induced apoptosis in NSCLC. Proc Natl Acad Sci U S A. 2015;112:8650–8655. | ||
Bean J, Brennan C, Shih JY, et al. MET amplification occurs with or without T790M mutations in EGFR mutant lung tumors with acquired resistance to gefitinib or erlotinib. Proc Natl Acad Sci U S A. 2007;104:20932–20937. | ||
Balak MN, Gong Y, Riely GJ, et al. Novel D761Y and common secondary T790M mutations in epidermal growth factor receptor–mutant lung adenocarcinomas with acquired resistance to kinase inhibitors. Clin Cancer Res. 2006;12:6494–6501. | ||
Laskin JJ, Sandler AB. State of the art in therapy for non-small cell lung cancer. Cancer Invest. 2005;23:427–442. | ||
Serru V, Dessen P, Boucheix C, Rubinstein E. Sequence and expression of seven new tetraspans. Biochim Biophys Acta. 2000;16:159–163. | ||
Zoller M. Tetraspanins: push and pull in suppressing and promoting metastasis. Nat Rev Cancer. 2009;9:40–55. | ||
Sala-Valdes M, Ailane N, Greco C, Rubinstein E, Boucheix C. Targeting tetraspanins in cancer. Expert Opin Ther Targets. 2012;16:985–997. | ||
Hemler ME. Tetraspanin functions and associated microdomains. Nat Rev Mol Cell Biol. 2005;6:801–811. | ||
Yanez-Mo M, Barreiro O, Gordon-Alonso M, Sala-Valdes M, Sanchez-Madrid F. Tetraspanin-enriched microdomains: a functional unit in cell plasma membranes. Trends Cell Biol. 2009;19:434–446. | ||
Poulter JA, Ali M, Gilmour DF, et al. Mutations in TSPAN12 cause autosomal-dominant familial exudative vitreoretinopathy. Am J Hum Genet. 2010;86:248–253. | ||
Junge HJ, Yang S, Burton JB, et al. TSPAN12 regulates retinal vascular development by promoting Norrin- but not Wnt-induced FZD4/beta-catenin signaling. Cell. 2009;139:299–311. | ||
Xu D, Sharma C, Hemler ME. Tetraspanin12 regulates ADAM10-dependent cleavage of amyloid precursor protein. Faseb J. 2009;23:3674–3681. | ||
Knoblich K, Wang HX, Sharma C, et al. Tetraspanin TSPAN12 regulates tumor growth and metastasis and inhibits beta-catenin degradation. Cell Mol Life Sci. 2014;71:1305–1314. | ||
Otomo R, Otsubo C, Matsushima-Hibiya Y, et al. TSPAN12 is a critical factor for cancer–fibroblast cell contact-mediated cancer invasion. Proc Natl Acad Sci U S A. 2014;111:18691–18696. | ||
Vousden KH, Prives C. Blinded by the light: the growing complexity of p53. Cell. 2009;137:413–431. | ||
Menendez D, Inga A, Resnick MA. The expanding universe of p53 targets. Nat Rev Cancer. 2009;9:724–737. | ||
Stegh AH. Targeting the p53 signaling pathway in cancer therapy – the promises, challenges and perils. Expert Opin Ther Targets. 2012;16:67–83. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.