Back to Journals » OncoTargets and Therapy » Volume 12
Triterpenoid Saponins from Anemone flaccida Suppress Tumor Cell Proliferation by Regulating MAPK, PD1/PDL1, and STAT3 Signaling Pathways and Altering Cancer Metabolism
Authors Han L, Yao S, Cao S, Mo G, Li J, Cao Y, Huang F
Received 17 April 2019
Accepted for publication 26 November 2019
Published 12 December 2019 Volume 2019:12 Pages 10917—10930
DOI https://doi.org/10.2147/OTT.S212666
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jianmin Xu
Lintao Han,1 Shiqi Yao,2 Sa Cao,2 Guoyan Mo,1 Jingjing Li,1 Yan Cao,2 Fang Huang1
1Key Laboratory of Traditional Chinese Medicine Resource and Prescription, Ministry of Education, Wuhan, Hubei 430061, People’s Republic of China; 2College of Pharmacy, Hubei University of Chinese Medicine, Wuhan, Hubei 430065, People’s Republic of China
Correspondence: Yan Cao
College of Pharmacy, Hubei University of Chinese Medicine, No. 1 Huang-Jia-Hu Road, Wuhan, Hubei 430065, People’s Republic of China
Tel/Fax +86 27 68896121
Email [email protected]
Fang Huang
Key Laboratory of Traditional Chinese Medicine Resource and Prescription, Ministry of Education, No. 1 Tan-Hua-Lin Road, Wuhan, Hubei 430061, People’s Republic of China
Tel/Fax +86 27 68890129
Email [email protected]
Purpose: Natural triterpenoid saponins isolated from Anemone flaccida Fr. Schmidt have exhibited anti-cancer properties and exerted remarkable inhibitory effects on tumor growth. Herein, we investigated the potential mechanism involved in the suppression of hepatocellular carcinoma (HCC) development by triterpenoid saponins in a mouse model.
Methods: An HCC model was established in H22 tumor-bearing mice and triterpenoid saponins were administered at various doses. Immunofluorescence, flow cytometry, and western blot were performed to analyze the effect of triterpenoid saponins on immune response in tumor tissues. Metabolomic analysis was carried out to assess the metabolites involved in mediating the effect of triterpenoid saponins on tumor tissues.
Results: Triterpenoid saponins induced anti-tumor immune response by decreasing the number of Treg cells, increasing that of B cells, natural killer cells, and CD3+/CD28+ T cells, and reducing the secretion of inflammatory factors including nuclear factor-κB, cyclooxygenase-2, and microsomal prostaglandin E synthase-1. In addition, triterpenoid saponins inhibited tumor growth and induced the apoptosis of HCC cells by blocking the activation of PD1/PD-L1, ERK1/2, p38 MAPK, JNK, and STAT3 signaling pathways. Furthermore, triterpenoid saponins regulated tumor immune response by upregulating a number of metabolites (including 1,3-diaminopropane, lauric acid, 2,4-diaminobutyric acid 2, and ribitol) and modulating the metabolism of histidine, arginine, proline, beta-alanine, glycine, serine, and threonine.
Conclusion: The findings suggested that triterpenoid saponins interfered with multiple signaling cascades involved in tumorigenesis and tumor metabolism and have potential applications in HCC therapy.
Keywords: triterpenoid saponins, hepatocellular carcinoma, immune response, MAPK, PD1/PD-L1, STAT3
Introduction
Hepatocellular carcinoma (HCC) is one of the leading causes of cancer-related deaths and the fifth most prevalent neoplasm worldwide,1 with more than 700,000 new cases diagnosed annually, most at an advanced stage.2 Despite remarkable advances in diagnosis and improvements in therapeutic strategies, the long-term survival of HCC patients remains unsatisfactory because of the high rates of intrahepatic and distal metastasis.3,4 It is critical to identify the potential molecular mechanisms underlying the progression and metastasis of HCC to provide novel therapeutic targets for cancer treatment.
Alternative medicine has emerged as a complement to conventional anti-cancer treatment schemes such as chemotherapy and radiotherapy. In particular, the components of natural herbal medicine have recently gained attention as natural compounds that act as anti-cancer agents with reduced side effects compared to those of clinical therapeutic drugs. Among these, Anemone flaccida Fr. Schmidt is a well-known medicinal plant that is widely distributed in China and has been used as a component in folk medicine to treat neuralgia and rheumatism. Saponins are a class of chemical compounds found in nature and function as bioactive constituents in many plants because of their anti-inflammatory, anti-viral, anti-bacterial, and anti-cancer properties.5 It was reported that saponins from Anemone david inhibited the proliferation of subcutaneous tumor S180 in mice,6 and saponins isolated from Rosa laevigata Michx showed significant hepatoprotective effects against carbon tetrachloride-induced acute liver damage in mice.7 Our previous study has demonstrated that triterpenoid saponins from A. flaccida exerted inhibitory effects on HeLa cell proliferation and induced HeLa cell apoptosis via activation of caspase-3.8
Recently, the effect of saponins on liver diseases, especially HCC, has elicited widespread concern. Specifically, the participation of saponins in mediating signaling pathways involved in various physiological phenomena is of interest. The dysregulation of certain pathways occurs in nearly half of all early and almost all advanced cases of HCC.9–11 For example, mitogen-activated protein kinases (MAPKs) are a highly evolutionarily conserved family of serine/threonine kinases that are involved in the regulation of tumor cell proliferation, differentiation, migration, survival, and angiogenesis.12 Members of the MAPK family include extracellular signal-regulated kinase (ERK) 1/2, c-Jun N-terminal kinase (JNK), and p38. Programmed cell death-1 (PD1) is a member of the CD28 receptor family, and its ligand PD-ligand 1 (PD-L1) plays a fundamental role in tumor immune escape by inhibiting immune effector functions. PD1 expression is induced in antigen-activated T cells and exhausted T and B cells, while PD-L1 is mainly expressed by antigen-presenting cells and non-hematopoietic cells.10 Likewise, signal transducer and activator of transcription 3 (STAT3) regulates multiple oncogenic pathways, including tumor cell survival, angiogenesis, and evasion from the immune system.13
The aim of the present study was to evaluate the effect of triterpenoid saponins from A. flaccida against HCC in mice by establishing a model of HCC using H22 hepatoma cells. The possible underlying mechanisms were evaluated to elucidate the potential protective effect of triterpenoid saponins against HCC via suppression of inflammation and apoptosis. A metabolomics approach was also employed to determined the involvement of tumor metabolism therein.
Materials and Methods
Plant Material and Preparation of Triterpenoid Saponins
The rhizome of A. flaccida Fr. Schmidt was obtained from Jiufeng County (Hubei province, China) and identified by Prof. Keli Chen (Department of Pharmacology, Hubei College of Traditional Chinese Medicine). Triterpenoid saponins were extracted according to a previously described method. Briefly, 5 kg of dried A. flaccida was extracted by CH3OH at room temperature. The extract was filtered, concentrated, and chromatographed. The residue obtained from the 30% EtOH fraction was further separated on a low-pressure reverse-phase C18 column using CH3OH2H2O. By similar procedures, triterpenoid saponins were obtained from the 50% EtOH fraction. The structures of the compounds were determined by chemical and spectral analysis. The following five compounds were identified (Figure S1): glycoside St-I4a (1), glycoside St-J (2), anhuienoside E (3), hederasaponin B (4), and flaccidoside II (5).
Animals and Experimental Design
All animal experiments were carried out according to the institutional guidelines for animal care and approved by the Animal Ethics Committee of Hubei University of Chinese Medicine. The mouse hepatoma model was established according to a previously reported method with some modifications.2 The murine hepatoma cell line H22 was obtained from BeNa Culture Collection (BNCC100746). Forty Balb/c mice weighing 20 ± 2 g were purchased from the Hubei Provincial Center for Disease and Prevention. The animals were maintained in a specific-pathogen-free environment at a temperature 23 ± 2°C in a 12/12-h light/dark cycle. Water and standard rodent chow were provided ad libitum. After one week of adaptive breeding, the mice were randomly divided into five groups (n = 8 in each group): control (CON), model (MOD), low-dose total saponins (LOW), medium-dose total saponins (MED), and high-dose total saponins (HIG).
H22 cells were diluted to a concentration of 1 × 107 cells/mL with phosphate-buffered saline (PBS) under sterile conditions. Then, 0.2 mL of cells were inoculated subcutaneously into the right armpit of each mouse to establish the hepatoma model (MOD). Mice in the LOW, MED, and HIG group were administered orally with total saponins daily at doses of 5 mg/kg, 10 mg/kg, and 15 mg/kg, respectively. Animals in the CON and MOD groups received equivalent volumes of solvent. Tumor size was measured on alternate days using a vernier caliper and mouse body weight was recorded once every two days. After 14 days of treatment, the mice were sacrificed and tumor tissue and blood were sampled. The tumor and spleen were isolated and weighed immediately after the mice were sacrificed. Immune organ index was calculated using the following equation: Immune organ index = mean organ weight (mg)/mean body weight (g).
Immunofluorescence
Fresh transplanted tumor tissues were extracted and embedded in paraffin wax. Tissue sections were prepared on microscopic slides and deparaffinized for staining. After antigen retrieval and endogenous peroxidase removal, the tissue sections were washed with PBS, fixed in 4% paraformaldehyde for 10 min, and permeabilized at room temperature. Non-specific binding was blocked with 1% bovine serum albumin for 1 h and the samples were incubated with primary antibodies against PD1 (ab214421, 1:100, Abcam) or PD-L1 (ab224030, 1:50, Abcam) overnight at 4°C. Thereafter, the sections were incubated with rabbit IgG conjugated to Alexa Fluor® 488 (ab15077, 1:200, Abcam) secondary antibody for 1 h at room temperature. Nuclei were stained with DAPI for 5 min at room temperature. Immunopositive cells were analyzed using a fluorescence microscope (Zeiss Axiophot II, Oberkochen, Germany) at 40× magnification (excitation 590 nm; emission 650 nm). The quantification of fluorescence images was performed using ImageJ software.
Flow Cytometry of Lymphocytes in Spleen and Tumor Tissues
Lymphocytes from mouse spleen and tumor tissues were isolated in sterile conditions. Spleen tissues were placed in 10 mL of PBS and then placed in a 200-mesh aseptic milling steel line. The tissues were repeatedly milled, filtered twice, centrifuged at 1500 rpm for 10 min at 4°C, and washed twice with PBS. Mouse lymphocyte separating medium was added and the tissues were centrifuged at 1500 rpm for 20 min to absorb the mononuclear cell layer. Cells were washed twice with PBS and the concentration was adjusted to 1 × 106 cells/mL. Flow cytometry data was acquired from a splenic cell suspension using the following antibodies: CD19-fluorescein isothiocyanate (FITC) (11-0193-82), CD25-phycoerythrin (PE)-cyanine 5 (Cy5) (15-0251-82), CD3-FITC (11-0031-82), CD4-FITC (11-0041-82), CD69-PE (12-0691-82), CD28-PE-Cy5 (15-0281-82), CD335-PE (11-3351-82), and Foxp3 (12-5773-80). All antibodies were purchased from eBioscience.
Western Blot
Western blot was performed to detect the expression of relevant proteins. Tumor samples were washed twice with PBS and homogenized in radioimmunoprecipitation assay lysis buffer (Beyotime, Wuhan, China) containing protease inhibitor at 4°C and centrifuged at 12000 × g for 15 min. The concentration of the proteins was measured by a bicinchoninic acid assay (Beyotime, Wuhan, China). Proteins (30 μg) were separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred onto polyvinylidene fluoride membranes (Millipore, USA). The membranes were blocked with 5% skim milk for 2 h at room temperature in Tris-buffered saline. Then, the membranes were incubated overnight at 4°C with primary antibodies against PD1 (ab36151, 1:250), PD-L1 (213480, 1:1000), nuclear factor (NF)-κβ (32360, 1:1000), cyclooxygenase-2 (COX-2, ab62331, 1:1000), p38 MAPK (ab170099, 1:1000), c-Jun N-terminal kinases (JNK, ab110724, 1:1000), p-JNK (ab4821, 1:1000), extracellular signal-regulated kinase 1/2 (ERK1/2, ab184699, 1:5000), p-ERK1/2 (ab214036, 1:1000), STAT3 (ab68153, 1:1000), p-STAT3 (ab76315, 1:20,000), and microsomal prostaglandin E synthase-1 (mPGES1, ab62050, 1:1000), all purchased from Abcam (Cambridge, UK). GAPDH (2118, 1:1000, CST, MA, USA) was selected as an internal reference. Then, the membranes were washed with Tris-buffered saline and incubated in goat IgG secondary antibody (PAB160011, 1:10,000, Bioswamp, Wuhan, China) for 2 h at room temperature. Immunoreactivity was visualized by colorimetric reaction using enhanced chemiluminescence substrate buffer (Millipore, MA, USA). Membranes were scanned with Gel Doz EZ imager (Bio-rad, USA).
Targeted Metabolomics Using Gas Chromatography Time-of-Flight Mass Spectrometry (GC-TOFMS)
Metabolomic analysis of tumor tissues from the MOD and HIG groups was performed using GC-TOFMS as previously reported.14 Tumor tissue samples were homogenized and extracted with 1 mL of degassed isopropanol/acetonitrile/water (3/3/2) at 4°C for 5 min. The extracts were subsequently dried and re-suspended in 50% aqueous acetonitrile to remove most of the complex lipids. After dry evaporation, the extracts were derivatized and subjected to GC-TOFMS (Leco Pegasus IV) fitted with automatic liner exchange-cold injection (Gerstel). Raw data were deconvoluted using ChromaTOF (Leco) and mass spectra were exported for further data processing by the BinBase database, including identification of metabolites. The impact of individual metabolic pathways was determined by topological analysis.
Statistical Analysis
Statistical analysis was performed by SPSS 19.0 software and the results are presented as the mean ± standard deviation (SD). Comparison between different groups was performed using one-way analysis of variance. P < 0.05 was considered to indicate a statistically significant difference.
Results
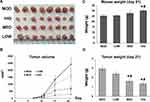
Triterpenoid Saponins Suppressed H22-Transplanted Tumor Growth
To determine the inhibitory effect of triterpenoid saponins on tumor growth in a macroscopic perspective, we monitored the tumor volume (Figure 1A and B) over 21 days and measured the mouse and tumor weight at the end of the experimental period (Figure 1C and D). Over the experimental period, tumor growth was suppressed by triterpenoid saponins to various extents depending on dose, with the HIG group showing the greatest growth inhibition. The average tumor weight in the HCC model mice was 2.92 ± 0.37 g. Compared with the MOD group, the average tumor weights of mice treated with medium and high doses of triterpenoid saponins decreased significantly to 1.62 ± 0.24 g and 1.34 ± 0.19 g, respectively, with corresponding tumor inhibition rates of 44.47% and 54.05%, respectively. Furthermore, the body weight of the mice treated with high-dose saponins was higher than that of mice in the model and low-dose groups.
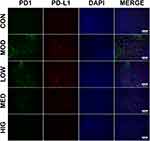
Triterpenoid Saponins Inhibited the Expression of PD1 and PD-L1 in H22 Tumors
Immunofluorescence was performed to detect the level and localization of PD1 and PD-L1 in H22 tumor tissues (Figure 2). Triterpenoid saponin treatment resulted in the downregulation of PD1 and PD-L1 in a dose-dependent manner, as indicated by the reduction in fluorescence level observed in the LOW, MED, and HIG groups compared to that of the MOD group. In particular, high-dose triterpenoid saponins showed the most drastic effect, as demonstrated by the lowest intensity of PD1- and PD-L1-positive staining in the tumor tissues.
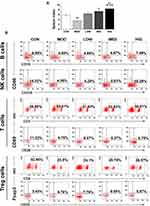
Triterpenoid Saponins Altered Spleen Index and Recruitment of Immune Effector Cells
To examine the effect of triterpenoid saponins on the immune system, we assessed the spleen index and recruitment of immune effector cells in the host animals. The results indicated that the spleen index of the HIG group was higher than that of the MOD group (Figure 3A), suggesting that triterpenoid saponins exerted beneficial influence on immune organs. Analysis of B cell and T cell populations from the spleen revealed that the proportion of B cells, natural killer (NK) cells, and CD3+/CD28+ cells decreased and that of CD4+/CD25+ Treg cells increased significantly in the MOD group compared with those in the control group. However, compared to the MOD group, HIG significantly increased the proportion of B cells, NK cells, and CD3+/CD28+ cells and decreased that of CD4+/CD25+ Treg cells (Figure 3B). The infiltration of immune effector cells was also evaluated in tumor tissues (Figure 4). Consistent with expression in the spleen, triterpenoid saponins increased the proportion of B cells and NK cells and decreased that of CD4+/CD25+ Treg cells in a dose-dependent manner. However, the proportion of CD3+/CD28+ T cells showed a decrease in tumor tissues.
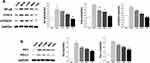
Triterpenoid Saponins Downregulated the Expression of Proteins Associated with Inflammation, Apoptosis, and MAPK Cascade
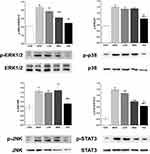
As shown in Figure 5A, the protein levels of COX-2, mPGES1, and NF-κβ in the MOD group were markedly increased compared with those in the control group. However, triterpenoid saponins decreased the levels of these proteins in a dose-dependent manner, with HIG exerting the most prominent effect. The protein expression of PD1 and PD-L1 showed similar trends (Figure 5B). We next determined whether triterpenoid saponins regulated the MAPK cascade including ERK1/2, p38, JNK, and STAT3 pathways (Figure 6). In HCC tumor tissues, the levels of phosphorylated ERK1/2, p38, JNK, and STAT3 relative to the respective total protein content were upregulated compared to those in the control group. Triterpenoid saponins downregulated the relative levels of p-ERK1/2, p-p38, p-JNK, and p-STAT3 in a dose-dependent manner, with high-dose exerting the greatest inhibitory effect.
Heat Map and Metabolomic Analysis of Changes in Primary Metabolite Levels
Tumor metabolism is actively involved in the process of cancer development and is tightly correlated with immune response and signal transduction.15 Thus, a metabolomic approach was employed to assess the participation of tumor metabolism underlying the therapeutic mechanism of triterpenoid saponins. The assessment of primary metabolites by GC-TOFMS is demonstrated by heat mapping (Figure 7). The results consist of annotated metabolites including saccharides, amino acids, and metabolites related to the tricarboxylic acid cycle and fatty acid metabolism. To evaluate the tumor tissues from a metabolite-based molecular approach, we analyzed the classifying power of each metabolite. The fold changes in these marker metabolites are shown in Table 1. We found that eight metabolites were increased and three were decreased in the HIG group. Furthermore, Figure 8 indicates that the differential metabolites were mainly enriched in histidine metabolism; arginine and proline metabolism; beta-alanine metabolism; and glycine, serine, and threonine metabolism.
 |
Table 1 Screening of Differentially Expressed Metabolites in Tumor Tissues Between MOD and HIG Groups |
Discussion
We have previously reported that triterpenoid saponins found in the rhizome of A. flaccida Fr. Schmidt exerted anti-tumor effects on HeLa cells by inducing apoptosis and reducing cell proliferation.8 Herein, we demonstrated the significant anti-tumor effect of triterpenoid saponins on HCC. Notably, this is the first study to demonstrate that triterpenoid saponin therapy negatively regulated the expression of PD1/PD-L1, ERK1/2, p38 MAPK, JNK, and STAT3 and inhibited inflammatory response by regulating immune effector cells and inflammatory mediator release.
The immune system plays an important role in immunosurveillance against tumor cells and acts as a primary defense against cancer.16 Triterpenoid saponins extracted from A. flaccida induced remarkably higher spleen indexes than that of non-treated mice subjected to HCC, indicating that triterpenoid saponins effectively boosted the immune system in H22 tumor-bearing mice. Furthermore, our studies uncovered novel roles of triterpenoid saponins as a positive regulator of B and T lymphocyte function, as triterpenoid saponin treatment increased the percentages of B cells, NK cells, and CD3+/CD28+ T cells. Meanwhile, the percentage of CD4+/CD25+ Treg cells, which play an important regulatory role in immune regulation, transplantation, and tumor immunity,17 was decreased. Tumor-associated immune suppressor cells such as Treg cells can effectively block anti-tumor immune response, representing an important obstacle for immunotherapy. Effective outcome of anti-tumor immunotherapy may be obtained by attenuating Treg suppressor function. It was reported that cyclophosphamide administration depleted Treg selectively and induced significant anti-tumor immune response in a murine HCC model.18 Moreover, antigen-hyperactivated T cells and exhausted T cells and B cells were found in the process of tumor development. In this study, triterpenoid saponins reduced the number of Treg cells and increased that of immune cells including B cells, NK cells, and CD3+/CD28+ T cells in spleen lymphocytes of H22 tumor-bearing mice, suggesting that triterpenoid saponins induced anti-H22 tumor immune response.
The ability of cancer cells to invade its surrounding is an important hallmark of tumor malignancy and results from aberrant cell signaling mechanisms. The signaling pathways involved in tumor metastasis are composed of a complex network of components. The STAT3 and NF-κβ signal pathways are constitutively activated in many malignant human tumor cells.19 Activated NF-κβ modulates pro-inflammatory mediators including interleukin-1β, interleukin-6, and tumor necrosis factor-α. In turn, tumor necrosis factor-α can increase the expression of COX-2, which induces the progress of tumor development along with pro-inflammatory cytokines.20 mPGES1, one of the PGE2 synthases, is co-localized and functionally coupled with COX-2.21 Induction of mPGES-1 was also accompanied by COX-2 induction in colon cancer tissues.20 In this study, we found that triterpenoid saponins significantly inhibited the expression of NF-κβ, COX-2, and mPGES1 in HCC. We inferred thus that triterpenoid saponins might exert anti-tumor effects by inhibiting immune factor release.
Several studies have defined the immune checkpoint receptor PD1/PD-L1 as a key signaling pathway regulating the critical balance between immune activation and tolerance.22–24 Recent studies have suggested that PD1/PD-L1 signaling enables escape from immune surveillance, transforming the tumor microenvironment into a tumor-protective and immune-suppressive milieu.25,26 In addition, Qin et al revealed that cisplatin mediated hepatoma drug resistance via the PD1/PD-L1 signaling pathway.10 In this study, H22-derived tumors showed elevated expression of PD1 and PDL-1, which was attenuated by triterpenoid saponins. In cancer cells, PD-L1 plays a crucial role in suppressing overall T-cell response and the development of cancer immunoresistance by binding to PD1 receptors on activated T cells, inhibiting the proliferation of tumor-reactive cytotoxic T cells and inducing the Treg phenotype.27 Blocking PD1/PD-L1 using clinically relevant PD1 monoclonal antibodies restored immune response and achieved remarkable clinical response in solid tumors including melanoma and lung cancer, providing a promising novel immunotherapeutic strategy.
The biological significance of the PD1/PD-L1 pathway is only beginning to be understood recently. Both the Janus kinase/STAT and MAPK signal pathways are known to be involved in PD1/PD-L1 activation.27 The importance of MAPK and STAT3 signaling in regulating various physiological processes in HCC treatment has been proposed. A study demonstrated that total saponins interfered with p38 signaling cascades involved in tumorigenesis and had potential application in cancer therapy.28 In addition, inhibiting the activation of reactive oxygen species-mediated p38/MAPK, Akt, and STAT3 signal pathways induced apoptosis in human hepatoma Hep3B cells.29 Our data indicated that the activation of ERK1/2, p38, JNK, and STAT3 signaling pathways in H22-induced hepatoma was diminished by triterpenoid saponins.
Furthermore, the results of metabolomic analysis revealed that triterpenoid saponins influenced various tumor metabolites. Tumor metabolism involves various phenomena such as glucose, amino acid, and lipid metabolism. The correlation between cancer metabolism, tumor immunity, and signal transduction is widely known, and the interplay between these phenomena affects malignant transformation and tumor control and clearance.30 In particular, the activation of T lymphocytes can alter glucose metabolism, in turn triggering changes in the tumor microenvironment and molecular signaling that affects tumor survival and progression.31,32 Nutrient availability and amino acid content are also factors that could lead to changes in immune response and tumor development via a variety of signaling pathways, such as PI3K/Akt/mTOR.33 Amino acids are the basic buildings blocks required to form proteins and are indispensable nutrients in all organisms that participate in many physiological processes. Studies have shown that abnormal amino acid metabolism is related to tumor progression34,35 and can affect tumor development and growth by regulating the immune system.36,37 In recent years, amino acid metabolic pathways have become important targets in anti-tumor immune therapy.38 In the analysis of metabolic pathways, we found that histidine, arginine, proline, beta-alanine, glycine, serine, and threonine metabolism were highly associated with and affected by different metabolites, and these amino acids might be involved in regulating tumor growth and immune response. Arginine is a non-essential amino acid that can be produced by proline, and tumor growth is promoted by feeding arginine in mice.39 It can improve immune function in patients with colorectal cancer after operation and enhance parenteral nutrition effect.40 According to our results, arginine metabolism was significantly affected by triterpenoid saponins. Taking into account previous studies that reported the relationship between arginine metabolism and inflammation,41–43 the regulation of tumor metabolism by traditional Chinese medicine can be clarified. In addition, the inhibition of glycine and serine metabolism by triterpenoid saponins could restrict tumor growth by reducing the production of nucleic acids and cell energy metabolism.44 Hence, we suggest that triterpenoid saponins exerted anti-tumor effect by interfering with the tumor immune system through mediating amino acid metabolism.
Conclusion
Our results proved that triterpenoid saponins dramatically inhibited transplanted tumor growth and induced anti-tumor immune response by regulating immunological effector cells, reducing the release of pro-inflammatory factors, and blocking the activation of PD1/PD-L1 signaling. Triterpenoid saponins also induced apoptosis and suppressed tumor cell invasion by blocking the MAPK and STAT3 pathways. In addition, triterpenoid saponins regulated tumor growth and immune response by modulating the metabolism of histidine, arginine, proline, beta-alanine, glycine, serine, and threonine. These results suggest that triterpenoid saponins have the potential to attenuate the occurrence of hepatoma. However, further studies on orthotopic human cancer are needed to validate the therapeutic use of this agent in humans.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30. doi:10.3322/caac.21442
2. Yang F, Li J, Zhu J, Wang D, Chen S, Bai X. Hydroxysafflor yellow A inhibits angiogenesis of hepatocellular carcinoma via blocking ERK/MAPK and NF-kappaB signaling pathway in H22 tumor-bearing mice. Eur J Pharmacol. 2015;754:105–114. doi:10.1016/j.ejphar.2015.02.015
3. van den Bosch MA, Defreyne L. Hepatocellular carcinoma. Lancet. 2012;380(9840):469–470. doi:10.1016/S0140-6736(12)61284-7
4. Maluccio M, Covey A. Recent progress in understanding, diagnosing, and treating hepatocellular carcinoma. CA Cancer J Clin. 2012;62(6):394–399. doi:10.3322/caac.21161
5. Zhang Y, He L, Meng L, Luo W. Taspine isolated from Radix et Rhizoma Leonticis inhibits proliferation and migration of endothelial cells as well as chicken chorioallantoic membrane neovascularisation. Vascul Pharmacol. 2008;48(2–3):129–137. doi:10.1016/j.vph.2008.01.008
6. Peng S, Xun L, Wang F, Ding L. Antitumor effect of the saponins from Anemone davidii. Nat Prod Res Develop. 2001;13:60–62.
7. Dong D, Zhang S, Yin L, et al. Protective effects of the total saponins from Rosa laevigata Michx fruit against carbon tetrachloride-induced acute liver injury in mice. Food Chem Toxicol. 2013;62:120–130. doi:10.1016/j.fct.2013.08.050
8. Han LT, Li J, Huang F, Yu SG, Fang NB. Triterpenoid saponins from Anemone flaccida induce apoptosis activity in HeLa cells. J Asian Nat Prod Res. 2009;11(2):122–127. doi:10.1080/10286020802573818
9. Ito Y, Sasaki Y, Horimoto M, et al. Activation of mitogen-activated protein kinases/extracellular signal-regulated kinases in human hepatocellular carcinoma. Hepatology. 1998;27(4):951–958. doi:10.1002/(ISSN)1527-3350
10. Qin X, Liu C, Zhou Y, Wang G. Cisplatin induces programmed death-1-ligand 1(PD-L1) over-expression in hepatoma H22 cells via Erk/MAPK signaling pathway. Cell Mol Biol (Noisy-Le-Grand). 2010;56(Suppl):Ol1366–1372.
11. Xiong H, Zhang ZG, Tian XQ, et al. Inhibition of JAK1, 2/STAT3 signaling induces apoptosis, cell cycle arrest, and reduces tumor cell invasion in colorectal cancer cells. Neoplasia. 2008;10(3):287–297. doi:10.1593/neo.07971
12. Li P, Xue WJ, Feng Y, Mao QS. Long non-coding RNA CASC2 suppresses the proliferation of gastric cancer cells by regulating the MAPK signaling pathway. Am J Transl Res. 2016;8(8):3522–3529.
13. Haura EB, Zheng Z, Song L, Cantor A, Bepler G. Activated epidermal growth factor receptor-Stat-3 signaling promotes tumor survival in vivo in non-small cell lung cancer. Clin Cancer Res. 2005;11(23):8288–8294. doi:10.1158/1078-0432.CCR-05-0827
14. Kind T, Wohlgemuth G, Lee DY, et al. FiehnLib: mass spectral and retention index libraries for metabolomics based on quadrupole and time-of-flight gas chromatography/mass spectrometry. Anal Chem. 2009;81(24):10038–10048. doi:10.1021/ac9019522
15. Singer K, Cheng WC, Kreutz M, Ho PC, Siska PJ. Immunometabolism in cancer at a glance. Dis Model Mech. 2018;11(8):dmm034272. doi:10.1242/dmm.034272
16. Yang J, Li X, Xue Y, Wang N, Liu W. Anti-hepatoma activity and mechanism of corn silk polysaccharides in H22 tumor-bearing mice. Int J Biol Macromol. 2014;64:276–280. doi:10.1016/j.ijbiomac.2013.11.033
17. Sakaguchi S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat Immunol. 2005;6(4):345–352. doi:10.1038/ni1178
18. Motoyoshi Y, Kaminoda K, Saitoh O, et al. Different mechanisms for anti-tumor effects of low- and high-dose cyclophosphamide. Oncol Rep. 2006;16(1):141–146.
19. Haftchenary S, Avadisian M, Gunning PT. Inhibiting aberrant Stat3 function with molecular therapeutics: a progress report. Anticancer Drugs. 2011;22(2):115–127. doi:10.1097/CAD.0b013e328341185b
20. Oshima H, Oshima M, Inaba K, Taketo MM. Hyperplastic gastric tumors induced by activated macrophages in COX-2/mPGES-1 transgenic mice. EMBO J. 2004;23(7):1669–1678. doi:10.1038/sj.emboj.7600170
21. Lazarus M, Kubata BK, Eguchi N, Fujitani Y, Urade Y, Hayaishi O. Biochemical characterization of mouse microsomal prostaglandin E synthase-1 and its colocalization with cyclooxygenase-2 in peritoneal macrophages. Arch Biochem Biophys. 2002;397(2):336–341. doi:10.1006/abbi.2001.2614
22. Ishida Y, Agata Y, Shibahara K, Honjo T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992;11(11):3887–3895. doi:10.1002/embj.1992.11.issue-11
23. Iwai Y, Ishida M, Tanaka Y, Okazaki T, Honjo T, Minato N. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci U S A. 2002;99(19):12293–12297. doi:10.1073/pnas.192461099
24. Freeman GJ, Long AJ, Iwai Y, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192(7):1027–1034. doi:10.1084/jem.192.7.1027
25. Duraiswamy J, Freeman GJ, Coukos G. Therapeutic PD-1 pathway blockade augments with other modalities of immunotherapy T-cell function to prevent immune decline in ovarian cancer. Cancer Res. 2013;73(23):6900–6912. doi:10.1158/0008-5472.CAN-13-1550
26. Duraiswamy J, Freeman GJ, Coukos G. Dual blockade of PD-1 and CTLA-4 combined with tumor vaccine effectively restores T-cell rejection function in tumors–response. Cancer Res. 2014;74(2):
27. Liu J, Hamrouni A, Wolowiec D, et al. Plasma cells from multiple myeloma patients express B7-H1 (PD-L1) and increase expression after stimulation with IFN-{gamma} and TLR ligands via a MyD88-, TRAF6-, and MEK-dependent pathway. Blood. 2007;110(1):296–304.
28. Zhan Y, Liu R, Wang W, Li J, Yang XO, Zhang Y. Total saponins isolated from Radix et Rhizoma Leonticis suppresses tumor cells growth by regulation of PI3K/Akt/mTOR and p38 MAPK pathways. Environ Toxicol Pharmacol. 2016;41:39–44. doi:10.1016/j.etap.2015.10.008
29. Liu C, Shen GN, Luo YH, et al. Novel 1,4-naphthoquinone derivatives induce apoptosis via ROS-mediated p38/MAPK, Akt and STAT3 signaling in human hepatoma Hep3B cells. Int J Biochem Cell Biol. 2018;96:9–19. doi:10.1016/j.biocel.2018.01.004
30. O’Sullivan D, Sanin DE, Pearce EJ, Pearce EL. Metabolic interventions in the immune response to cancer. Nat Rev Immunol. 2019;19(5):324–335. doi:10.1038/s41577-019-0140-9
31. Allison KE, Coomber BL, Bridle BW. Metabolic reprogramming in the tumour microenvironment: a hallmark shared by cancer cells and T lymphocytes. Immunology. 2017;152(2):175–184. doi:10.1111/imm.12777
32. Cascone T, McKenzie JA, Mbofung RM, et al. Increased tumor glycolysis characterizes immune resistance to adoptive T cell therapy. Cell Metab. 2018;27(5):977–987 e974. doi:10.1016/j.cmet.2018.02.024
33. Sugiura A, Rathmell JC. Metabolic Barriers to T Cell Function in Tumors. J Immunol. 2018;200(2):400–407. doi:10.4049/jimmunol.1701041
34. Wise DR, DeBerardinis RJ, Mancuso A, et al. Myc regulates a transcriptional program that stimulates mitochondrial glutaminolysis and leads to glutamine addiction. Proc Natl Acad Sci U S A. 2008;105(48):18782–18787. doi:10.1073/pnas.0810199105
35. Zhao E, Ding J, Xia Y, et al. KDM4C and ATF4 cooperate in transcriptional control of amino acid metabolism. Cell Rep. 2016;14(3):506–519. doi:10.1016/j.celrep.2015.12.053
36. Cruz M, Maldonado-Bernal C, Mondragon-Gonzalez R, et al. Glycine treatment decreases proinflammatory cytokines and increases interferon-gamma in patients with type 2 diabetes. J Endocrinol Invest. 2008;31(8):694–699. doi:10.1007/BF03346417
37. Bansal V, Ochoa JB. Arginine availability, arginase, and the immune response. Curr Opin Clin Nutr Metab Care. 2003;6(2):223–228. doi:10.1097/00075197-200303000-00012
38. Ananieva E. Targeting amino acid metabolism in cancer growth and anti-tumor immune response. World J Biol Chem. 2015;6(4):281–289. doi:10.4331/wjbc.v6.i4.281
39. Yerushalmi HF, Besselsen DG, Ignatenko NA, et al. Role of polyamines in arginine-dependent colon carcinogenesis in Apc(Min) (/+) mice. Mol Carcinog. 2006;45(10):764–773. doi:10.1002/mc.20246
40. Song JX, Qing SH, Huang XC, Qi DL. Effect of parenteral nutrition with L-arginine supplementation on postoperative immune function in patients with colorectal cancer. Acad j First Med Coll PLA. 2002;22(6):545–547.
41. Geiger R, Rieckmann JC, Wolf T, et al. L-arginine modulates T cell metabolism and enhances survival and anti-tumor activity. Cell. 2016;167(3):829–842 e813. doi:10.1016/j.cell.2016.09.031
42. Feldmeyer N, Wabnitz G, Leicht S, et al. Arginine deficiency leads to impaired cofilin dephosphorylation in activated human T lymphocytes. Int Immunol. 2012;24(5):303–313. doi:10.1093/intimm/dxs004
43. Kesarwani P, Kant S, Prabhu A, Chinnaiyan P. The interplay between metabolic remodeling and immune regulation in glioblastoma. Neuro Oncol. 2017;19(10):1308–1315. doi:10.1093/neuonc/nox079
44. Amelio I, Cutruzzola F, Antonov A, Agostini M, Melino G. Serine and glycine metabolism in cancer. Trends Biochem Sci. 2014;39(4):191–198. doi:10.1016/j.tibs.2014.02.004
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.