Back to Journals » Drug Design, Development and Therapy » Volume 14
Triptolide Inhibits the Proliferation of HaCaT Cells Induced by IL22 via Upregulating miR-181b-5p
Authors He Q, Zhang B, Hu F, Long J, Shi Q, Pi X, Chen H, Li J
Received 25 March 2020
Accepted for publication 29 June 2020
Published 22 July 2020 Volume 2020:14 Pages 2927—2935
DOI https://doi.org/10.2147/DDDT.S254466
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Tuo Deng
Qi He,1,2,* Bo Zhang,1,2,* Feng Hu,3 Jianwen Long,1,2 Quan Shi,1,2 Xianming Pi,1,2 Hongxiang Chen,4 Jiawen Li4
1Department of Dermatology, Hubei Provincial Hospital of Traditional Chinese Medicine, Wuhan, Hubei 430061, People’s Republic of China; 2Department of Dermatology, Hubei Province Academy of Traditional Chinese Medicine, Wuhan, Hubei 430074, People’s Republic of China; 3Department of Dermatology, Wuhan No.1 Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei 430022, People’s Republic of China; 4Department of Dermatology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei 430022, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Quan Shi
Department of Dermatology, Hubei Provincial Hospital of Traditional Chinese Medicine, No. 4 Huanyuanshan, Wuchang District, Wuhan, Hubei 430061, People’s Republic of China
Email [email protected]
Background: Evidence has been shown that triptolide was effective in the treatment of psoriasis; however, the mechanisms remain poorly understood. Thus, this study aimed to investigate the role of triptolide on the proliferation and differentiation of HaCaT cells which are treated with IL22 to mimic abnormal proliferation/differentiation in keratinocyte of psoriasis.
Materials and Methods: HaCaT cells were transfected with miR-181b-5p antagomir for 24 h, and then exposed to 10 μM Triptolide for 24 h, following by 100 ng/mL of IL22 for 24 h. In addition, the proliferation and cell cycle distribution in HaCaT cells were assessed by immunofluorescence or flow cytometry assays, respectively.
Results: Triptolide obviously upregulated the level of miR-181b-5p in HaCaT cells. In addition, triptolide significantly inhibited IL22-induced proliferation of HaCaT cells via inducing cell cycle arrest. Moreover, IL22 markedly inhibited the differentiation of HaCaT cells, and this phenomenon was reversed by triptolide treatment. In contrast, the effects of triptolide on the proliferation and differentiation in IL22-stimulated HaCaT cells were notably reversed by miR-181b-5p antagomir. Moreover, dual-luciferase assay showed that E2F5 was the direct target of miR-181b-5p in HaCaT cells. Meanwhile, upregulation of miR-181b-5p obviously decreased the level of E2F5 in HaCaT cells.
Conclusion: In this study, we found that triptolide could inhibit the proliferation and promote the differentiation in IL22-stimulated keratinocytes via upregulating miR-181b-5p. These data indicated that triptolide may be a potential agent for the treatment of psoriasis.
Keywords: psoriasis, triptolide, keratinocytes, miR-181b, differentiation
Introduction
Psoriasis is an immune-mediated chronic, noncommunicable cutaneous disorder, which is characterized by erythema and scaling.1,2 Psoriasis affects between 2% and 3% of the population over the world.3 Clinically, environmental and genetic factors play a vital role in the pathogenesis of psoriasis.4 In addition, abnormal keratinocyte differentiation/proliferation, development of new blood vessels and inflammatory cell infiltration are the cutaneous manifestations of psoriasis.4,5 Moreover, psoriasis is a T cell-mediate disease, which could result in abnormalities in immune cells, and then induce excessive proliferation of keratinocytes.6,7 In recent years, physiotherapy, topical therapy, and systemic therapy are the main therapeutic strategies for the treatment of psoriasis.8 However, the treatment options for treating patients with psoriasis remain unsatisfactory due to the low response rate and high recurrence rate.8 Therefore, it is important to explore better therapeutic strategies for the treatment of psoriasis.
Triptolide was extracted from a traditional Chinese herb Tripterygium wilfordii Hook F. (TwHF).9 TwHF is effective for treating a number of immunological disorders, including psoriasis, lupus erythematosus and rheumatoid arthritis.9–11 In addition, triptolide has been revealed to exhibit a number of biological functions, including anti-inflammatory, anti-tumor, and immunosuppressive activities.12–14 Hongqin et al found that triptolide could inhibit inflammatory response via suppressing IFNGAMMA signaling in HaCaT keratinocytes.15 However, the role of triptolide in psoriasis is still unclear and needs to be illuminated.
MicroRNAs (miRNAs) are a class of short, single-stranded, noncoding RNAs, which are 22–25 nucleotides in length.16 Evidence has been indicated that miRNAs are capable of negatively regulate gene expression by direct binding to 3ʹ-untranslated regions (3ʹ-UTRs) of target mRNAs at the transcriptional and post-transcriptional levels.17 It has been reported that miRNAs play vital roles in several cellular processes, such as cells proliferation, differentiation and apoptosis.18 Meanwhile, miRNAs have emerged as important factors in the development of epidermal disorders, including psoriasis.8 Zheng et al indicated that miR-181b-5p could inhibit the keratinocytes proliferation in psoriasis.19 This study aimed to investigate the mechanisms by which triptolide regulate the proliferation and differentiation of IL22 stimulated HaCaT cells.
Materials and Methods
Cell Culture
HaCaT cells were obtained from the Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China). Human Epidermal Keratinocytes (HEK) were purchased from ScienCell (Carlsbad, CA, USA). Cells were maintained in DMEM (Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 10% fetal bovine serum (Thermo Fisher Scientific) and 100 U/mL penicillin (Sigma Aldrich, St. Louis, MO, USA), and incubated at 37°C with 5% CO2.
Cell Counting Kit-8 (CCK-8)
Cell Counting Kit-8 (CCK-8, Dojindo, Kuma-moto, Japan) was applied to assess the cell proliferation. The HaCaT cells (10,000 cells/well) were plated into 96-well plates, and incubated overnight at 37°C. Later on, cells were transfected with miR-181b-5p antagomir and exposed to 10 μM triptolide (MedChemExpress, Monmouth Junction, NJ, USA) for 24 h, then treated with 100 ng/mL of IL22 (Sigma-Aldrich, St. Louis, MO, USA) for 24 h. After that, 10 μL CCK-8 reagent was added into each well, and the plates were incubated for another 2 h at 37°C. Subsequently, a microplate Reader (Bio-Rad, Hercules, CA, USA) was applied to evaluate the absorbance of each well at 450 nM. Triptolide was dissolved with DMSO (20 mM store solution), and then diluted with DMSO medium for cell assays.
Western Blot Assay
Cells were lysed in RIPA buffer at 4°C for 15 min. Total proteins were quantified using BCA method (Thermo Fisher Scientific). Then, equal amounts of proteins (30 μg) in the lysate were separated by 10% SDS-PAGE. Later on, the proteins were transferred onto polyvinylidene difluoride membrane (Thermo Fisher Scientific). After that, the membrane was blocked in 5% skimmed milk at room temperature for 1 h, and then probed with the primary antibodies against Filaggrin (1:1000, Abcam Cambridge, MA, USA), KERATIN 1 (1:1000, Abcam), P21 (1:1000, Abcam), CYCLIN E1 (1:1000, Abcam), cyclin-dependent kinase 2 (CDK2, 1:1000, Abcam), INVOLUCRIN (1:1000, Abcam), KERATIN 10 (1:1000, Abcam), E2F5 (1:1000, Abcam) and BETAACTIN (1:1000, Abcam) at 4°C overnight. Subsequently, goat anti-Rabbit IgG secondary antibodies (1:3000, Abcam Cambridge, MA, USA) were incubated with the membranes at room temperature for 1 h. Finally, the membrane was scanned using an electrochemiluminescence (Thermo Fisher Scientific). β-actin was acted as the internal control.
Reverse Transcription-Quantitative Polymerase Chain Reaction (RT-qPCR)
Total RNA samples from HaCaT and HEK cells were isolated using the TRIzol reagent according to the manufacturer’s instructions. For reverse transcription of miRNA, the TaqMan MicroRNA Reverse Transcription Kit (Applied Biosystems, Foster City, Ca, USA) was applied to reverse transcribe the total RNA to cDNA. For reverse transcription of mRNA, RNAs were reversely transcribed into cDNAs using the Prime ScriptTMRT reagent Kit (Takara Bio Inc. Shiga, Japan). Then, RT-qPCR analysis was performed using the SYBR® Premix Ex TaqTM II (Takara), under the following conditions: 3 min 95°C, followed by 40 cycles of 10 s 95°C, 30 s 58°C, and 30 s 72°C. U6 and actin were used as the internal control for normalizing miRNAs and mRNA expressions, respectively. MiR-125b, forward: 5ʹ-CTTCCCTGAGACCCTAACTTGTG-3ʹ; reverse: 5ʹ-CTCAACTGGTGTCGTGGAGTC-3ʹ. MiR-181b-5p, forward: 5ʹ-CAACTGAATTGCCGACTCCAC-3ʹ; reverse: 5ʹ-CTCAACTGGTGTCGTGGAGTC-3ʹ. MiR-223, forward: 5ʹ-TCGTCAGTTTGTCAAATACCCC-3ʹ; reverse: 5ʹ-CTCAACTGGTGTCGTGGAGTC-3ʹ. MiR-744, forward: 5ʹ-GGCTAACAGCACTCAACTGAATT-3ʹ; reverse: 5ʹ-CTCAACTGGTGTCGTGGAGTC-3ʹ. U6, forward: 5ʹ-CTCGCTTCGGCAGCACAT-3ʹ; reverse: 5ʹ-AACGCTTCACGAATTTGCGT-3ʹ. E2F5, forward: 5ʹ-GGGCTGCTCACTACCAAGTTC-3ʹ; reverse: 5ʹ-CACCTACACCTTTCCACTGGATAC-3ʹ. Actin, forward: 5ʹ-GTCCACCGCAAATGCTTCTA-3ʹ; reverse: 5ʹ-TGCTGTCACCTTCACCGTTC-3ʹ. Data were analyzed using the 2–ΔΔCT method.
Cell Transfection
MiR-181b-5p agomir, miR-181b-5p antagomir and negative control (NC) were obtained from RiboBio (Guangzhou, China). HaCaT cells were transfected with miR-181b-5p agomir, miR-181b-5p antagomir or NC using Lipofectamine 2000 reagent (Thermo Fisher Scientific) for 24 h according to the manufacturer’s instructions.
KI67 and 5-Ethynyl-2ʹ-Deoxyuridine (EdU) Immunofluorescence Assays
HaCaT or HEK cells were fixed in 4% paraformaldehyde, followed by washing twice with PBS. Subsequently, HaCaT or HEK cells were incubated with primary antibody against KI67 (1:200) or EdU (1:200) overnight at 4°C, respectively. And then, they were incubated with fluorescein-conjugated goat anti-rabbit IgG antibody (1:200, Abcam) at room temperature for 1 h. The nuclei were counterstained with DAPI for 5 min. Finally, cells were observed under BX53 fluorescence microscope (Olympus, Japan). For each experimental group, at least five images were captured from random fields. KI67 or EdU positive cell/DAPI positive cell percentage was quantified.
Flow Cytometry
Cells were re-suspended and fixed in 70% ethanol overnight at 4°C. After that, cells were stained with 1 mg/mL of PI/RNase Staining Buffer (BD Biosciences, Franklin Lakes, NJ, USA) in the dark for 30 min according to the instructions. Later on, the FACScan™ flow cytometer (BD Biosciences) was used to the populations in G0-G1, S and G2-M phases.
Dual-Luciferase Reporter Assay
E2F5 segment was synthesized with either wild-type (WT) or mutant (MT) seed region and then cloned into the psiCHECK-2 vector. After that, cells were co-transfected with WT-E2F5 or MT-E2F5 plasmid, with miR-181b-5p agomir or NC respectively using Lipofectamine 2000. Subsequently, the luciferase activity in cell lysate was detected at 48 h using the Dual Luciferase Reporter Assay System (Promega, Madison, USA) according to the manufacturer’s protocol with renilla luciferase activity as endogenous control.
Statistical Analysis
All data were repeated in triplicate. Data are presented as the mean ± SD. All statistical analyses were performed using GraphPad Prism software (version 7.0, La Jolla, CA, USA). One-way and Two-way analysis of variance (ANOVA) followed by Tukey’s tests were carried out for multiple group comparisons. Differences were considered to be significant at *P < 0.05.
Results
Triptolide Inhibited the Proliferation of HaCaT Cells Induced by IL22 via Promoting Cell Differentiation
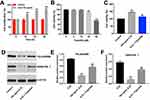
Evidence suggested that IL22 could promote keratinocyte proliferation which plays an important role during the pathogenesis of psoriasis.20 As revealed in Figure 1A, 100 ng/mL IL22 significantly promoted the proliferation of HaCaT cells in a time-dependent manner. When HaCaT cells exposed to IL22 for 24 h, cell proliferation rate was increased to 150% compared with control group (Figure 1A). Therefore, this treatment condition was used in the following experiments.
Next, to explore the effect of triptolide at different doses on the proliferation of HaCaT cells, CCK-8 assay was used. As indicated in Figure 1B, triptolide (20 and 40 μM) markedly inhibited the proliferation of HaCaT cells, while 10 μM triptolide had very limited effect on cell proliferation. Therefore, 10 μM triptolide was utilized in the following experiments. Additionally, triptolide notably inhibited the pro-proliferative effect of IL22 on HaCaT cells (Figure 1C). Moreover, IL22 exposure significantly reduced the expressions of differentiation markers FILAGGRIN and KERATIN 1 in HaCaT cells, and these phenomenons were notably reversed in the presence of triptolide (Figure 1D–F). These data indicated that triptolide could inhibit the proliferation of HaCaT cells induced by IL22 via promoting cell differentiation.
Triptolide Inhibited the Proliferation of HaCaT Cells Induced by IL22 via Upregulating miR-181b-5p
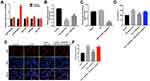
Previous studies reported that some miRNAs play an important role during the development of psoriasis, such as miR-125b-5p, miR-181b-5p, miR-187, miR-223, and miR-744.3,19,21,22 However, the functional interaction between triptolide and miRNAs in IL22-stimulated HaCaT cells remains unclear. As shown in Figure. 2A, triptolide markedly elevated the level of miR-181b-5p in HaCaT cells. In contrast, the level of miR-181b-5p in HaCaT cells was notably decreased after IL22 exposure (Figure 2B). However, IL22 induced miR-181b-5p downregulation was significantly reversed by triptolide treatment in HaCaT and HEK cells (Figure 2B and Supplementary Figure 1A). As expected, the level of miR-181b-5p was significantly decreased following transfection with miR-181b-5p antagomir (Figure 2C). Meanwhile, the effect of triptolide against IL22-induced proliferation in HaCaT cells was notably inhibited by miR-181b-5p antagomir (Figure 2D–F and Supplementary Figure 1B–D). Additionally, miR-181b-5p antagomir itself could increase the proliferation of HEK cells (Supplementary Figure 1E). All these data illustrated that triptolide could inhibit the proliferation of HaCaT cells induced by IL22 via upregulating miR-181b-5p.
Triptolide Restrained the Cell Cycle in IL22-Stimulated HaCaT via Upregulating miR-181b-5p
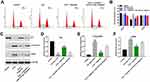
Next, flow cytometry was applied to determine whether triptolide could impact the cell cycle distribution in IL22-stimulated HaCaT cells. As shown in Figure 3A and B, the proportion of cells in the G0-G1 phase was significantly decreased in IL22-stimulated HaCaT cells, while the proportions of cells in the S phases were increased, compared with the control group (Figure 3A and B). Specifically, the percentage of G0/G1 cells was increased from 33.0% in IL22 treatment group to 40% in IL22 + triptolide group, along with a decrease in cells in the S phase from 34% to 25%, indicating that triptolide could induce G0/G1 arrest in IL22-stimulated HaCaT cells (Figure 3A and B). In contrast, triptolide-induced cell cycle arrest in IL22-stimulated HaCaT cells was reversed by miR-181b-5p antagomir (Figure 3A and B).
As we know, the eukaryotic cell cycle is mediated by the activities of cyclin-dependent kinases and cyclins.23 Meanwhile, the regulatory protein P21 could interact with the cyclin/CDK complex to inhibit cell cycle progression.24 In the current study, triptolide obviously upregulated the level of P21 and downregulated the expressions of CYCLIN E1 and CDK2 in IL22-stimulated HaCaT cells. However, these phenomena were notably reversed following transfection with miR-181b-5p antagomir (Figure 3C–F). These data suggested that triptolide could induce cell cycle arrest in stimulated HaCaT cells via upregulating miR-181b-5p.
Triptolide Induced the Differentiation of IL22-Stimulated HaCaT Cells via Upregulating miR-181b-5p
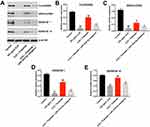
Next, Western blot was performed to measure whether triptolide could impact the differentiation of IL22-stimulated HaCaT cells. As shown in Figure 4A–E, IL22 markedly downregulated the expressions of FILAGGRIN, INVOLUCRIN, KERATIN 1 and KERATIN 10 in HaCaT cells, and these phenomena were notably reversed by triptolide treatment. In contrast, triptolide-induced FILAGGRIN, INVOLUCRIN, KERATIN 1 and KERATIN 10 proteins increases in IL22-stimulated HaCaT cells were significantly reversed by miR-181b-5p antagomir (Figure 4A–F). These data illustrated that triptolide could induce the differentiation of IL22-stimulated HaCaT cells via upregulating miR-181b-5p.
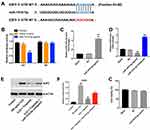
E2F5 Was the Direct Target of miR-181b-5p in IL22-Stimulated HaCaT Cells
Next, targetScan dataset (http://www.targetscan.org/vert_71/) and miRDB (http://www.mirdb.org/) were used to predict the potential binding targets of miR-181b-5p. The outcome indicated that E2F5 might be a potential target of miR-181b-5p (Figure 5A). In addition, dual luciferase reporter assay validated that miR-181b-5p agomir suppressed the luciferase activity of psiCHECK-2-E2F5-WT (Figure 5B). Moreover, overexpression of miR-181b-5p significantly increased the level of miR-181b-5p in HaCaT cells (Figure 5C). Meanwhile, overexpression of miR-181b-5p significantly decreased the level of E2F5 in HaCaT cells, while knockdown of miR-181b-5p significantly increased the level of E2F5 (Figure 5D). These results indicated that E2F5 was a binding target of miR-181b-5p. Furthermore, IL22 obviously upregulated the expression of E2F5 in HaCaT cells, while this phenomenon was notably reversed in the presence of triptolide (Figure 5E and F). In consistently, triptolide-induced E2F5 protein decrease in IL22-stimulated HaCaT cells was significantly reversed by miR-181b-5p antagomir (Figure 5E and F). Furthermore, miR-181b-5p agomir had very limited effect on the viability of HaCaT cells (Figure 5G). These results revealed that triptolide could inhibit IL22-induced keratinocyte proliferation via regulation of miR-181b-5p/E2F5 axis.
Discussion
Psoriasis is a chronic inflammatory skin disorder, which is characterized by uncontrolled proliferation and poor differentiation of keratinocytes.25 In addition, excessive hyperproliferation of keratinocytes, abnormal differentiation of keratinocytes, and infiltration of inflammatory cells into the epidermis are three different processes of cellular alteration in skin.26 Evidence has been shown that IL22 could promote the proliferation of keratinocytes and inhibit keratinocyte differentiation, which were consistent with our study.27,28 Moreover, IL36GAMMA could inhibit the differentiation of keratinocytes via reducing the expressions of FILAGGRIN, INVOLUCRIN, KERATIN 1 and KERATIN 5.29 Consistent with the previous study, our data found that IL22 significantly downregulated the expressions of FILAGGRIN, INVOLUCRIN, KERATIN 1 and KERATIN 10 in HaCaT cells. These results indicated that IL22 could promote the proliferation and suppress the differentiation of keratinocytes.
Currently, natural products have been recognized as the effective drugs for the treatment of psoriasis.30 Sun et al indicated that berberine could inhibit the proliferation of keratinocytes.30 In addition, triptolide exhibited an anti-inflammatory role in the treatment of psoriasis.9 However, the mechanism by which triptolide regulates hyperplasia of the epidermis in psoriasis remains unclear. In this study, we found that triptolide inhibited the proliferation in IL22-stimulated HaCaT cells via inducing the cell cycle arrest. In addition, triptolide could promote the differentiation of IL22-stimulated HaCaT. These data indicated that triptolide could inhibit the proliferation and promote the differentiation in IL22-stimulated HaCaT cells.
Recent study indicated that trichosanthes kirilowii, a traditional Chinese herb, exerted a protective role for the treatment of skin diseases via regulating the level of miR-142.31 Previous studies indicated that some miRNAs play a vital role in the development of psoriasis, such as miR-125b-5p, miR-181b-5p, miR-187, miR-223, and miR-744.3,19,21,22 Our data indicated that triptolide had very limited effect on the levels of miR-125b-5p, miR-187, miR-223, and miR-744 in HaCaT cells. However, triptolide markedly upregulated the level of miR-181b-5p in HaCaT cells. In contrast, the level of miR-181b-5p was significantly decreased in IL22-stimulated HaCaT cells, which was consistent with previous study.19 In addition, the inhibitory effect of triptolide against IL22-induced proliferation in HaCaT cells was reversed by miR-181b-5p antagomir. Moreover, triptolide-induced upregulation of differentiation-associated markers in IL22-stimulated HaCaT cells was significantly inhibited in by miR-181b-5p antagomir. All these results illustrated that triptolide regulated the proliferation and differentiation of IL22-stimulated keratinocytes via upregulating miR-181b-5p.
Afterwards, targetScan, miRDB dataset and luciferase reporter assays validated that E2F5 was a direct target of miR-181b-5p. E2F5, a member of E2F family, which play an important role in cell cycle, cell differentiation and cell death.32 Jiang et al indicated that downregulation of E2F5 could suppress the growth of hepatocellular carcinoma cells.33 In addition, wong et al indicated that upregulation of E2F isoforms could suppress the expressions of differentiation-associated markers in keratinocytes.34 In this study, we found that IL22 markedly upregulated the expression of E2F5 in keratinocytes, leading to promotion of proliferation and inhibition of differentiation. In contrast, triptolide notably reversed IL22-induced upregulation of E2F5 in keratinocytes. These data indicated that triptolide could inhibit the proliferation and promote the differentiation in IL22-stimulated keratinocytes via regulation of miR-181b-5p/E2F5 axis.
Conclusion
In summary, we found that triptolide could inhibit the proliferation and promote the differentiation in keratinocytes during IL22 stimulation via upregulation of miR-181b-5p. These data indicated that triptolide may be a potential agent for the treatment of psoriasis.
Abbreviations
TwHF, Tripterygium wilfordii Hook F; 3ʹ-UTRs, 3ʹ-untranslated regions; HEK, human epidermal keratinocytes; CCK-8, Cell Counting Kit-8; CDK2, cyclin-dependent kinase 2; RT-qPCR, reverse transcription-quantitative polymerase chain reaction; NC, negative control; EdU, 5-ethynyl-2ʹ-deoxyuridine; WT, wild-type; MT, mutant.
Disclosure
Qi He and Bo Zhang should be considered as co-first authors. The authors declare no competing financial interests.
References
1. Zhao X, Li R, Qiao M, Yan J, Sun Q. MiR-548a-3p promotes keratinocyte proliferation targeting PPP3R1 after being induced by IL22. Inflammation. 2018;41(2):496–504. doi:10.1007/s10753-017-0705-3
2. Zhou Q, Yu Q, Gong Y, et al. Construction of a lncRNA-miRNA-mRNA network to determine the regulatory roles of lncRNAs in psoriasis. Exp Ther Med. 2019;18(5):4011–4021. doi:10.3892/etm.2019.8035
3. Wang C, Zong J, Li Y, Wang X, Du W, Li L. MiR-744-3p regulates keratinocyte proliferation and differentiation via targeting KLLN in psoriasis. Exp Dermatol. 2019;28(3):283–291. doi:10.1111/exd.13888
4. Greb JE, Goldminz AM, Elder JT, et al. Psoriasis. Nat Rev Dis Primers. 2016;2(1):16082. doi:10.1038/nrdp.2016.82
5. Ogawa E, Sato Y, Minagawa A, Okuyama R. Pathogenesis of psoriasis and development of treatment. J Dermatol. 2018;45(3):264–272. doi:10.1111/1346-8138.14139
6. Di Cesare A, Di Meglio P, Nestle FO. The IL-23/Th17 axis in the immunopathogenesis of psoriasis. J Invest Dermatol. 2009;129(6):1339–1350. doi:10.1038/jid.2009.59
7. Kupetsky EA, Mathers AR, Ferris LK. Anti-cytokine therapy in the treatment of psoriasis. Cytokine. 2013;61(3):704–712. doi:10.1016/j.cyto.2012.12.027
8. Feng S, Wang L, Liu W, Zhong Y, Xu S. MiR-126 correlates with increased disease severity and promotes keratinocytes proliferation and inflammation while suppresses cells’ apoptosis in psoriasis. J Clin Lab Anal. 2018;32(9):e22588. doi:10.1002/jcla.22588
9. Han R, Rostami-Yazdi M, Gerdes S, Mrowietz U. Triptolide in the treatment of psoriasis and other immune-mediated inflammatory diseases. Br J Clin Pharmacol. 2012;74(3):424–436. doi:10.1111/j.1365-2125.2012.04221.x
10. Huang L, Feng S, Wang H. Decreased bone mineral density in female patients with systemic lupus erythematosus after long-term administration of Tripterygium Wilfordii Hook. F. Chin Med J. 2000;113(2):159–161.
11. Lv QW, Zhang W, Shi Q, et al. Comparison of Tripterygium wilfordii Hook F with methotrexate in the treatment of active rheumatoid arthritis (TRIFRA): a randomised, controlled clinical trial. Ann Rheum Dis. 2015;74(6):1078–1086. doi:10.1136/annrheumdis-2013-204807
12. Wang X, Matta R, Shen G, Nelin LD, Pei D, Liu Y. Mechanism of triptolide-induced apoptosis: effect on caspase activation and Bid cleavage and essentiality of the hydroxyl group of triptolide. J Mol Med. 2006;84(5):405–415. doi:10.1007/s00109-005-0022-4
13. Li P, Yang X, Yang Y, et al. Synergistic effect of all-trans-retinal and triptolide encapsulated in an inflammation-targeted nanoparticle on collagen-induced arthritis in mice. J Control Release. 2019.
14. Song JM, Molla K, Anandharaj A, et al. Triptolide suppresses the in vitro and in vivo growth of lung cancer cells by targeting hyaluronan-CD44/RHAMM signaling. Oncotarget. 2017;8(16):26927–26940. doi:10.18632/oncotarget.15879
15. Hongqin T, Xinyu L, Heng G, Lanfang X, Yongfang W, Shasha S. Triptolide inhibits IFN-gamma signaling via the Jak/STAT pathway in HaCaT keratinocytes. Phytother Res. 2011;25(11):1678–1685. doi:10.1002/ptr.3471
16. Huang RY, Li L, Wang MJ, Chen XM, Huang QC, Lu CJ. An exploration of the role of MicroRNAs in psoriasis: a systematic review of the literature. Medicine. 2015;94(45):e2030. doi:10.1097/MD.0000000000002030
17. Ivey KN, Srivastava D. microRNAs as developmental regulators. Cold Spring Harb Perspect Biol. 2015;7(7):a008144. doi:10.1101/cshperspect.a008144
18. Shenoy A, Blelloch RH. Regulation of microRNA function in somatic stem cell proliferation and differentiation. Nat Rev Mol Cell Biol. 2014;15(9):565–576. doi:10.1038/nrm3854
19. Zheng Y, Cai B, Li X, Li D, Yin G. MiR-125b-5p and miR-181b-5p inhibit keratinocyte proliferation in skin by targeting Akt3. Eur J Pharmacol. 2019;862:172659.
20. Shen H, Zeng B, Wang C, et al. MiR-330 inhibits IL22-induced keratinocyte proliferation through targeting CTNNB1. Biomed Pharmacother. 2017;91:803–811. doi:10.1016/j.biopha.2017.05.005
21. Tang L, He S, Zhu Y, et al. Downregulated miR-187 contributes to the keratinocytes hyperproliferation in psoriasis. J Cell Physiol. 2019;234(4):3661–3674. doi:10.1002/jcp.27135
22. Wang R, Wang FF, Cao HW, Yang JY. MiR-223 regulates proliferation and apoptosis of IL22-stimulated HaCat human keratinocyte cell lines via the PTEN/Akt pathway. Life Sci. 2019;230:28–34. doi:10.1016/j.lfs.2019.05.045
23. Boxem M. Cyclin-dependent kinases in C. elegans. Cell Div. 2006;1(1):6. doi:10.1186/1747-1028-1-6
24. Li W, Sanki A, Karim RZ, et al. The role of cell cycle regulatory proteins in the pathogenesis of melanoma. Pathology. 2006;38(4):287–301. doi:10.1080/00313020600817951
25. Arul S, Dayalan H, Jegadeesan M, Damodharan P. Induction of differentiation in psoriatic keratinocytes by propylthiouracil and fructose. BBA Clin. 2016;6:82–86. doi:10.1016/j.bbacli.2016.06.002
26. Chandra A, Ray A, Senapati S, Chatterjee R. Genetic and epigenetic basis of psoriasis pathogenesis. Mol Immunol. 2015;64(2):313–323. doi:10.1016/j.molimm.2014.12.014
27. Peng C, Zhang S, Lei L, et al. Epidermal CD147 expression plays a key role in IL22-induced psoriatic dermatitis. Sci Rep. 2017;7(1):44172. doi:10.1038/srep44172
28. Hao JQ. Targeting interleukin-22 in psoriasis. Inflammation. 2014;37(1):94–99. doi:10.1007/s10753-013-9715-y
29. Wang W, Yu X, Wu C, Jin H. IL-36gamma inhibits differentiation and induces inflammation of keratinocyte via Wnt signaling pathway in psoriasis. Int J Med Sci. 2017;14(10):1002–1007. doi:10.7150/ijms.20809
30. Sun S, Zhang X, Xu M, et al. Berberine downregulates CDC6 and inhibits proliferation via targeting JAK-STAT3 signaling in keratinocytes. Cell Death Dis. 2019;10(4):274.
31. Joo JH, Hong IK, Kim NK, Choi E. Trichosanthes kirilowii extract enhances repair of UVB radiation induced DNA damage by regulating BMAL1 and miR1423p in human keratinocytes. Mol Med Rep. 2018;17(1):877–883. doi:10.3892/mmr.2017.7932
32. Manicum T, Ni F, Ye Y, Fan X, Chen BC. Prognostic values of E2F mRNA expression in human gastric cancer. Biosci Rep. 2018;38(6). doi:10.1042/BSR20181264
33. Jiang Y, Yim SH, Xu HD, et al. A potential oncogenic role of the commonly observed E2F5 overexpression in hepatocellular carcinoma. World J Gastroenterol. 2011;17(4):470–477. doi:10.3748/wjg.v17.i4.470
34. Wong CF, Barnes LM, Dahler AL, et al. E2F modulates keratinocyte squamous differentiation: implications for E2F inhibition in squamous cell carcinoma. J Biol Chem. 2003;278(31):28516–28522. doi:10.1074/jbc.M301246200
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.