Back to Journals » Transplant Research and Risk Management » Volume 6
Triple antiviral therapy with telaprevir after liver transplantation: a case series
Authors Knapstein J, Grimm D, Wörns M, Galle P, Lang H, Zimmermann T
Received 7 April 2014
Accepted for publication 18 June 2014
Published 9 September 2014 Volume 2014:6 Pages 73—78
DOI https://doi.org/10.2147/TRRM.S65651
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Johanna Knapstein,1 Daniel Grimm,1 Marcus A Wörns,1 Peter R Galle,1 Hauke Lang,2 Tim Zimmermann1
11st Department of Internal Medicine, Johannes Gutenberg-University, Mainz, Germany; 2Department of General, Visceral and Transplantation Surgery, Johannes Gutenberg-University, Mainz, Germany
Introduction: Hepatitis C virus (HCV) reinfection occurs universally after liver transplantation, with accelerated cirrhosis rates of up to 30% within 5 years after liver transplantation. Dual antiviral therapy with pegylated interferon-2a (peg-IFN) and ribavirin (RBV) only reaches sustained virological response rates of ~30% after liver transplantation. With the approval of viral NS3/4A protease inhibitors telaprevir (TVR), boceprevir, and simeprevir and the NS5B polymerase inhibitor sofosbuvir, combination therapy offers new therapeutic options for HCV-infected patients, resulting in considerably higher sustained virological response rates in the nontransplant setting.
Case presentation: We report three cases of TVR-based triple antiviral therapy in HCV genotype 1 reinfected patients after liver transplantation, of whom a 57-year-old Caucasian female and a 43-year-old Caucasian male were therapy naïve, and a 49-year-old Caucasian male patient was pretreated ineffectively. After 4 weeks of therapy, viral load decreased one to three log10 and became negative in weeks 6 to 8 in the therapy naïve patients. The pretreated patient showed a negative viral load in week 4. TVR was administered over 12 weeks, 750 mg thrice daily. Doses of immunosuppression with cyclosporine were reduced four to six fold. Initial peg-IFN and RBV doses ranged from 135–180 µg/week and 800–1,200 mg/day, according to the patient's body weight. Doses of peg-IFN and RBV were adapted to 90–135 µg/week and 400–800 mg/day after 2 to 12 weeks of protease inhibitor therapy. Dual therapy was continued for 36 weeks with total treatment duration of 48 weeks in the therapy naïve patients leading to a sustained virological response 12 weeks after the end of therapy. In the pretreated patient a breakthrough was detected in week 24 and therapy was discontinued. Overall, antiviral therapy was well tolerated. Side effects included dysgeusia and anemia leading to erythropoietin application and blood transfusions.
Conclusion: This case series emphasizes that triple therapy with TVR is an efficient treatment for therapy naïve HCV genotype 1 reinfected patients after liver transplantation. But therapeutic options for pretreated patients require improvement.
Keyword: cyclosporine, interferon, ribavirin, hepatitis C, protease inhibitor
Introduction
Hepatitis C (HCV) reinfection occurs universally after liver transplantation (LT).1 During immunosuppression the time course of recurrent hepatitis C is accelerated, with cirrhosis rates of up to 30% within 5 years of LT.2 So far, antiviral therapy has been limited to a combination of pegylated interferon-2a (peg-IFN) and ribavirin (RBV), with sustained virological response (SVR) rates of ~30% post LT.3–5 With the approval of the first generation viral NS3/4A protease inhibitors (PI), telaprevir (TVR), and boceprevir (BOC) and the second generation inhibitor simeprevir (SIM), as well as the NS5B RNA-dependent RNA polymerase inhibitor (RdRpI) sofosbuvir (SOF), combination therapy offers new therapeutic options, resulting in considerably higher SVR rates of up to 90% in treatment naïve patients in the nontransplant setting.6–10 However, the management of HCV reinfection after LT is complicated by drug interactions, tolerability, and pretreatment.11,12 Therefore, an individualized treatment regimen is often required. We present three cases of TVR-based antiviral triple therapy in two therapy naïve and one inefficiently pretreated HCV genotype 1 reinfected patient after LT.
Case presentation
Case 1
A 57-year-old Caucasian female underwent LT in 2011 due to hepatocellular carcinoma (HCC) based on chronic hepatitis C genotype 1b-associated liver cirrhosis, after LT bile duct anastomosis stenosis occurred and repetitive stenting was necessary. Nine months after LT, HCV reinfection was detected in liver biopsy accompanied by mild fibrosis. HCV-RNA measured 6.8×103 IU/mL (COBAS® TaqMan® HCV test, version 2.0; Hoffman-La Roche Ltd, Basel, Switzerland. Lower limit of quantification, 25 IU/mL; lower limit of detection, 10 IU/mL). Serum transaminases and bilirubin were slightly elevated (alanine transaminase [ALT] 73 U/L, upper limits of normal [ULN] <50 IU/L, aspartate transaminase [AST] 100 U/L, ULN 35 IU/L, bilirubin 1.3 mg/dL ULN 1.2 mg/dL).
All other liver values were within the normal range (alkaline phosphatase, gamma-glutamyl transferase, international normalized ratio [INR], and albumin). Hemoglobin (Hb), leukocyte, and thrombocyte counts were 12.1 mg/dL, 3.2/nL and 250/nL, respectively (Figure 1A).
The patient was therapy naïve and revealed the unfavorable IL28B C/T genotype. Immunosuppression included 50 mg cyclosporine A (CsA) twice a day (BID; CsA blood level: 78 ng/mL) and 500 mg/BID mycophenolate mofetil (MMF). Comedication was calcium, vitamin D3, and 50 μg L-thyroxin per day. The patient presented in a good state of general health with a body mass index (BMI) of 25.6 (170 cm, 74 kg).
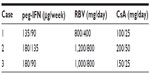
Triple therapy was started with 135 μg peg-IFN-alpha-2a once a week, 800 mg RBV/day, and 750 mg TVR thrice daily. IFN and RBV doses were reduced to 90 μg/week and 400 mg/day in week 12 (Table 1). The CsA dosage was adapted to 25 mg/day (CsA blood level: 49 ng/mL) (Table 1) and blood levels were stable during the course of therapy (Figure 1A). The viral load declined three log10 after 4 weeks of triple therapy and became negative in week 8, resulting in SVR 12. Bilirubin blood level normalized during the course of the triple therapy unrelated to any stenting (Figure 1A). Therefore, it is likely that the elevation of bilirubin blood levels was caused by HCV-activity rather than cholestasis. Side effects included dyspnea and anemia, leading to blood transfusion once every other week and the application of 30 μg erythropoietin (EPO) once a week. The decline in Hb-levels from 12.1 mg/dL initially to a minimum of 7.2 mg/dL is shown in Figure 1A. Red blood cell, leukocyte and platelet counts as well as CsA blood levels were checked weekly. At the end of PI therapy the CsA dosage was set back to 50 mg BID (CsA blood level: 91 ng/mL).
Case 2
A 43-year-old Caucasian male patient was liver-transplanted in 2010 due to chronic hepatitis C genotype 1 and alcohol associated liver cirrhosis. Histological analysis of liver biopsy one year after LT showed the early stages of fibrosis (Desmet stage F1–F2) due to HCV reinfection. Viral load measured 6.7×106 IU/mL (COBAS TaqMan HCV test: lower limit of quantification, 25 IU/mL; lower limit of detection, 10 IU/mL). Serum transaminases were considerably elevated (ALT 420 U/L, ULN <50 IU/L, AST 190 U/L, ULN 35 IU/L). All other liver values were within the normal range (alkaline phosphatase, gamma-glutamyl transferase, bilirubin, INR, and albumin). Hb, leukocyte, and thrombocyte counts were 16.3 mg/dL, 4.2/nL, and 142/nL, respectively (Figure 1B). The patient was therapy naïve and revealed an unfavorable IL28B C/T genotype.
Side diagnoses included psoriasis vulgaris and schizophrenia. Aripiprazole was discontinued 3 months before starting antiviral therapy. Immunosuppression was switched from 4 mg BID tacrolimus (Tac) to 100 mg BID CsA due to better controllability (CsA blood level, 52 ng/mL). MMF at 500 mg BID was continued. The patient presented in a good state of general health with a BMI of 29.4 (176 cm, 91 kg).
We started a 4-week lead-in period with 180 μg peg-IFN-alpha-2a once a week and 1,200 mg RBV/day to consider the virological response, tolerability and compliance. Within the lead-in, the viral load decreased one log10 and serum transaminases normalized (Figure 1B). Therapy was well tolerated with good compliance. We started 750 mg three times a day (TID) TVR in week 5. CsA, RBV, and IFN doses were reduced to 25 mg BID, 800 mg/day and 135 μg/week, respectively (CsA blood level: 36 ng/mL) (Table 1). The viral load became negative in week 6 resulting in SVR 12. CsA blood levels were stable (Figure 1B). Side effects included mild anemia and a stage II rash, which was manageable by the application of external steroids. Red blood cell, leukocyte, and platelet counts as well as CsA blood levels were checked once a week. At the end of PI therapy the CsA dosage was set back to 100 mg BID (CsA blood level, 109 ng/mL).
Case 3
A 49-year-old Caucasian male patient underwent LT in 2008 due to HCC, based on chronic hepatitis C genotype 1b-associated liver cirrhosis since 1998. Histological analysis of liver biopsy 3 years after LT proved HCV reinfection with plurisaptate fibrosis (Desmet stage F3). The viral load measured 1.16×106 IU/mL (COBAS TaqMan HCV test: lower limit of quantification, 25 IU/mL; lower limit of detection, 10 IU/mL). Serum transaminases and bilirubin blood levels were elevated with frequent variations (ALT 124 U/L, ULN <50 IU/L, AST 96 U/L, ULN 35 IU/L, bilirubin 1.1 mg/dL, ULN 1.2 mg/dL). All other liver values were in the normal range (alkaline phosphatase, gamma-glutamyl transferase, INR and albumin). Hb, leukocyte, and thrombocyte counts were 13.6 mg/dL, 2.3/nL, and 117/nL, respectively (Figure 1C). The patient was pretreated with dual antiviral therapy twice, prior to and after LT, both resulting in non-response. His IL28B genotype was T/T. Side diagnoses included hypertension. Comedication with amlodipine 5 mg/day was discontinued to avoid drug interactions. Due to better controllability, immunosuppression with 8 mg BID Tac was switched to 75 mg BID CsA (CsA blood level, 50 ng/mL). MMF was continued at 500 mg BID. The patient presented in a good state of general health with a BMI of 28.4 (184 cm, 96 kg), justifying another attempt at antiviral therapy.
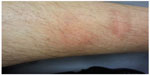
We initiated triple therapy with 180 μg peg-IFN-alpha-2a once a week, 1,000 mg RBV/day and 750 mg TVR TID. The CsA dose was adjusted to 25 mg/day during PI therapy (CsA blood level, 48 ng/mL) (Table 1). RBV and peg-IFN doses were reduced to 800 mg/day, and 90 μg/week, respectively in week 8 (Table 1). Viral load became negative in week 4, transaminases improved significantly and bilirubin blood levels normalized (Figure 1C). Unfortunately a breakthrough occurred in week 24 and therapy was discontinued. Side effects included reflux symptoms, hypertension, mild anemia, moderate leukopenia, and erythema nodosum along both lower legs (Figure 2) with serological proof of atypical anti-neutrophil cytoplasmic antibodies. All side effects were manageable: hypertension and reflux symptoms by application of 3,125 mg BID carvedilol and 20 mg BID esomeprazole; leukopenia improved on administration of 30 million IU granulocyte colony-stimulating factor (G-CSF). The anemia and erythema nodosum abated when therapy was discontinued. The decline in white blood cell count from an initial 2.3/nL to a minimum of 1.2/nL is presented in Figure 1C. Red blood cell, leukocyte, and platelet counts as well as CsA blood levels were checked once a week. At the end of PI therapy the CsA dosage was set back to 100 mg BID (CsA blood level, 45 ng/mL).
Rejection or impairment of liver function did not occur in any of the cases during triple therapy. No additional liver biopsies were performed.
Discussion
Antiviral therapy of recurrent hepatitis C after LT is an issue of great interest, particularly as the time course of fibrosis progression is accelerated under immunosuppression.2 Improved antiviral therapy is urgently required, as SVR rates for dual therapy in HCV-positive patients after LT are quite poor.3,4 Combination therapy with PIs and RdRpIs in non-LT HCV cohorts leads to significantly higher SVR rates than dual therapy.6–10 But management of hepatitis C post LT is complicated by drug interactions, side effects and pretreatment.11,12
However, little data exist on HCV combination therapy after LT. Coilly et al presented preliminary results of triple therapy with BOC or TVR in terms of efficacy and safety in LT recipients and reported their experience with BOC in five liver transplant genotype 1 patients, with an estimated oral clearance reduction of 50% with CsA and up to 80% with Tac.13,14
Coilly et al reported the first multicenter study of 29 patients with HCV genotype 1 recurrence treated with triple therapy after LT in five French liver transplant centers, with a complete early virological response in 71% of patients treated with BOC and 73% of patients treated with TVR.15 However the final results of this study showed SVR 12 in only 20% of TVR and 71% of BOC treated patients.16 The American three center study by Pungpapong et al showed SVR 24 in 67% of TVR and 45% of BOC treated patients.17 Regarding combination therapy with SOF after LT, to date there exist only a few case reports.18,19 One larger study presented during the 2014 European liver congress with compassionate use of SOF in 104 patients after LT reported 62% SVR 12.20
We report successful TVR-based triple therapy of recurrent HCV infection after LT in two therapy naïve HCV genotype 1 infected patients. In one pretreated genotype 1 reinfected patient after LT occurred under antiviral triple therapy with TVR.
In all three cases IL28B polymorphism was determined as a predictor of response to therapy. Previous studies have shown that, after LT, patients harboring IL28B C alleles are more susceptible to successful antiviral therapy.21 However, in the presented cases the patients had the unfavorable genotypes C/T and T/T.
During triple therapy, the immunosuppressive agent should be changed to CsA whenever possible17 due to better controllability. A four-fold dose reduction in CsA is recommended with TVR.15
Anemia is reported to be the most important adverse event of antiviral therapy in LT patients, mainly due to RBV-induced hemolysis.13 In the presented cases anemia was manageable by RBV dose reduction, application of EPO and blood transfusions, which did not affect SVR. Leukopenia could be controlled by the administration of G-CSF and no serious infection occurred. Red blood cell counts, leukocytes, platelet counts, and CsA blood levels were checked once a week. Therefore, very early therapeutic interventions, involving dose reductions, especially of RBV and CsA, were possible.
However, monitoring of RBV plasma concentration could predict decreases in hemoglobin and avoid transfusions, since increased plasma concentrations of RBV as a result of renal dysfunction in HCV patients treated with TVR were found.22,23 TVR drug monitoring could also be important after LT in order to maximize SVR rates and minimize side effects and drug–drug interactions.24
In the presented cases none of the patients developed kidney failure under triple therapy. Chronic HCV infection has been described in association with various skin disorders and some skin lesions have been reported as side effects of IFN.25 In particular, erythema nodosum seems to be related to IFN therapy.26 Dermatological toxicity is also known as a specific side effect of PI. In case two a grade II rash occurred and patient three developed erythema nodosum. Both improved at the end of therapy. Overall, therapy with TVR was well tolerated without major complications.
Conclusion
TVR-based antiviral triple therapy in two therapy naïve HCV genotype 1 reinfected patients after LT was well tolerated without major complications, resulting in SVR 12. In one former non-responder a breakthrough occurred after 24 weeks of triple therapy. Doses of immunosuppressive drugs need to be adapted. Continuous monitoring of red blood cell counts, leukocytes, platelet counts, and blood levels of immunosuppressive agents should be performed regarding dose reductions. This case series emphasizes that triple therapy with TVR is an effective treatment for therapy naïve HCV genotype 1 reinfected patients after liver transplantation. But therapeutic options for pretreated patients need to be improved, and larger studies with second generation PIs and RdRpIs in combination with peg-IFN and RBV and all-oral peg-IFN-free regimens as well as upcoming direct acting antivirals are needed.
Disclosure
The authors received lecture fees and travel grants from Hoffman-Roche, Janssen Pharmaceutica, and MSD Sharp and Dohme GmbH. The authors report no other conflicts of interest in this work.
References
Garcia-Retortillo M, Forns X, Feliu A, et al. Hepatitis C virus kinetics during and immediately after liver transplantation. Hepatology. 2002;35(3):680–687. | |
Gane EJ. The natural history of recurrent hepatitis C and what influences this. Liver Transpl. 2008;14(Suppl 2):36–44. | |
Berenguer M. Systematic review of the treatment of established recurrent hepatitis C with pegylated interferon in combination with ribavirin. J Hepatol. 2008;49(2):274–287. | |
Wang CS, Ko HH, Yoshida EM, Yoshida EM, Marra CA, Richardson K. Interferon-based combination anti-viral therapy for hepatitis C virus after liver transplantation: a review and quantitative analysis. Am J Transplant. 2006;6(7):1586–1599. | |
Xirouchakis E, Triantos C, Manousou P, et al. Pegylated interferon and ribavirin in liver transplant candidates and recipients with HCV cirrhosis: systematic review and meta-analysis of prospective controlled studies. J Viral Hepat. 2008;15(10):699–709. | |
Kwo PY, Lawitz EJ, McCone J, et al. Efficacy of boceprevir, an NS3 protease inhibitor, in combination with peg-interferon alpha-2b and ribavirin in treatment-naïve patients with genotype 1 hepatitis C infection (SPRINT-1): an open-label, randomised, multicenter phase 2 trial. Lancet. 2011;376:705–716. | |
Jacobson IM, McHutchinson JG, Dusheiko G, et al. Telaprevir for previously untreated chronic hepatitis C virus infection. N Engl J Med. 2011;364(25):2405–2416. | |
Lawitz E, Mangia A, Wyles D, et al. Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med. 2013;368(20):1878–1887. | |
Jacobson I, Foster GR, Fried MW, et al. Simeprevir (TMC435) with peginterferon/ribavirin for chronic HCV genotype-1 infection in treatment-naïve patients: results from QUEST-1, a phase III trial. J Hepatol. 2013;58:574. | |
Manns M, Poordad F, Alfonso de Araujo ES. Simeprevir (TMC435) with peginterferon/ribavirin for treatment of chronic HCV genotype-1 infection in treatment-naïve patients: results from QUEST-2, a phase III trial. J Hepatol. 2013;58:568. | |
Terrault N. Liver transplantation in the setting of chronic HCV. Best Pract Res Clin Gastroenterol. 2012;26(4):531–548. | |
Kiser JJ, Burton JR, Anderson PL, Everson GT. Review and management of drug interactions with boceprevir and telaprevir. Hepatology. 2012;55(5):1620–1628. | |
Coilly A, Roche B, Samuel D. Current management and perspectives for HCV recurrence after liver transplantation. Liver Int. 2013;33(Suppl 1):56–62. | |
Coilly A, Furlan V, Roche B, et al. Practical Management of Boceprevir and Immunosuppressive Therapy in Liver Transplant Recipients with Hepatitis C Virus Recurrence. Antimicrob Agents Chemother. 2012;56(11):5728–5734. | |
Coilly A, Roche B, Botta-Fridlund D, et al. Efficacy and safety of protease inhibitors for severe hepatitis C recurrence after liver transplantation: a first multicentric experience. J Hepatol. 2012;56:21. | |
Coilly A, Roche B, Dumontier J, et al. Safety and efficacy of protease inhibitors to treat hepatitis C after liver transplantation: A multicenter experience. J Hepatol. 2014;60(1):78–86. | |
Pungpapong S, Aqel BA, Koning L, et al. Multicenter experience using telaprevir or boceprevir with peginterferon and ribavirin to treat hepatitis C genotype 1 after liver transplantation. Liver Transpl. 2013;19(7):690–700. | |
Kim B, Trivedi A, Thung SN, Grewal P. Case report of successful treatment of fibrosing cholestatic hepatitis C with sofosbuvir and ribavirin after liver transplantation. Semin Liver Dis. 2014;34(1):108–112. | |
Fontana RJ, Hughes EA, Bifano M, Appelman H, Dimitrova D, Hindes R, Symonds WT. Sofosbuvir and daclatasvir combination therapy in a liver transplant recipient with severe recurrent cholestatic hepatitis C. Am J Transplant. 2013;13(6):1601–1605. | |
Forns X, Prieto M, Charlton M, et al. Sofosbuvir compassionate use program for patients with severe reccurent hepatitis c including fibrosing cholestatic hepatitis following liver transplantation. J Hepatol. 2014;60:26. | |
Charlton MR, Thompson A, Veldt BJ, et al. Interleukin-28B polymorphisms are associated with histological recurrence and treatment response following liver transplantation in patients with hepatitis C virus infection. Hepatology. 2011;53(1):317–324. | |
Tempestilli M, D’Offizi G, Lionetti R, et al. Increased plasma concentration of Ribavirin as a result of renal dysfunction in HCV patients treated with telaprevir. Hepatology. In press 2013. | |
D’Avolio A, Pensi D, Baietto L, Di Perri G. Therapeutic drug monitoring of intracellular anti-infective agents. J Pharm Biomed Anal. In press 2014. | |
Farnik H, Zimmermann T, Herrmann E, et al. Telaprevir drug monitoring during antiviral therapy of hepatitis C graft infection after liver transplantation. Liver Int. In press 2014. | |
Calista D, Landi G. Lichen planus, erythema nodosum, and erythema multiforme in patient with chronic hepatitis C. Cutis. 2001;67(6):454–456. | |
Ionescu C, Micu L, Constantinescu I, et al. Prolonged Treatment with Interferon Alpha and Peginterferon Induces Rheumatoid Arthritis Syndrome and Erythema Nodosum. J Gastrointestin Liver Dis. 2008;17(2):211–212. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.