Back to Journals » Infection and Drug Resistance » Volume 15
Trends of Rifampicin Resistance in Patients with Pulmonary Tuberculosis: A Longitudinal Analysis Based on Drug Resistance Screening in Eastern China Between 2015 and 2019
Authors Ren Y, Chen B , Zhao J, Tan X, Chen X , Zhou L, Wang F, Peng Y, Jiang J
Received 19 October 2022
Accepted for publication 14 December 2022
Published 28 December 2022 Volume 2022:15 Pages 7707—7717
DOI https://doi.org/10.2147/IDR.S394089
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Yanli Ren,1 Bin Chen,2 Jiaying Zhao,3 Xiaohua Tan,1 Xinyi Chen,2 Lin Zhou,2 Fei Wang,2 Ying Peng,2 Jianmin Jiang1,2
1School of Public Health, Hangzhou Normal University, Hangzhou, People’s Republic of China; 2Zhejiang Center for Disease Control and Prevention, Hangzhou, People’s Republic of China; 3School of Public Health, Xiamen University, Fujian, People’s Republic of China
Correspondence: Ying Peng; Jianmin Jiang, Email [email protected]; [email protected]
Objective: To understand the trend of overall rifampicin resistance rates for tuberculosis in Zhejiang Province between 2015 and 2019.
Methods: The basic demographic information of patients with tuberculosis who were screened for drug resistance in Zhejiang Province between January 1, 2015 and December 31, 2019 was collected through the national Tuberculosis Information Management System. The data were processed and analyzed using IBM SPSS 26.0 and GeoDa 1.14 software.
Results: The total rifampicin resistance rate was 5.9% in 53,893 validated cases of drug resistance screening conducted in patients with pulmonary tuberculosis in Zhejiang Province during the study period. There was a decreasing trend in the rifampicin resistance rate in both initial and re-treated patients (P< 0.001), but the rifampicin resistance rate was higher in re-treated TB patients than in TB patients receiving their initial treatment (11.4% vs 4.2%). The rate of drug resistance steadily decreased in all prefectures, and there was a significant upward trend in the use of the Xpert MTB/RIF rapid assay. An increasing trend was also identified in the rate of rifampicin and ofloxacin co-resistance (P< 0.001).
Conclusion: The overall rate of rifampin resistance in patients with tuberculosis in Zhejiang Province in the past five years has shown a decreasing trend, but the rate of resistance to ofloxacin was high. Resistance testing to fluoroquinolones should be carried out as early as possible in patients whose diagnosis results indicate rifampin resistance, and more effective second-line treatment plans should be developed based on the results of this testing.
Keywords: rifampicin, drug-resistant tuberculosis, ofloxacin, trend analysis
Introduction
Tuberculosis (TB) is a chronic infectious disease caused by Mycobacterium tuberculosis. It remains one of the major diseases threatening public health globally. Multidrug-resistant tuberculosis (MDR-TB) refers to Mycobacterium tuberculosis that is resistant to at least isoniazid and rifampicin. According to the Global Tuberculosis Report released by the World Health Organization (WHO), there were 16,343 multidrug-resistant/rifampicin-resistant tuberculosis (MDR/RR-TB) patients in China in 2020.1 The development of drug resistance in patients may be caused by irregular drug use or acquired after exposure to drug-resistant Mycobacterium tuberculosis. Studies have shown that in Shanghai, 73% of MDR-TB is caused by the transmission of MDR-TB between patients.2 Rifampicin resistance is the main factor influencing the outcome of anti-tuberculosis treatment. Typically, over 80% of rifampicin-resistant strains are also resistant to isoniazid, so rifampicin resistance is gradually becoming a marker of multidrug-resistant tuberculosis.3 The treatment of drug-resistant TB is more complicated and requires a longer regimen than that of drug-sensitive TB. Drug-resistant TB is a challenge to the prevention and control of TB worldwide, especially in low- and middle-income countries.
China has a high burden of tuberculosis relative to other countries worldwide, and the number of tuberculosis cases in China is second only to India.1 There is still a long way to go towards the goal of ending TB. The TB epidemic status crucially depends on regional geographic, socioeconomic, and cultural variations.4 Therefore, the prevalence of tuberculosis in different regions also varies. For example, in Shandong Province in northern China, the rifampicin-resistance rate in newly diagnosed tuberculosis cases was 4.43%, while the rifampicin-resistance rate in retreated patients was 11.26%.5 Between 2012 and 2019, the rifampicin-resistance rate in Dalian City decreased from 14.59% to 7.31% in newly treated patients and from 52.44% to 15.15% in retreated patients.6 In southern China’s Guizhou province, the rate of rifampicin resistance has shown an upward trend, from 12.8% in 2015 to 17.5% in 2018.4 In other countries, such as Uganda, the rate of rifampicin resistance was 10.2% in newly treated patients and 23.5% in re-treated patients in 2018–2019.7 In Ethiopia, the laboratory-detected rifampicin resistance rate was 6.3%.8 As a representative province in eastern China, Zhejiang Province also has its own unique TB drug resistance rate. A study of drug resistance based on a random sample of 30 sites in Zhejiang Province showed that from 1998 to 2013, the rate of rifampicin resistance in newly diagnosed patients decreased from 6.3% to 5.2% and that in previously treated patients decreased from 43.9% to 29.4%.9 From 2013 to 2014, the total drug resistance rate for tuberculosis was 30.88%, based on the results of all drug resistance screening in Zhejiang Province. The prevalence of rifampicin resistance in patients receiving initial TB treatment was 4.39%, and the prevalence of rifampicin resistance in retreated TB patients was 24.47%.10 However, the prevalence of rifampicin resistance and its changing trends have not been studied in recent years, which has prevented us from knowing the effectiveness of prevention and control methods during this time period. The aim of this study was to analyze the prevalence of RR-TB using the TB case cohort screened and registered in Zhejiang province between 2015 and 2019. Through an analysis of the epidemiological characteristics and trends of rifampicin-resistance rates, we aimed to provide an evidence base for the assessment of existing TB interventions and a guide for future TB control in eastern China.
Methods
Study Setting
Zhejiang Province is located in the Yangtze River Delta region in eastern China and includes 11 prefectures and 90 county-level administrative regions. It is a province with a relatively good economic development in China, with a permanent population of more than 60 million.11 In 2014, Zhejiang Province started to establish a MDR-TB prevention and control system with the support of the Zhejiang Provincial Financial MDR-TB Special Fund. Until 2016, molecular or cultural drug susceptibility screening was carried out mainly for patients with TB at high-risk of drug resistance, but gradually expanded to include standard patients with TB. Since the launch of the “National Health Commission of China-Bill & Melinda Gates Foundation TB Project” in 2017, all laboratory-confirmed tuberculosis patients are referred to undergo Xpert MTB/RIF at the county level TB labs and/or Drug Susceptible Test (DST) at prefecture-level labs. The patients’ screening information is reported in the national Tuberculosis Information Management System (TBIMS). If diagnosed with RR-TB, these patients are registered as such in the TBIMS database for diagnosed RR-TB patients.12 Therefore, not only high-risk groups are screened for drug resistance; infectious patients with initial positive bacterial test results have gradually become the subjects of drug resistance screening.
Data Sources and Population
This study was a retrospective study based on TBIMS data. Routine information on tuberculosis detection and management was collected through the TBIMS, including information on TB patients that had completed rifampicin susceptibility testing that was reported in the 11 prefectures in Zhejiang Province (Hangzhou, Huzhou, Jiaxing, Jinhua, Lishui, Ningbo, Quzhou, Shaoxing, Taizhou, Wenzhou, and Zhoushan). Inclusion criteria: There were 56,030 TB patients with suspected drug-resistant tuberculosis and high-risk retreatment tuberculosis patients registered in TBIMS of Zhejiang Province between January 1, 2015 and December 31, 2019. Exclusion criteria: 2137 cases of non-tuberculous Mycobacterium were excluded. Finally, a total of 53,893 cases were included for analysis, including their basic demographic information and drug sensitivity test outcomes.
Definition of Related Terms
Rifampicin-resistant tuberculosis (RR-TB): Mycobacterium tuberculosis that is resistant to rifampicin regardless of whether it is resistant to other anti-TB drugs.
Initial treatment: Patients who have never been treated with anti-tuberculosis drugs for tuberculosis in the past; or those who are taking regular drugs with standard chemotherapy regimens for less than one month; or those who have had irregular chemotherapy for less than one month.
Retreatment: Patients who have been treated with anti-tuberculosis drugs irregularly for more than 1 month; or patients who have failed initial treatment.
Rapid test method: The rapid test method refers to Xpert MTB/RIF technology, which has been recommended as a rapid molecular test to detect Mycobacterium tuberculosis and rifampin resistance mutations by the WHO since 2011.13 This molecular technique features a rapid, fully-automated system with high accuracy compared to traditional phenotypic DST,14 and it has been shown to increase the detection of RR-TB two-to-eight fold in recent years.15
Conventional test method: All positive Mycobacterium tuberculosis isolates were transported to prefecture-level laboratories for conventional DST. Indirect drug susceptibility of the culture-positive isolates was detected by the proportion method according to the recommendation of the WHO. Sputum samples were added to the liquid medium for culture and if no bacteria appeared by week 8, the results were recorded as negative. If bacteria were present, the result was recorded as positive. Indirect drug susceptibility of the culture-positive isolates was detected by the proportion. The concentrations of the four first-line anti-tuberculosis drugs were determined by the proportional method as follows: 0.2 μg/mL for isoniazid, 4 μg/mL for streptomycin, 40 μg/ mL for rifampicin, and 2 μg/mL for ethambutol. The strain was declared resistant to the corresponding drug when the growth rate was higher than 1% compared to the control. This approach usually takes 2 to 3 months and can have a serious impact on the timely diagnosis of drug-resistant TB.
Data Analysis
The basic information, clinical data, and drug susceptibility results of the research subjects were obtained from the TBIMS and then imported into SPSS 24.0 (IBM SPSS Statistics for Windows, Version 24.0; IBM Corp., Armonk, NY, USA) for statistical analysis, and the count data were represented by the composition ratio. A χ2 trend test was carried out on the changing trend of the rifampicin resistance rate. The GeoDa software (Version 1.14) was used to draw the spatial distribution map of the rifampicin resistance rate, and the maps of various cities in Zhejiang Province were superimposed with the rifampicin-resistance rates between 2015 and 2019, and the changes in the rifampicin-resistance rates were represented by gradient colors.
Ethical Approval
The data in this article were derived from TBIMS, and the Ethics Review Committee of the Zhejiang Provincial Center for Disease Control and Prevention had reviewed the study and issued an ethical review waiver for informed consent. Data were accessed using a secure form to prevent unauthorized access.
Results
Demographic Characteristics and Drug Resistance Rate Analysis of RR-TB Patients
The demographic and clinical data of 53,893 potentially drug-resistant tuberculosis cases in Zhejiang Province between 2015 and 2019 were analyzed, the overall rate of rifampin resistance was 5.9% (3182/53,893) using the drug susceptibility test. Of the 39,046 males and 14,847 females (Table 1), 5.9% of both males and females developed rifampicin-resistant TB. The rate of rifampicin resistance was significantly higher in youth and adults than in other age groups. During the 5-year period, 1276 ethnic minorities were screened for drug resistance, and the prevalence of rifampicin resistance in ethnic minorities was significantly lower than that in Han people (4.5% vs 5.9%). Healthcare workers had higher rates of rifampin resistance than people in other occupations. Those who migrated within Zhejiang Province had lower rates of rifampicin resistance than those who immigrated from other provinces. Among all patients who underwent drug susceptibility testing, 78.44% (42,272/53,893) of TB patients were classified as receiving initial treatment and 21.56% (11,621/53,893) were classified as receiving retreatment. The resistance rate of rifampicin in patients receiving initial TB treatment was 4.2% (Figure 1A), while the prevalence of rifampicin resistance in retreated TB was 11.4% (Figure 1B). The prevalence of rifampicin resistance in association with both first treatment and retreatment showed a downward trend over the years (initial treatment χ2trend = 46.929, P<0.001; retreatment χ2trend = 253.311, P<0.001).
 |
Table 1 Analysis of Basic Demographic Characteristics of Rifampicin-Resistant and Potentially RRTB Patients |
Geospatial Distribution of Rifampicin Resistance Rates
Figure 2 shows the geographical location of each city in Zhejiang Province. Different colors represent the prevalence of rifampicin resistance in each city. The darkest colors in Zhoushan indicate the highest RR rates, while the colors in Quzhou, Shaoxing, and Jiaxing were relatively light. The rifampicin resistance rate in eastern Zhejiang was higher than that in southwest Zhejiang. From the results of a year-by-year analysis of various cities, the rifampicin resistance rate decreased in all cities except Quzhou, which always remained at a low level (Figure 3). However, the number of potential RR-TB patients screened has doubled since 2017 (Appendix Table 1), indicating that rifampicin resistance is gradually being controlled. Although Zhoushan City has a high rate of rifampicin resistance, the overall number of RR-TB patients identified was small and the number of patients who received rifampicin screening was also relatively small compared to other cities.
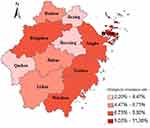 |
Figure 2 Geospatial distribution of 5-year overall rifampicin resistance rates in 11 cities in Zhejiang Province. The map data of cities in Zhejiang were obtained from the aliyun website (http://datav.aliyun.com/portal/school/atlas/area_selector). The map in this article is used only for data representation and does not represent a real administrative map. |
 |
Figure 3 Epidemiological trend of rifampicin resistance rates in various cities in Zhejiang Province between 2015 and 2019. |
Comparison of Rifampicin Resistance Rates in TB Patients Diagnosed Using Conventional and Rapid Detection methods
Table 2 shows the rifampicin resistance rates detected using different testing methods, including conventional and rapid testing. The number of suspected drug-resistant patients using rapid test methods for drug susceptibility testing has increased year by year, showing a clear upward trend (χ2trend=20,388.54, P<0.001), indicating that the use of Xpert MTB/RIF technology is gradually being promoted. The prevalence of RR has experienced a downward trend using both traditional and rapid detection methods (traditional χ2trend =59.47, P<0.001; rapid χ2trend = 13.4, P<0.001).
 |
Table 2 Analysis of Rifampicin Resistance Rate Using Different Detection Methods in Zhejiang Province from 2015 to 2019 |
Co-Resistance Rate of Rifampicin and Ofloxacin
Among the effective cases of TB drug resistance screening between 2015 and 2019, a total of 9612 cases were screened for ofloxacin resistance based on the completion of rifampicin resistance screening. The overall five-year co-resistance rate of rifampicin and ofloxacin was 6.2% (Table 3), and the co-resistance rate showed an upward trend (χ2trend = 124.126, P<0.001). Ofloxacin, one of the fluoroquinolone drugs, is the drug of choice for second-line anti-TB treatment, but the current prevalence of ofloxacin resistance has increased from 6.3% in 2015 to 11.9% in 2019. The situation of co-resistance is also not optimistic, with the co-resistance rate increasing from 2.5% in 2015 to 10.3% in 2019.
 |
Table 3 Co-Resistance Analysis of RFP and OFX in Zhejiang Province from 2015 to 2019 |
Discussion
Our study collected data on all 53,893 individuals registered for drug resistance screening in Zhejiang Province between 2015 and 2019, of which 3182 patients had documented resistance to rifampicin (5.9%), including 4.2% of patients receiving initial treatment and 11.4% of patients receiving re-treatment. Compared with other provinces in China, drug resistance control in Zhejiang Province was shown to be at a lower level. For example, in Heilongjiang province in the northern region, the initial rifampicin resistance rate was 11.99% and the re-treatment resistance rate was 29.41% from 2017 to 2020;16 in Xinjiang province in the west, the multi-drug resistance rate was 15.5% in 2019.17 In countries with low TB prevalence, such as Japan, the rate of rifampicin resistance was only 1%.18 And in countries with a high TB burden, such as India, rifampicin resistance rates were as high as 40.3%.19 The results of this study showed that the rifampicin resistance rate of the screening population in Zhejiang Province showed a decreasing year-on-year trend. Between 2015 and 2019, the expansion of drug resistance screening led to a significant increase in the number of individuals being screened. The number of patients screened in 2015 was only 5513, but 11,781 patients were screened each year since 2017, and the number of patients screened in 2019 was almost three times that of 2015. However, the number of patients with RR-TB did not multiply in a corresponding manner. The rifampicin resistance rate of the retreated patients in Zhejiang Province was three times that of the newly treated patients, and the drug resistance rate was significantly different, which was consistent with the results of related studies in other regions such as Shandong Province in northern China.5 Retreated TB patients tend to have longer treatment times and poorer compliance with anti-TB courses, and are more likely to discontinue treatment than patients receiving their initial treatment. Therefore, re-treated patients are at an increased risk of developing resistance to rifampicin. During the course of treatment, it is necessary to improve patient compliance by enhancing health education for patients and stressing the importance of taking the correct medication to patients to make them take medication responsibly and regularly, thereby reducing the risk of developing acquired drug resistance.
The present study found no difference in the prevalence of rifampicin resistance between males and females, which was inconsistent with the findings of the Myanmar study in southern Asia.20 The prevalence of rifampicin resistance was highest in the 20- to 60-year-old group. As the main labor force of society, middle-aged patients are characterized by rich social activities, strong mobility, large public influence, and low medication compliance, which makes them more likely to cause drug resistance. Therefore, more attention should be paid to such patients and their management should be strengthened. The prevalence of rifampicin resistance in the Han ethnicity participants was higher than that in other ethnicities, which may be due to cultural differences. The rifampicin resistance rates of healthcare workers and workers in the service industry were higher than those of other occupations. Healthcare workers have higher resistance rates due to occupational exposure to more drug-resistant patients while workers in the service industry are exposed to a wide variety of people. Health workers should comply with the sterilization and isolation system in the treatment work. It is recommended to reduce the incidence of occupational infections by enhancing training in infection protection and raising the awareness of healthcare workers about post-exposure prophylaxis. The rifampicin resistance rate of the floating population in the Zhejiang province was lower than that of the floating population inside and outside of Zhejiang. Most of the population migrating in and out of the province are workers, whose poor economic conditions and poor compliance with treatment can easily lead to irregular treatment, thus causing drug resistance.
From the perspective of geographical and spatial distribution, the rifampicin resistance rates in Quzhou, Shaoxing, and Jiaxing were significantly lower than those in other regions; in particular, Quzhou was stable at a lower level. Zhoushan City is composed of various islands with a small population density and a small number of rifampicin-resistant patients in total, but the drug resistance rate has been at a high level due to the small number of people screened. The level of economic development and health resources may also have an impact on the drug resistance rates in various regions.4,21,22 For example, in Hangzhou and Ningbo, due to their developed economies and abundant health resources, the local population tend to be highly mobile, rich in social interactions, and complex in interpersonal activities, which may lead to rifampicin resistance rates at high levels. Even so, the rate of drug resistance in each municipality was decreasing year by year, and the province was on a steady downward trend.
The Xpert MTB/RIF test method is the WHO recommended rapid test that simultaneously detects tuberculosis and rifampicin resistance in people with signs and symptoms of tuberculosis at lower health system levels.23,24 It allows the detection of Mycobacterium tuberculosis complex and simultaneously the most common resistance mutations to rifampicin in less than 2 h.25–27 It is unaffected by the external environment and other factors, which is conducive to timely treatment of patients and reduces the risk of social transmission.28 However, Xpert MTB/RIF is expensive, and resources were limited, and thus it was not popularized in Zhejiang Province until 2017. Therefore, very few patients accepted this method over DST before 2017. Since the implementation of the “National Health Commission of China-Bill & Melinda Gates Foundation TB Project” in Zhejiang Province, the number of screened patients using the molecular rapid detection method has increased exponentially. The results of this study show that the promotion and use of rapid diagnosis technology has achieved promising results. In 2019, 88.97% of patients have been tested using the Xpert MTB/RIF method, and most patients can get the DST of rifampicin results the same day, which greatly shortens the diagnosis time compared to traditional drug susceptibility testing that requires more than one month for the diagnosis to be confirmed. It is beneficial to help clinicians know the patient’s DST of the key drugs in time and then design a more appropriate clinical treatment plan for the patient to achieve early diagnosis and treatment.
This study found that the co-resistance rate of rifampicin and ofloxacin is gradually increasing, and co-resistance will cause huge difficulties to the treatment of patients with TB. In the guidelines for the treatment of drug-resistant tuberculosis issued by the WHO,29,30 it was recommended that rifampicin-resistant TB patients should be treated with second-line anti-TB treatment, and fluoroquinolones should be the first choice; these are broad-spectrum antibiotics for the treatment of multidrug-resistant tuberculosis that can effectively deal with infections caused by common respiratory pathogens and were widely used in clinical practice before.31 However, if the patient is resistant to both rifampicin and ofloxacin, there will be problems such as the need for a more complicated regimen, higher treatment costs, longer treatment cycles, lower cure rates, and higher incidence of adverse drug reactions.32,33 Fluoroquinolones should not be added arbitrarily in the absence of first-line drug susceptibility results or drug history in tuberculosis patients.34 Although only ofloxacin was resistant in this study, fluoroquinolones have cross-resistance properties, and therefore ofloxacin resistance cannot totally represent the current resistance trend of fluoroquinolones. It is suggested that, for the rifampicin-resistant patients with TB, consideration should be given to gradually advancing the screening of fluoroquinolone resistance to all rifampicin-resistant TB patients to further standardize the diagnosis and treatment procedures.
The main limitation of this study is that the data in the paper were collected from an information management system. Although we carried out quality control and most of the data were correctly registered, it is still difficult to avoid some incorrect entries due to the different understanding of concept definitions by the coding staff in different places. Second, the information obtained from the system data was relatively limited, and it was not possible to do a more detailed analysis of the influencing factors.
Conclusion
This study suggests that between 2015 and 2019, the overall rifampicin resistance rate in Zhejiang Province showed a trend to decrease, and the rifampicin resistance rate in all municipalities also showed a decreasing trend. The expansion of drug resistance testing has also led to effectiveness in curbing the spread of rifampicin-resistant tuberculosis in the population of Zhejiang Province. However, the rate of fluoroquinolone resistance is increasing, and it is necessary to conduct fluoroquinolone resistance testing for patients with confirmed rifampicin resistance to clarify treatment options. The problem of co-resistance requires high attention, and it is recommended that multi-drug resistance testing for first-line and second-line tuberculosis be carried out early in the course of clinical diagnosis and treatment to improve the effectiveness of treatment and reduce the occurrence of drug resistance.
Acknowledgments
This study was supported by the National Health and Health Commission Scientific Research Fund (WKJ-ZJ-2118).
Disclosure
The authors declare no conflicts of interest in this work.
References
1. World Health Organization. Global tuberculosis report 2021; 2021. Available from: https://apps.who.int/iris/handle/10665/346387.
2. Yang C, Luo T, Shen X, et al. Transmission of multidrug-resistant Mycobacterium tuberculosis in Shanghai, China: a retrospective observational study using whole-genome sequencing and epidemiological investigation. Lancet Infect Dis. 2017;17(3):275–284. doi:10.1016/S1473-3099(16)30418-2
3. Murase LS, Perez de Souza JV, Meneguello JE, et al. Possible binding of piperine in Mycobacterium tuberculosis RNA polymerase and rifampin synergism. Antimicrob Agents Chemother. 2019;63(11):1. doi:10.1128/AAC.02520-18
4. Chen L, Fu X, Tian P, et al. Upward trends in new, rifampicin-resistant and concurrent extrapulmonary tuberculosis cases in northern Guizhou Province of China. Sci Rep. 2021;11(1):18023. doi:10.1038/s41598-021-97595-8
5. Song W-M, Li Y-F, Ma X-B, et al. Primary drug resistance of mycobacterium tuberculosis in Shandong, China, 2004–2018. Respir Res. 2019;20(1):223. doi:10.1186/s12931-019-1199-3
6. Du L, Zhang Y, Lv X, et al. Prevalence of multidrug-resistant tuberculosis in Dalian, China: a retrospective study. Infect Drug Resist. 2021;14:1037–1047. doi:10.2147/IDR.S294611
7. Micheni LN, Kassaza K, Kinyi H, et al. Rifampicin and isoniazid drug resistance among patients diagnosed with pulmonary tuberculosis in southwestern Uganda. PLoS One. 2021;16(10):e0259221. doi:10.1371/journal.pone.0259221
8. Diriba G, Kebede A, Tola HH, et al. Surveillance of drug resistance tuberculosis based on reference laboratory data in Ethiopia. Infect Dis Poverty. 2019;8(1):54. doi:10.1186/s40249-019-0554-4
9. Liu Z, Zhang M, Wang J, et al. Longitudinal analysis of prevalence and risk factors of rifampicin-resistant tuberculosis in Zhejiang, China. Biomed Res Int. 2020;2020:3159482. doi:10.1155/2020/3159482
10. Songhua C, Wu BB, Liu ZW, et al. An analysis on the epidemic characteristics of tuberculosis drug resistance in Zhejiang Province. Prev Med. 2016;28(8):757–761+765.
11. China national Bureau of statistics. 2020 China statistical yearbook; 2021. Available from: http://www.stats.gov.cn/tjsj/ndsj/2020/indexch.htm.
12. Jiang W, Peng Y, Wang X, et al. Policy changes and the screening, diagnosis and treatment of drug-resistant tuberculosis patients from 2015 to 2018 in Zhejiang Province, China: a retrospective cohort study. BMJ Open. 2021;11(4):e047023. doi:10.1136/bmjopen-2020-047023
13. Falzon D, Jaramillo E, Schunemann HJ, et al. WHO guidelines for the programmatic management of drug-resistant tuberculosis: 2011 update. Eur Respir J. 2011;38(3):516–528. doi:10.1183/09031936.00073611
14. Ioannidis P, Papaventsis D, Karabela S, et al. Cepheid GeneXpert MTB/RIF assay for Mycobacterium tuberculosis detection and rifampin resistance identification in patients with substantial clinical indications of tuberculosis and smear-negative microscopy results. J Clin Microbiol. 2011;49(8):3068–3070. doi:10.1128/JCM.00718-11
15. Albert H, Nathavitharana RR, Isaacs C, et al. Development, roll-out and impact of Xpert MTB/RIF for tuberculosis: what lessons have we learnt and how can we do better? Eur Respir J. 2016;48(2):516–525. doi:10.1183/13993003.00543-2016
16. Cuiyu L, Suping L, Baoshan P, et al. Drug resistance in pulmonary tuberculosis patients in Jiamusi City, Heilongjiang Province, 2017–2020. Dis Surveilll. 2021;36(10):1052–1056.
17. Maimaitiaili A, Huang QL, Guerseman A, Renati A. Analysis of drug-resistance to rifampicin and isoniazid in 1307 patients with pulmonary tuberculosis in Kashgar, Xinjiang. Chin J Antituberculosis. 2020;42(11):1209–1213.
18. Mizukoshi F, Kobayashi N, Kirikae F, et al. Molecular epidemiology of drug-resistant Mycobacterium Tuberculosis in Japan. MSphere. 2021;6(4):e0097820. doi:10.1128/mSphere.00978-20
19. Jain A, Singh PK, Chooramani G, et al. Drug resistance and associated genetic mutations among patients with suspected MDR-TB in Uttar Pradesh, India. Int J Tuberc Lung Dis. 2016;20(7):870–875. doi:10.5588/ijtld.15.0874
20. Seifert M, Aung HT, Besler N, et al. Age and sex distribution of Mycobacterium tuberculosis infection and rifampicin resistance in Myanmar as detected by Xpert MTB/RIF. BMC Infect Dis. 2021;21(1):781. doi:10.1186/s12879-021-06296-0
21. Liang L, Wu Q, Gao L, et al. Factors contributing to the high prevalence of multidrug-resistant tuberculosis: a study from China. Thorax. 2012;67(7):632–638. doi:10.1136/thoraxjnl-2011-200018
22. Li Q, Zhao G, Wu L, et al. Prevalence and patterns of drug resistance among pulmonary tuberculosis patients in Hangzhou, China. Antimicrob Resist Infect Control. 2018;7:61. doi:10.1186/s13756-018-0348-7
23. Horne DJ, Kohli M, Zifodya JS, et al. Xpert MTB/RIF and Xpert MTB/RIF ultra for pulmonary tuberculosis and rifampicin resistance in adults. Cochrane Database Syst Rev. 2019;6(6):Cd009593. doi:10.1002/14651858.CD009593.pub4
24. Nasiri MJ, Zamani S, Pormohammad A, et al. The reliability of rifampicin resistance as a proxy for multidrug-resistant tuberculosis: a systematic review of studies from Iran. Eur J Clin Microbiol Infect Dis. 2018;37(1):1. doi:10.1007/s10096-017-3079-4
25. Vergara Gómez A, González-Martín J, García-Basteiro AL. Xpert® MTB/RIF: usefulness for the diagnosis of tuberculosis and resistance to rifampicin. Med Clin. 2017;149(9):399–405. doi:10.1016/j.medcli.2017.06.007
26. Steingart KR, Schiller I, Horne DJ, et al. Xpert® MTB/RIF assay for pulmonary tuberculosis and rifampicin resistance in adults. Cochrane Database Syst Rev. 2014;2014(1):CD009593. doi:10.1002/14651858.CD009593.pub3
27. Shao Y, Peng H, Chen C, et al. Evaluation of GeneXpert MTB/RIF for detection of pulmonary tuberculosis at peripheral tuberculosis clinics. Microb Pathog. 2017;105:260–263. doi:10.1016/j.micpath.2017.02.040
28. Cox H, Dickson-Hall L, Ndjeka N, et al. Delays and loss to follow-up before treatment of drug-resistant tuberculosis following implementation of Xpert MTB/RIF in South Africa: a retrospective cohort study. PLoS Med. 2017;14(2):e1002238. doi:10.1371/journal.pmed.1002238
29. World Health Organization. WHO Guidelines Approved by the Guidelines Review Committee, in WHO Treatment Guidelines for Drug-Resistant Tuberculosis, 2016 Update. World Health Organization; 2016.
30. Falzon D, Schünemann HJ, Harausz E, et al. World Health Organization treatment guidelines for drug-resistant tuberculosis, 2016 update. Eur Respir J. 2017;49(3):4. doi:10.1183/13993003.02308-2016
31. Sulochana S, Mitchison DA, Kubendiren G, et al. Bactericidal activity of moxifloxacin on exponential and stationary phase cultures of Mycobacterium tuberculosis. J Chemother. 2009;21(2):127–134. doi:10.1179/joc.2009.21.2.127
32. Gandhi NR, Nunn P, Dheda K, et al. Multidrug-resistant and extensively drug-resistant tuberculosis: a threat to global control of tuberculosis. Lancet. 2010;375(9728):1830–1843. doi:10.1016/S0140-6736(10)60410-2
33. Marks SM, Flood J, Seaworth B, et al. Treatment practices, outcomes, and costs of multidrug-resistant and extensively drug-resistant tuberculosis, United States, 2005–2007. Emerg Infect Dis. 2014;20(5):812–821. doi:10.3201/eid2005.131037
34. Ginsburg AS, Grosset JH, Bishai WR. Fluoroquinolones, tuberculosis, and resistance. Lancet Infect Dis. 2003;3(7):432–442. doi:10.1016/S1473-3099(03)00671-6
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

