Back to Journals » Cancer Management and Research » Volume 12
Trefoil Factor 2 Regulates Proliferation and Apoptosis of Pancreatic Cancer Cells and LPS-Induced Normal Pancreatic Duct Cells by β-Catenin Pathway
Authors Zhou Y, Zhang Y, Wang J
Received 29 July 2020
Accepted for publication 11 September 2020
Published 29 October 2020 Volume 2020:12 Pages 10705—10713
DOI https://doi.org/10.2147/CMAR.S274578
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Eileen O'Reilly
Yun Zhou,* Yan Zhang,* Jia Wang
Department of Clinical Laboratory Medicine, Shanghai Tenth People’s Hospital of Tongji University, Shanghai 200072, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jia Wang
Department of Clinical Laboratory Medicine, Tenth People’s Hospital of Tongji University, 301 Yanchang Middle Road, Jingan District, Shanghai 200072, People’s Republic of China
Tel +86 21-66306756
Email [email protected]
Introduction: Pancreatic cancer (PC) is a malignant tumor with poor prognosis. This study aimed to determine the role of trefoil factor 2 (TFF2) in the proliferation and apoptosis of LPS-induced normal pancreatic duct cells and pancreatic cancer cells through β-catenin pathway.
Methods: TFF2 expression in normal pancreatic duct cells, pancreatic cancer cells and LPS-induced normal pancreatic duct cells was detected by RT-qPCR analysis and Western blot analysis. The transfection effects in pancreatic cancer cells and LPS-induced normal pancreatic duct cells were analyzed by RT-qPCR analysis. After indicated transfection, proliferation, apoptosis and inflammation of these cells were respectively detected by CCK-8 assay, TUNEL assay and certain ELISA kits. Expression of β-catenin pathway-related proteins was analyzed by Western blot analysis. Co-immunoprecipitation assay determined the combination of TFF2 and β-catenin.
Results: TFF2 expression was increased in pancreatic cancer cells and LPS-induced HPDE cells compared with HPDE cells. According to TFF2 expression in these cells, PanC-1 cells and 5 μg/mL LPS were selected. In addition, TFF2 interference decreased the proliferation and promoted the apoptosis of PanC-1 cells and LPS-induced HPDE cells. However, TFF2 interference did not obviously change the levels of TNF-α, IL-1β and IL-6 in PanC-1 cells and LPS-induced HPDE cells. Furthermore, TFF2 interference suppressed the expression of β-catenin, c-Myc, Cyclin D1 and BIRC5 in PanC-1 cells and LPS-induced HPDE cells. TFF2 was demonstrated to combine with β-catenin.
Discussion: TFF2 interference inhibits proliferation and promotes apoptosis of PanC-1 cells and LPS-induced HPDE cells by suppressing β-catenin pathway.
Keywords: trefoil factor 2, pancreatic duct cancer cells, LPS, normal pancreatic duct cells, β-catenin pathway
Introduction
Pancreatic cancer (PC) is a malignant tumor of the digestive system which gathers high degree of malignancy, and the difficulty in early diagnosis of PC leads to its rapid development with poor prognosis. Pancreatic ductal adenocarcinoma (PDAC) is the most common type of PC, accounting for more than 90% of all PC patients.1,2 In China, chronic pancreatitis (CP) is the most clear and important PDAC risk factor. Study has shown that the relative risk of CP patients developing into PDAC is as high as 13.3%.3 About 5% of CP patients will develop pancreatic cancer after 20 years, and the use of non-steroidal drugs (NSAID) such as aspirin can reduce the risk of CP from developing into pancreatic cancer.4 Therefore, it is important to further clarify the mechanism of pancreatitis-cancer transformation.
Trefoil factor (TFF) family includes three members, which are TFF1, 2 and 3 in mammals. They can maintain biological activity in the complex physiological environment of gastrointestinal tract and play the role of mucosal protection and injury repair.5 Through GEPIA (Gene Expression Profiling Interactive Analysis), TFF2, unlike TFF1 and TFF3, is found not to be significantly elevated in other tumors except for pancreatic cancer. Therefore, TFF2 is more likely to be a marker with high specificity for pancreatic cancer. Study on the mechanism of TFF2 in pancreatic cancer has reported that TFF2 may promote the immune escape and drug resistance of tumor by adsorbing immature dendritic cells (DC) and affecting the maturation of DC.6 Study has also found that CXCR4 may be the true acting receptor of TFF2 which regulates inflammatory immunity and causes tumorigenesis in vivo through CXCR4.7 Wnt/β-catenin pathway is demonstrated to be important in the development of pancreatic cancer. The growth of pancreatic cancer was inhibited by Huaier extract through suppressing Wnt/β-catenin pathway.8 FAM84B overexpression promoted cell proliferation, apoptosis, mitochondrial function, and glycolysis, which was alleviated by inhibiting Wnt/β-catenin pathway.9 ARHGAP30 overexpression suppressed pancreatic cancer progression by blocking the β-catenin pathway.10 Through KEGG, tumor-promoting mechanism of CXCR4 is closely related to Wnt/β-catenin pathway. STRING also shows a possible interaction between TFF2 and β-catenin. Unlike TFF3, TFF1 and TFF2 seem to be associated with gastritis, especially Helicobacter pylori (HP) infection.11–13 Studies have reported that pancreatic cancer is also related to HP infection.14,15 Therefore, TFF2 has greater potential as a marker for early detection of pancreatic cancer risk factors and prevention of pancreatitis-cancer transformation.
Above all, this study aimed to determine the role of TFF2 in the proliferation and apoptosis of LPS-induced pancreatic duct cells and pancreatic cancer cells through β-catenin pathway.
Materials and Methods
Cell Culture
HPDE cells were achieved from Shanghai Yagi Biological Technology Co., Ltd (Shanghai, China). SW1990, PanC-1 and CFPAC were obtained from American Type Culture Collection (Rockville, MD, USA) and T3M4 cells were brought from CoBioer Biosciences Co., Ltd (Nanjing, China). All cells were cultured in DMEM medium containing 10% fetal bovine serum (FBS) and 100 U/mL penicillins in an incubator of 5% CO2 and 37°C.
LPS Induction
HPDE cells in logarithmic phase were seeded into 6-well plate (5×105 cells/well). Then, HPDE cells were respectively treated with LPS at different concentrations (0.1 μg/mL, 1 μg/mL and 5 μg/mL) for 24 h.
Cell Transfection
Pancreatic cancer cells and LPS-induced HPDE cells were inoculated in a 6-well plate until the cell density was 70%. Then, pancreatic cancer cells and LPS-induced HPDE cells were transfected with ShRNA NC, ShRNA-TFF2-#1 (CCGGACTTCATCTTTGAAGTGCCCTCTCGAGAGGGCACTTCAAAGATGAAGTTTTTTG) and ShRNA-TFF2-#2 (CCGGTTTGACAATGGATGCTGTTTCCTCGAGGAAACAGCATCCATTGTCAAATTTTTG) with LipofectamineTM2000 transfection reagent for 48 h.
RT-qPCR Analysis
Pancreatic cancer cells and LPS-induced HPDE cells were taken and TRIzol lysate was added to the cells. The extracted RNA was dissolved in diethyl pyrocarbonate (DEPC) water. cDNA was synthesized using the Transcriptor First Strand cDNA Synthesis Kit, following the standard procedure of the kit. Then, TFF2 was determined with SYBR Green Realtime PCR kit, and the internal reference was GAPDH. At the end of the experiment, Ct values of each reaction were recorded and TFF2 level was calculated with 2−ΔΔCt method with GAPDH as reference.
Western Blot Analysis
Cells were washed with PBS for 3 times, and lysates containing RIPA were added to the cells for 20 min on ice, which was centrifuged at 4°C. After the protein was quantified by BCA method, the proteins were mixed with the sample buffer and boiled for 5 min. Each lane was loaded with 50 μg proteins which were separated by 10% SDS-PAGE. The proteins were transferred to PVDF membrane at a fixed voltage of 100 V for 2 h. Then, membrane was sealed in 5% skim milk powder for 1 h and incubated with β-catenin, c-Myc, Cyclin D1 and BIRC5 at 4°C overnight. After washing with buffer, membrane was then incubated with horseradish peroxidase-conjugated antibody at 25°C for 1 h. In the end, protein bands were observed by the enhanced chemiluminescence system (Bio-Rad Clarity Western ECL, USA).
CCK-8 Assay
The cell density was adjusted to 5×105 cells/mL, 24 h after cell transfection. 100 μL cells were inoculated into 96-well plates and cultured for 24 h. 10 μL CCK-8 solution was added to the cells at 0 h, 24 h, 48 h and 72 h for another incubation for 4 h. The absorbance value of each well was measured at the wavelength of 450 nm with the enzyme marker.
TUNEL Assay
The cells growing in the cultured pores of the glass tablet were fixed with 2% methylal for 30 min. TUNEL staining was performed according to the instructions of the TUNEL kit. Finally, FITC-labeled apoptotic cells in green fluorescence were observed under an inverted fluorescence microscope with an excitation wavelength of 450 nm and emission wavelength of 550 nm.
ELISA Assay
Cells were cultured after indicated treatment for 24 h and cell supernatant was collected. The levels of TNF-α, IL-6 and IL-1β in cell supernatant were respectively detected by TNF-α ELISA kit, IL-6 ELISA kit and IL-1β ELISA kit (Beyotime, Shanghai, China).
Co-Immunoprecipitation Assay
Pancreatic cancer cells were lysed with 1mL RIPA buffer containing 50 μL PMSF on ice, which was centrifuged at 12,000 r/min for 10 min at 4°C to obtain the supernatant. 1 μg β-catenin antibody and 1 μg TFF2 antibody were respectively added to the supernatant, which were incubated and shaken slowly at 4°C overnight. Agarose beads were added to above solution to be incubated and shaken slowly at 4°C for 4 h. After immunoprecipitation reaction, agarose beads were centrifuged to the tube bottom at 12,000 r/min for 10 min at 4°C. Supernatant was carefully removed and agarose beads were rinsed with 1 mL lysis buffer for three times, followed by adding with 15~20 μL 1×SDS loading buffer to be boiled for 5 min. The above samples were subjected to 10% SDS-PAGE and Western blot analysis.
Statistical Analysis
SPSS 23.0 statistical software was used for statistical analysis and the form of mean ± SD was used to represent the experimental data. One-way analysis of variance was used for the comparison of mean values between groups. P<0.05 was considered statistically significant.
Results
TFF2 Expression in Pancreatic Cancer Cells and LPS-Induced Normal Pancreatic Duct Cells
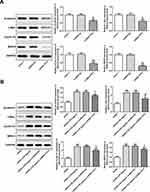
The protein expression and mRNA expression of TFF2 in SW1990, PanC-1, T3M4 and CFPAC cells were elevated compared with HPDE cells, and TFF2 expression in PanC-1 cells was the highest, which was why PanC-1 cells were selected for the subsequent experiments (Figure 1A). The protein expression and mRNA expression of TFF2 in LPS-induced HPDE cells were increased compared with HPDE cells. With the increasing concentration of LPS, the mRNA expression of TFF2 in LPS-induced HPDE cells was gradually increased. The protein expression of TFF2 in LPS-induced HPDE cells was gradually increased when LPS concentration was changed from 0.1 μg/mL to 1 μg/mL, and it did not further increase when LPS concentration was changed from 1 μg/mL to 5 μg/mL (Figure 1B). The concentration of LPS at 5 μg/mL was chosen for the next experiments.
Transfection Effects are Verified
After PanC-1 cells were transfected with ShRNA NC, ShRNA-TFF2-#1 and ShRNA-TFF2-#2, TFF2 expression was declined in PanC-1 cells transfected with ShRNA-TFF2-#1 and ShRNA-TFF2-#2 (Figure 2A). The LPS-induced HPDE cells were transfected with ShRNA NC, ShRNA-TFF2-#1 and ShRNA-TFF2-#2, and TFF2 expression was also declined in LPS-induced HPDE cells transfected with ShRNA-TFF2-#1 and ShRNA-TFF2-#2 (Figure 2B). TFF2 expression was the lowest in PanC-1 cells and LPS-induced HPDE cells, which were transfected with ShRNA-TFF2-#1.
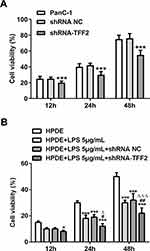
TFF2 Interference Inhibits Proliferation of Pancreatic Cancer Cells and LPS-Induced Normal Pancreatic Duct Cells
The proliferation of PanC-1 cells transfected with ShRNA-TFF2 was decreased at 12 h, 24 h and 48 h compared with PanC-1 group and ShRNA NC group (Figure 3A). As shown in Figure 3B, TFF2 interference inhibited proliferation of LPS-induced HPDE cells compared with that of LPS-induced HPDE cells and LPS-induced HPDE cells transfected with ShRNA NC at 24 h and 48 h. The proliferation of LPS-induced HPDE cells transfected with ShRNA-TFF2 was suppressed compared with that of HPDE cells.
TFF2 Interference Promotes Apoptosis of Pancreatic Cancer Cells and LPS-Induced Normal Pancreatic Duct Cells
As shown in Figure 4A, the apoptosis of PanC-1 cells transfected with ShRNA-TFF2 was increased compared with that of PanC-1 cells and PanC-1 cells transfected with ShRNA NC. After LPS induction, the apoptosis of LPS-induced HPDE cells was increased compared with that of HPDE cells. After LPS-induced HPDE cells were transfected with ShRNA-TFF2, the apoptosis of LPS-induced HPDE cells was further increased compared with that of LPS-induced HPDE cells and LPS-induced HPDE cells transfected with ShRNA NC (Figure 4B).
Effect of TFF2 Interference on Inflammation in Pancreatic Cancer Cells and LPS-Induced Normal Pancreatic Duct Cells
PanC-1 cells were transfected with ShRNA NC and ShRNA-TFF2. The levels of TNF-α, IL-1β and IL-6 in PanC-1 cells transfected with ShRNA-TFF2 were not statistically changed compared with that in PanC-1 cells and PanC-1 cells transfected with ShRNA NC (Figure 5A). After LPS induction, the levels of TNF-α, IL-1β and IL-6 in LPS-induced HPDE cells and LPS-induced HPDE cells transfected with ShRNA NC were obviously increased. And TFF2 interference did not obviously change the levels of TNF-α, IL-1β and IL-6 in LPS-induced HPDE cells (Figure 5B).
TFF2 Interference Suppresses β-Catenin Pathway
The expression of β-catenin, c-Myc, Cyclin D1 and BIRC5 in PanC-1 cells transfected with ShRNA-TFF2 was declined compared with that in PanC-1 cells and PanC-1 cells transfected with ShRNA NC (Figure 6A). The expression of β-catenin, c-Myc, Cyclin D1 and BIRC5 in LPS-induced HPDE cells and LPS-induced HPDE cells transfected with ShRNA NC was elevated compared with HPDE cells and TFF2 interference obviously down-regulated the expression of β-catenin, c-Myc, Cyclin D1 and BIRC5 in LPS-induced HPDE cells (Figure 6B).
TFF2 Can Combine with β-Catenin
When β-catenin antibody was added to lysates of PanC-1 cells, protein expression of β-catenin and TFF2 was detected in lysates of PanC-1 cells. The protein expression of β-catenin and TFF2 was also detected in lysates of PanC-1 cells added with TFF2 antibody (Figure 7).
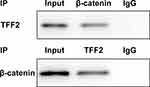 |
Figure 7 TFF2 can combine with β-catenin. (n=3). |
Discussion
Inflammation can cause DNA damage and chromosome instability and mutation of cancer-promoting genes, and tumor suppressor gene is the initial reaction to induce tumor. Persistent CP is highly correlated with KRAS mutations, which may induce uncontrolled proliferation of pancreatic cells that gradually advanced from chronic inflammatory cells to invasive cancer cells.3 Studies have shown that chronic inflammation increases the incidence of tumors, especially in pancreatic and gastrointestinal tumors. Chronic inflammation of the pancreas can release cytokines that affect the pancreas, exposing the pancreas to inflammatory microenvironment for a long time, ultimately increasing the risk of pancreatic cancer about 20 times higher than that of the normal population.16 Therefore, it is important to find early markers of PC to prevent the pancreatitis-cancer transformation.
Recent studies have shown that chronic active gastritis caused by HP can develop into cancer through chronic atrophic gastritis and intestinal metaplasia.17–19 Wang et al found that in gastric fundic mucosa infected with H. felis, the TFF2 was expressed in mucous neck cells.20 In addition, TFF2 expression was enhanced with the severity of atrophy of mucous membrane.21 A large number of research findings have showed that the occurrence of pancreatitis has a certain relationship with HP infection. HP infection can produce some urease that is hydrolyzed to produce ammonia, CO2 and water, causing certain damage to gastric mucous membrane and affecting the normal physiological function of the pancreas. The mechanism of TFF2 leading to pancreatic cancer has been partially identified.6,7 The role of TFF2 in LPS-induced pancreatic ductal epithelium and pancreatic cancer cells was explored in this study. In this study, we found that TFF2 expression was also increased in LPS-induced HPDE cells and PanC-1 cells. Furthermore, TFF2 interference could suppress the proliferation and promote the apoptosis of LPS-induced HPDE cells and PanC-1 cells.
Activation of Wnt/β-catenin pathway is closely related to the occurrence and development of pancreatic cancer and other malignant tumors.22,23 Previous study has confirmed that the abnormal activation of Wnt/β-catenin signaling pathway will lead to the degradation of extracellular matrix and the uncontrolled proliferation and differentiation of cells, leading to the development of pancreatic cancer.24 Lu et al demonstrated that inhibition of Wnt/β-catenin signaling pathway significantly inhibited the metastasis and proliferation of pancreatic cancer cells.25 In this study, we found that TFF2 interference inhibited the β-catenin expression, thereby inhibiting the expression of downstream effector proteins of β-catenin.
In conclusion, TFF2 expression was increased in LPS-induced HPDE cells and PanC-1 cells. TFF2 interference suppressed the proliferation and promoted the apoptosis of LPS-induced HPDE cells and PanC-1 cells by inhibiting β-catenin pathway. However, this study has some limitations, and several cancer cell lines and even animal models will be used to further study this topic.
Funding
There is no funding to report.
Disclosure
The authors declare they have no competing interests.
References
1. Zhang L, Sanagapalli S, Stoita A. Challenges in diagnosis of pancreatic cancer. World J Gastroenterol. 2018;24(19):4–17. doi:10.3748/wjg.v24.i19.2047
2. Liu M, Ji S, Xu X, Yu X. Advances in basic research, clinical diagnosis and treatment of pancreatic cancer in 2019. Chin Oncol. 2020;30(01):1–10.
3. Dai JJ, Jiang MJ, Wang XP, Tian L. Inflammation-related pancreatic carcinogenesis: mechanisms and clinical potentials in advances. Pancreas. 2017;46(8):973–985. doi:10.1097/MPA.0000000000000886
4. Jiang MJ, Dai JJ, Gu DN, Huang Q, Tian L. Aspirin in pancreatic cancer: chemopreventive effects and therapeutic potentials. Biochim Biophys Acta Rev Cancer. 2016;1866(2):163–176. doi:10.1016/j.bbcan.2016.08.002
5. Andoh A, Kinoshita K, Rosenberg IM, Podolsky DK. Intestinal trefoil factor induces decay-accelerating factor expression and enhances the protective activities against complement injury in intestinal epithelial cells. Gastroenterology. 2001;120(5):A182–A182. doi:10.1016/S0016-5085(01)80900-8
6. Sung GH, Chang H, Lee JY, et al. Pancreatic-cancer-cell-derived trefoil factor 2 impairs maturation and migration of human monocyte-derived dendritic cells in vitro. Animal Cells Syst (Seoul). 2018;22(6):368–381. doi:10.1080/19768354.2018.1527721
7. Dubeykovskaya Z, Dubeykovskiy A, Solal-Cohen J, et al. Secreted trefoil factor 2 activates the CXCR4 receptor in epithelial and lymphocytic cancer cell lines. J Biol Chem. 2009;284(6):3650–3662. doi:10.1074/jbc.M804935200
8. Zhou C, Li J, Qian W, et al. Huaier extract restrains pancreatic cancer by suppressing Wnt/β-catenin pathway. Biomed Pharmacother. 2020;127:110126. doi:10.1016/j.biopha.2020.110126
9. Zhang X, Xu J, Yan R, et al. FAM84B, amplified in pancreatic ductal adenocarcinoma, promotes tumorigenesis through the Wnt/β-catenin pathway. Aging. 2020;12(8):6808–6822. doi:10.18632/aging.103044
10. Zhou Y, Hua Z, Zhu Y, et al. Upregulation of ARHGAP30 attenuates pancreatic cancer progression by inactivating the β-catenin pathway. Cancer Cell Int. 2020;20(1):225. doi:10.1186/s12935-020-01288-7
11. Alvarez MC, Fernandes J, Michel V, et al. Effect of helicobacter pylori infection on GATA-5 and TFF1 regulation, comparison between pediatric and adult patients. Dig Dis Sci. 2018;63(11):2889–2897. doi:10.1007/s10620-018-5223-0
12. Hu GY, Yu BP, Dong WG, et al. Expression of TFF2 and helicobacter pylori infection in carcinogenesis of gastric mucosa. World J Gastroenterol. 2003;9(5):910–914. doi:10.3748/wjg.v9.i5.910
13. Johns CE, Newton JL, Westley BR, et al. The diurnal rhythm of the cytoprotective human trefoil protein TFF2 is reduced by factors associated with gastric mucosal damage: ageing, helicobacter pylori infection, and sleep deprivation. Am J Gastroenterol. 2005;100(7):1491–1497. doi:10.1111/j.1572-0241.2005.41859.x
14. Xiao M, Wang Y, Gao Y, et al. Association between helicobacter pylori infection and pancreatic cancer development: a meta-analysis. PLoS One. 2013;8(9):e75559.
15. Ai F, Hua X, Liu Y, et al. Preliminary study of pancreatic cancer associated with helicobacter pylori infection. Cell Biochem Biophys. 2015;71(1):397–400. doi:10.1007/s12013-014-0211-2
16. Liu ST, Pham H, Pandol SJ, Ptasznik A. Src as the link between inflammation and cancer. Front Physiol. 2014;4:416. doi:10.3389/fphys.2013.00416
17. Guarner J, Herrera-Goepfert R, Mohar A, et al. Gastric atrophy and extent of intestinal metaplasia in a cohort of helicobacter pylori–infected patients. Hum Pathol. 2001;32(1):31–35. doi:10.1053/hupa.2001.20889
18. El‐Zimaity HM, Ota H, Graham DY, et al. Patterns of gastric atrophy in intestinal type gastric carcinoma. Cancer. 2002;94(5):1428–1436. doi:10.1002/cncr.10375
19. Hatakeyama M. Helicobacter pylori and gastric carcinogenesis. J Gastroenterol. 2009;44(4):239–248. doi:10.1007/s00535-009-0014-1
20. Wang TC, Goldenring JR, Dangler C, et al. Mice lacking secretory phospholipase A2 show altered apoptosis and differentiation with helicobacter felis infection. Gastroenterology. 1998;114(4):675–689. doi:10.1016/S0016-5085(98)70581-5
21. Fang Z. Expression of TFF2 in Fundic Gland of Gastric Carcinoma and Peptic Ulcer. Yan’an University; 2006.
22. Shiah SG, Shieh YS, Chang JY. The role of Wnt signaling in squamous cell carcinoma. J Dent Res. 2016;95(2):129–134. doi:10.1177/0022034515613507
23. Zhu S, Wang Z, Wang J, et al. Aberrant expression of CXCR4 and β-catenin in pancreatic cancer. Anticancer Res. 2013;33(9):4103–4110.
24. Clevers H, Nusse R. Wnt/β-Catenin signaling and disease. Cell.2012;149(6):1192–1205. doi:10.1016/j.cell.2012.05.012
25. Liu L, Zhi Q, Shen M, et al. FH535, a β-catenin pathway inhibitor, represses pancreatic cancer xenograft growth and angiogenesis. Oncotarget. 2016;7(30):47145–47162. doi:10.18632/oncotarget.9975
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.