Back to Journals » Therapeutics and Clinical Risk Management » Volume 15
Treatment with Saccharomyces boulardii and Escherichia coli Nissle is safe and associated with reduced nosocomial transmission of vanB vancomycin-resistant Enterococcus faecium on an early rehabilitation ward in Germany: a retrospective analysis
Authors Borgmann S, Rieß B, Siegmund R, Werner G, Klare I
Received 4 August 2018
Accepted for publication 23 December 2018
Published 27 February 2019 Volume 2019:15 Pages 343—354
DOI https://doi.org/10.2147/TCRM.S179208
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Professor Garry Walsh
Stefan Borgmann,1 Beate Rieß,1 Rabea Siegmund,1 Guido Werner,2 Ingo Klare2
1Department of Infectious Diseases and Infection Control, Ingolstadt Hospital, Ingolstadt, Germany; 2National Reference Centre for Staphylococci and Enterococci, Robert Koch Institute, Wernigerode Branch, Wernigerode, Germany
Purpose: According to the WHO vancomycin-resistant Enterococcus faecium (VRE) belongs to the microorganisms for which new antibiotics are urgently needed. Depending on the type of vancomycin resistance vanA gene VRE is differentiated from vanB VRE and other types. In this retrospective analysis the results of VRE surveillance performed at a German tertiary hospital with approximately 1,200 beds between 2013 and 2017 are shown.
Patients and methods: Rectal screening swabs were taken at admission and once per week on the early rehabilitation ward of Ingolstadt Hospital (ERWIN) but not at other wards. The number of VRE colonized patients was evaluated by using appropriate computer software (LabCentre, Hybase). The Hybase program was also used to find out the number of Saccharomyces boulardii and multi-susceptible Escherichia coli Nissle in blood cultures of patients at ERWIN. The mechanism of vancomycin resistance was examined by PCR and clonality of VRE strains was analyzed by pulsed-field gel electrophoresis.
Results: Between 2013 and 2015 the number of VRE increased from 30 to 78 per year whereas in 2016 and 2017 the number declined to 51. Systematic analysis of the laboratory data revealed that this increase was driven by oligoclonal transmission of vanB VRE on ERWIN until August 2016 despite performing intensified infection control measures. However, afterward the number of VRE decreased at ERWIN and subsequently at the other wards. While searching for the reason behind this beneficial development we noticed that at ERWIN, patients treated with antibiotics received two probiotic medications simultaneously (S. boulardii, E. coli Nissle) for the duration of the antibiotic therapy plus an additional 2 days. There was no indication of side effects caused by these microorganisms, particularly no infections.
Conclusion: Application of S. boulardii and E. coli Nissle was safe and associated with reduced transmission of VRE from patient to patient at ERWIN. Therefore, in our setting, probiotic treatment of patients receiving antibiotics contributed to the increase of patients’ safety.
Keywords: microbiota, oligoclonal spread, outbreak, probiotics, vanB, bacterial spread, circuit model of bacterial transmission, vanA
Introduction
Probiotics are live microorganisms that render beneficial effects once consumed. While in obese individuals probiotics seem favorable for losing weight, their usage was controversially discussed for the treatment of various diseases, including inflammatory bowel diseases, Clostridium difficile infections, and allergic diseases.1–5 Apart from mediating beneficial effects, probiotics themselves should not cause disadvantageous effects, in particular not infections. A multitude of microorganisms have been used, among them lactobacilli, bifidobacteria, Streptococcus (ie, Streptococcus thermophiles), Escherichia coli Nissle, and Saccharomyces boulardii.2,6–9
S. boulardii exhibits anti-inflammatory capacities and inhibits binding of pathogenic bacteria to the epithelium.10 Similar effects were described following application of E. coli Nissle.9 In clinical settings S. boulardii reduced the development of antibiotic-associated diarrhea including diarrhea caused by C. difficile.11
In 2018 the WHO updated the list indexing bacterial species for which new antibiotics are urgently needed, among them vancomycin-resistant Enterococcus faecium (VRE).12 Within the past years nosocomial spread of VRE has occurred with increasing frequency.13,14 Fortunately, the bacteria often causes colonization but not infections.15 However, infections in severely ill patients who frequently receive antibiotic treatment have become more common, contributing to a rise in fatal outcomes and increased hospital costs.14,16–18
Eight genes mediating vancomycin resistance have been described of which vanA and vanB are relevant in clinical practice. In laboratory tests vanA VRE is resistant to vancomycin and teicoplanin while vanB VRE is susceptible to teicoplanin. Apart from glycopeptide resistance, VRE often contains various pathogenicity factors, eg, the enterococcal surface protein and hyaluronidase encoded by the genes esp and hyl. The esp gene resides on an E. faecium pathogenicity island as part of the chromosome in hospital-associated strain types, whereas the so-called hylEfm gene is part of another set of mobile genetic elements mainly residing on an E. faecium megaplasmid.19,20
In the present study, we report the results of the surveillance of multi-resistant bacteria in a tertiary care hospital in Germany. This observational study focused on the epidemiology of VRE in an early rehabilitation ward.
Patients and methods
Ingolstadt Hospital is a tertiary care hospital with approximately 1,200 beds located in the center of Bavaria (South-East Germany). The hospital contains an early rehabilitation ward. Characteristics of the ward have been described earlier in detail.21,22 In brief, the early rehabilitation ward of Ingolstadt Hospital (ERWIN) has 22 beds. Patients predominantly suffer from neurological deficits (mostly stroke) and trauma (at least two injuries). Due to an outbreak of carbapenem-resistant Klebsiella pneumoniae (CKP) in the winter of 2015, ERWIN was closed for several weeks.21 After intensively cleaning and disinfecting the ward, ERWIN was reopened in week 8 of 2015 (February 2015).
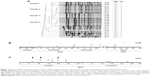
A time line showing the measures most likely influencing VRE incidence is shown in Figure 1.
Between May 2014 and November 2016 rectal swabs were taken from each patient treated at ERWIN at admission and once per week to identify colonization with VRE. The first VRE isolate of each patient was systematically sent to the German Reference Centre for Staphylococci and Enterococci at the Robert Koch Institute (RKI).
In the hospital, patients colonized/infected with VRE either resided in a single room or underwent cohort isolation with a sex-matched VRE colonized room-mate. Staff and visitors in contact with VRE colonized patients wore gloves and coats and were instructed to consistently maintain basic hygiene measures, primarily hand disinfection. However, exceptions occurred when a colonized patient suffered extremely from isolation and consequently standard infection control measures were performed. Before the CKP outbreak these exceptions were also allowed at ERWIN but not after reopening the ward in February 2015.
Starting in September 2015 patients at ERWIN received two probiotic drugs while receiving antibiotics. Probiotics were S. boulardii 375 mg/hard capsule, (Eubiol; CNP-Pharma GmbH, Fürstenzell, Germany) and E. coli Nissle 2.5–25×109 bacteria/capsule (Mutaflor; Ardeypharm GmbH, Herdecke, Germany). S. boulardii was given once a day (1-0-0) and E. coli Nissle twice a day (1-0-1). Probiotics were applied during antibiotic treatment and for and additional 2 days. Probiotics were not systematically applied to patients on the other wards in the hospital.
The laboratory in Ingolstadt Hospital performed diagnostic microbiology as described earlier.21,23 In brief, species’ identification and antibiotic susceptibility were examined with the Vitek 2 compact (BioMerieux, Nürtingen, Germany). VRE from screening swabs was cultured on a selective agar plate (Brilliance VRE agar; Oxoid, Wesel, Germany). Vancomycin resistance was confirmed by Etest analysis (Biomerieux). Minimal inhibitory concentration ≤4 mg/L for vancomycin was considered as susceptible. Proof of VRE was communicated by the laboratory staff to the physicians treating the patients and also to the Department of Infectious Diseases and Infection Control. The results of microbiological analyses were sent to the computer program Hybase (EpiNetAG, Bochum, Germany). This program allows the identification of patients colonized/infected with a certain bacterium. In our setting E. faecium and E. faecium VRE were encoded as two different species. This allowed easy access and fast identification of VRE colonized patients and the date of bacterial isolation in a certain period, eg, 2013–2017 by using the Hybase program. Negative screening results of VRE colonized patients were obtained with the laboratory software LabCentre l.i.c. (i-SOLUTION Health GmbH, Mannheim, Germany). Number of patient days were received from the computer program industry solutions health care module (SAP/IS-H; Siemens, Munich, Germany), adapted to the requirements of the hospital.
The standard analysis portfolio at the RKI contained an extended antibiotic susceptibility testing of 18 substances by broth microdilution and using European Committee on Antimicrobial Susceptibility Testing recommendations and criteria (EUCAST clinical breakpoints version 7.0; in case of missing breakpoints the corresponding epidemiological cut-off value was used for a classification susceptible [S]-intermediate [I]-resistant [R]). Genomic DNA was isolated using a DNeasy Blood and Tissue Kit (Qiagen Hilden, Germany) and markers vanA and vanB as well as esp and hyl were amplified in two separate multiplex PCRs as described elsewhere.20,24
For pulsed-field gel electrophoresis (PFGE), bacterial cells were fixed in agarose plugs and DNA was extracted using a standard protocol.24 SmaI-digested genomic DNA was resolved in an agarose gel in an alternating electric field provided in a CHEF III PFGE apparatus (Bio-Rad Laboratories Inc., Hercules, CA, USA). Band patterns were analyzed using BioNumerics version 7.6 (Applied Maths, Sint-Martens-Latem, Belgium) with the following settings: optimization 0.5 and position tolerance 1.0.
Data about hand disinfection practices were obtained from the purchasing department of the hospital. When a bottle of hand disinfectant was empty or had reached the date of expiration the cleaning assistants replaced it with a new bottle. The cleaning assistants also assigned this replacement to a particular cost center. However, due to the fact that two wards form a spatial unit, these assignments were not very reliable. Therefore, hand disinfectant consumption was recorded for a unit consisting of two wards, respectively. Volume of consumed hand disinfectant was related to the number of patient days. Number of patient days on ERWIN and the neighboring palliative ward, as well as the number of patient days of all wards were obtained from the controlling department (Figure S1).
Ethics statement
The study does not contain clinical studies or patient data allowing identification of patients or individual clinical course. According to the ethics committee of the Bavarian Medical Association (Bayerische Landesärztekammer), which has jurisdiction over medical matters in our state (Federal State Bavaria), ethics approval was not necessary for this analysis. This study was conducted in accordance with the Declaration of Helsinki.
Results
In order to perform surveillance of multi-resistant bacteria in our hospital, the number of VRE colonized patients treated between 2013 and 2017 were retrospectively examined. As shown by Figure 2, in 2013, 23 patients had been colonized with VRE. In 2015 this number increased to 78 while in 2016 and 2017 it declined to 51, respectively. From 2013–2014 at ERWIN the number of VRE colonized patients increased from four to 27 and remained high in 2015 (N=29) and 2016 (N=26). As shown by Table 1, incidence at ERWIN increased up to 32 times higher than that of the other wards. As the VRE burden of ERWIN appeared similar between 2014 and 2016, it seems that the situation at ERWIN had not changed within this period.
However, this impression changed when the number of VRE colonized patients identified within a certain month and also the genotype of glycopeptide resistance were considered (Figure 3). VRE exhibiting vanA-encoded resistance was only sporadically isolated between 2013 and 2016. However, the number of vanA VRE markedly increased within the last quarter of 2016, especially due to the fact that in October and November 2016 an outbreak occurred at ERWIN (Figure 3A), supposedly affecting eleven patients. Results of PFGE analysis showed that six of the eleven isolates were not identical to other isolates, as the corresponding banding patterns were unique (Figure 4). Two of the five vanA VRE exhibiting an identical banding pattern (cluster 2) had been isolated at patient’s admission to ERWIN indicating that both patients had acquired the bacteria earlier. Therefore, at most, three of the eleven vanA VRE could have been acquired at ERWIN. By performing intensified infection control measures, there was no further vanA VRE cases in December 2016.
In contrast to vanA VRE, vanB VRE was rarely found in the hospital before December 2013 (Figure 3B). However, afterward, the burden of vanB VRE increased, and ERWIN was overproportionately affected: from 2014–2016 50% of all vanB VRE isolated in our hospital had been isolated at ERWIN (60/120) and this percentage rose to 76% (22 of 29) within the period from May–August 2015. To normalize VRE burden, the number of VRE colonized patients was referred to the number of patient days. As shown by Table 1, VRE burden at ERWIN was about tenfold higher in comparison to other wards. The tracking of the screening results showed that nearly all vanB VRE colonized patients treated at ERWIN had acquired VRE colonization in that ward (Figure S2). To examine whether vanB VRE was clonal, PFGE of vanB VRE isolates was performed. In the winter of 2015, the ward was closed because of an outbreak of CKP. Isolates obtained within the weeks before and after the closure were analyzed. As shown by Figure 4, only one of the eleven vanB VRE exhibited a unique banding pattern. The other isolates belonged to three distinct clusters (clusters 1, 3, and 4) suggesting oligoclonal spread of vanB VRE. VRE belonging to clusters 1 and 4 was isolated before and after the closure of ERWIN, indicating presence of an unidentified reservoir that persisted, despite extended hygiene measures during closure and markedly increased usage of hand disinfectant from 2014–2016 (Figure S1). Two of the cluster 3 VRE colonized patients also exhibited CKP, suggesting an association of VRE cluster 3 and CKP transmission.
After August 2015 the number of vanB VRE colonized patients decreased, and in the second half of 2016, as well as in 2017, VRE colonized patients were not identified at ERWIN. As there were no apparent changes in infection control measures, neither at the hospital nor at ERWIN, the responsible consultant was asked if treatment procedures had been changed. Interestingly, since September 2015 each patient at ERWIN had received two probiotic preparations (E. coli Nissle, S. boulardii) when being treated with antibiotics. Probiotics had been applied for the duration of antibiotic treatment and for an additional 2 days. This measure purportedly prevented transmission of vanB VRE. Surprisingly, together with the decreased number of vanB VRE at ERWIN, this decrease was also observed on the other wards of the hospital (Figure S3).
esp was prevalent in more than 90% of VRE. By contrast, presence of hyl gene was lower. Amounts of 76.2% of vanA and 48.3% of vanB VRE isolated from patients at ERWIN showed hyl gene as did 66.2% of vanA and 59.7% of vanB VRE from patients on other wards (Figure S4). As shown by Figure 4, most of the isolates examined by PFGE exhibited genes of both pathogenicity factors.
Between 2013 and 2017 VRE was isolated from blood cultures of ten patients. Four isolates were vanA and six were vanB VRE. One of these vanB VRE patients was treated at ERWIN. At ERWIN, eight VRE were isolated from urine and a further VRE from ascites. Therefore, the vast majority of VRE isolations from patients at ERWIN were colonization (Figure S5).
From the patients treated with probiotics, S. boulardii was isolated only in stool samples or in swabs taken from the throat and notably not in blood cultures. Between 2013 and 2017 seven blood cultures were found to be positive for E. coli. Four cases occurred within the “probiotic period” from September 2015–December 2017 (28 months, 1.74 cases/12 months) and three cases within the preceding observation period from January 2013–August 2015 (32 months, 1.12 cases/12 months) (Figure S6, left panel). Analysis of patients’ documents revealed that one of these patients had not received probiotics before (Figure S6, right panel). Apart from E. coli in blood culture, two patients concurrently exhibited E. coli in urine (>100,000/μL) suggesting urogenital infection as the cause of blood-stream infection and a patient had suffered from perforation of the gall bladder before developing blood-stream infection with E. coli. In the laboratory, practice does not allow multi-susceptible bacteria isolated from blood cultures to be stored. Therefore, it is not possible from a retrospective standpoint to exclude E. coli Nissle as the cause of blood-stream infections in three patients. However, as demonstrated previously, this seems very unlikely.
Discussion
In this retrospective monocentric analysis we observed that the application of two probiotics in patients treated with antibiotics was associated with a lower spread of vanB VRE at an early rehabilitation ward. On that ward patients underwent VRE screening at admission and once per week from May 2014–November 2016. To examine whether patients had been colonized with VRE, rectal swabs were taken from each patient and processed at the microbiological laboratory of the hospital. In a US study sensitivity of positive rectal swabs depended on the concentration of VRE in feces. As some of the patients analyzed in that study exhibited comparably low concentrations, sensitivity of rectal swabs was low to identify VRE colonization.25 Therefore, we cannot exclude the possibility that some colonized patients were not detected. However, an aim of infection control measures is to prevent infections caused by multi-drug resistant bacteria. In the present study, VRE was isolated from clinical samples (urine, blood culture) only when a high number of rectal swabs were present (Figure S5). Furthermore, each patient with VRE in urine and blood culture exhibited VRE in corresponding rectal swabs. Therefore, in our analysis, rectal swabs were sufficient in identifying clinically relevant colonization. A similar observation had been reported earlier.26 Analysis of the screening results showed that nearly all of the colonized patients had acquired vanB VRE while residing at ERWIN. After ERWIN had been closed due to an outbreak of CKP in January 2015, all VRE colonized patients were consequently isolated without exceptions to prevent further spread of VRE. However, transmission of VRE continued, resulting in acquisition of VRE by up to six patients per month. Due to the preceding CKP outbreak in January 2015, infection control measures were consistently performed and the staff was very motivated to avoid mistakes. As these efforts failed, the consultant at ERWIN started application of S. boulardii and E. coli Nissle for all patients treated with antibiotics. Subsequently, the number of vanB VRE colonized patients continuously decreased in the following months and from May 2016 on there were no patients colonized with vanB VRE. Most likely, the application of probiotics successfully contributed to limiting the spread of vanB VRE, while intensified isolation of VRE colonized patients proved insufficient.
In October 2016 an outbreak of vanA VRE occurred. We assumed that due to limitation of vanB VRE spread, the staff did not perform basic hygiene measures as intensely as before. In total, eleven vanA VRE colonized patients were identified in autumn 2016 suggesting acquisition of the bacteria by ten patients. However, upon reviewing screening results, as well as PFGE analysis, it was revealed that at most, three patients acquired the bacteria at ERWIN. Nevertheless, this scenario showed that application of probiotics does not substitute the need for basic infection control measures. In contrast to long-lasting spread of vanB VRE within the non-probiotic period, spread of vanA VRE was rapidly limited indicating that application of probiotics was supportive for limiting the outbreak.
Surprisingly, a decreased number of vanB VRE at ERWIN in 2016 was accompanied by a decreased number of vanB VRE on the other wards. In the hospital, patients are usually moved from other wards to ERWIN but not the other way around. While the nursing staff is assigned exclusively to ERWIN, speech and physiotherapists visiting ERWIN are working on several wards. On the other hand, it is not conclusive that the decreased transmission of VRE via therapists was a consequence of probiotic treatment of patients. Therefore, we have no mechanistic model to explain this parallel development. A solution for this situation may be to regard VRE transmission not as a single event caused by another event, but as a current which depends on the voltage of the electric circuit. In such a model a high number of VRE colonized patients would represent the force (voltage) driving transmission of VRE to another patient. Consequently, a high number of VRE will lead to an increased probability of VRE acquisition. Apart from the driving force, the number of transmissions will also depend on the resistor. In our case, a parallel circuit comprising of two resistors seems to be present, with ERWIN being resistor 1 and the other wards resistor 2. Upon increasing the resistance of one resistor, the current in the whole circuit decreases as observed in the present scenario. Although the resistance of the second resistor will remain unchanged, the current flowing through resistor 2 will subsequently decrease because the voltage of the circuit has been lowered due to a lower number of colonized patients. On the other hand, the number of VRE has increased in Germany over the past several years, most likely leading to a higher number of VRE colonized patients admitted at German hospitals.16,27 This general increase explains why the number of vanA and vanB VRE colonized patients increased in 2017 on the other wards.
Two of the three vanB VRE strains isolated at the ward in autumn 2014 were present after the reopening in February 2015. When considering VRE transmission as a result of a certain infection control failure this finding can only be explained by the assumption that unidentified members of the staff had been a VRE reservoir. However, in this case there would be no explanation as to why consumption of probiotics stopped VRE transmission, despite the reservoir still being present on the ward. In contrast, according to the electric circuit model of VRE transmission, a reservoir somewhere in the hospital will drive VRE transmission at ERWIN as long as the resistor at the ward remains unchanged.
There are two main reasons why multi-resistant bacteria often spread on early rehabilitation wards. First, in comparison to other hospitalized patients outside intensive care and the haemato-oncologic wards, patients at ERWIN receive more (broad spectrum) antibiotics. Patients at ERWIN mostly suffer from stroke, often regurgitating food, especially when fed by their relatives with non-appropriate nourishment. Subsequently, the patients develop pneumonia and are treated with antibiotics. According to this theory, antibiotic treatment was previously identified as the major risk factor for acquisition of multi-resistant bacteria.16,28 Second, patients at ERWIN have more contact with staff members, especially with physiotherapists and this contact is often more intensive than contact experienced by patients on other wards. However, physiotherapeutic treatment regime remained unchanged within the study period. Since the number of VRE transmissions decreased despite therapists continuing to work with the patients, it is not likely that spread of VRE predominantly resulted from this contact. Therefore, it is most likely that the application of probiotics prevented colonization with VRE by moderating the effect of antibiotic treatment on patients’ microbiota.
It is not clear whether the beneficial effect was exclusively mediated by the microorganisms or by other components of the preparations. Apart from E. coli Nissle, Mutaflor contains the laxative, macrogol, which might facilitate the action of the microorganisms. It is also not clear whether one of the probiotics mediated the beneficial effect alone or if both species worked together. There exists only one study in which E. coli Nissle was used to limit preexisting VRE colonization of mice. In that study E. coli Nissle failed to reduce the VRE density.29 In other studies lactobacilli had predominantly been used to clear VRE colonization. Although in some studies no beneficial effect was observed, in most studies application of lactobacilli at least reduced VRE burden, while in some examinations VRE was eradicated.30 Conflicting results were observed when applying lactobacilli to mice and humans. While Lactobacillus rhamnosus Lcr35 lowered VRE density in mice there was no effect in VRE colonized patients.29 Apart from lactobacilli Barnesiella sp. and Bacteroides thetaiotaomicron reduced the density of intestinal VRE in mice, raising the question whether anaerobic bacteria might also be effective probiotics.31,32 Interestingly, Bacteroides recovery after antibiotic treatment was facilitated by applying Lactobacillus paracasei CNCM I-3689 to VRE (E. faecalis) colonized mice.33
Various mechanisms that mediate probiotic benefits against VRE have been discussed.30 For instance, L. rhamnosus strain GG expresses pili competing with those of VRE for mucus binding sites.34 Probiotics were also shown to stimulate enteral immunity. From Lactobacillus plantarum WCFS1 derived extracellular vesicles protected Caenorhabditis elegans against VRE infection perhaps increasing the transcription rate of the host defense proteins cpr-1 and clec-60.35 In mice, L. paracasei CNCM I-3689 supplementation increased ileal expression of cathelicidin antimicrobial peptide.33
While in most of the analyses probiotics mediated beneficial effects, application of a probiotic multi-species cocktail coincided with an extended VRE outbreak happening between February 2012 and August 2013 at a Turkish neonatal intensive care unit.36 Recently, the Drug Commission of the German Medical Association (AKDA) published a Dear-doctor-letter restricting the usage of S. boulardii/Saccharomyces cerevisiae due to occurrence of severe infections caused by these microorganisms in critically ill patients.37
At ERWIN, probiotics were applied for more than 2 years in patients treated with antibiotics. During this time, no patient developed infection caused by S. boulardii. However, in blood cultures from four patients, multi-susceptible E. coli was isolated. Since molecular analyses comparing those isolates with probiotic E. coli Nissle were not performed, it remains speculative if two of these infections were actually caused by the probiotic E. coli.
In December 2016 VRE screening was terminated. The reason for the termination was that rehabilitation departments and nursing homes were refusing care for patients colonized with multi-resistant bacteria. Within the past years the authors contacted the Bavarian Medical Association and also the Bavarian health authorities to support treatment of VRE colonized patients in those facilities. However, neither authority believes it has the expertise for such action. Therefore, VRE screening was stopped to avoid systemic discrimination of VRE colonized patients.
Limitations
To start, the study is a retrospective analysis and was not performed under controlled conditions. We do not know with any certainty whether probiotics mediated adverse side effects. It seems improbable, however, that probiotic microorganisms themselves caused infections as described previously. On the other hand, we have no proof of that. Furthermore, all patients tolerated probiotic application well.
Conclusion
The results of our study show that application of probiotics to patients treated with antibiotics might be supportive to contain transmission of VRE from patient to patient. In our setting usage of both types of probiotics seemed to be safe.
Acknowledgment
The authors thank Carolyn BonDurant Wack for language editing. This study was not funded.
Disclosure
The authors report no conflicts of interest in this work.
References
John G, Wang L, Nanavati J, Twose C, Singh R, Mullin G. Dietary alteration of the gut microbiome and its impact on weight and fat mass: a systematic review and meta-analysis. Genes. 2018;9(3):167. | ||
Sivananthan K, Petersen AM. Review of Saccharomyces boulardii as a treatment option in IBD. Immunopharmacol Immunotoxicol. 2018;11:1–11. | ||
Miele E, Shamir R, Aloi M, et al. Nutrition in pediatric inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2018;66(4):687–708. | ||
Hedge DD, Strain JD, Farver DR. New advances in the treatment of Clostridium difficile infection (CDI). TCRM. 2008;4(5):949–964. | ||
Hendaus M, Jomha F, Ehlayel M. Allergic diseases among children: nutritional prevention and intervention. TCRM. 2016;12:361. | ||
Markowiak P, Śliżewska K. Effects of probiotics, prebiotics, and synbiotics on human health. Nutrients. 2017;9(9):1021. | ||
Vitetta L, Vitetta G, Hall S. Immunological tolerance and function: associations between intestinal bacteria, probiotics, prebiotics, and phages. Front Immunol. 2018;9:2240. | ||
Kandasamy S, Vlasova AN, Fischer DD, et al. Unraveling the differences between gram-positive and gram-negative probiotics in modulating protective immunity to enteric infections. Front Immunol. 2017;8(9):1262. | ||
Sonnenborn U, Robertson L. Escherichia coli strain Nissle 1917-from bench to bedside and back: history of a special Escherichia coli strain with probiotic properties. FEMS Microbiol Lett. 2016;363(19):fnw212. | ||
Stier H, Bischoff SC. Saccharomyces boulardii CNCM I-745 on the gut-associated immune system. 2016;9:269–279. | ||
McFarland LV. Meta-analysis of probiotics for the prevention of antibiotic associated diarrhea and the treatment of Clostridium difficile disease. Am J Gastroenterol. 2006;101(4):812–822. | ||
Tacconelli E, Carrara E, Savoldi A, et al. Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect Dis. 2018;18(3):318–327. | ||
O’Driscoll T, Crank CW. Vancomycin-resistant enterococcal infections: epidemiology, clinical manifestations, and optimal management. Infect Drug Resist. 2015;8:217–230. | ||
Ulrich N, Vonberg R-P, Gastmeier P. Outbreaks caused by vancomycin-resistant Enterococcus faecium in hematology and oncology departments: A systematic review. Heliyon. 2017;3(12):e00473. | ||
Borgmann S, Niklas DM, Klare I, et al. Two episodes of vancomycin-resistant Enterococcus faecium outbreaks caused by two genetically different clones in a newborn intensive care unit. Int J Hyg Environ Health. 2004;207(4):386–389. | ||
Remschmidt C, Behnke M, Kola A, et al. The effect of antibiotic use on prevalence of nosocomial vancomycin-resistant enterococci- an ecologic study. Antimicrob Resist Infect Control. 2017;6(1):95. | ||
Scheich S, Lindner S, Koenig R, et al. Clinical impact of colonization with multidrug-resistant organisms on outcome after allogeneic stem cell transplantation in patients with acute myeloid leukemia. Cancer. 2018;124(2):286–296. | ||
Puchter L, Chaberny IF, Schwab F, Vonberg RP, Bange FC, Ebadi E. Economic burden of nosocomial infections caused by vancomycin-resistant enterococci. Antimicrob Resist Infect Control. 2018;7(1):1. | ||
Top J, Sinnige JC, Majoor EA, Bonten MJ, Willems RJ, van Schaik W. The recombinase IntA is required for excision of esp-containing ICEEfm1 in Enterococcus faecium. J Bacteriol. 2011;193(4):1003–1006. | ||
Laverde Gomez JA, van Schaik W, Freitas AR, et al. A multiresistance megaplasmid pLG1 bearing a hylEfm genomic island in hospital Enterococcus faecium isolates. Int J Med Microbiol. 2011;301(2):165–175. | ||
Borgmann S, Pfeifer Y, Becker L, Rieß B, Siegmund R, Sagel U. Findings from an outbreak of carbapenem-resistant Klebsiella pneumoniae emphasize the role of antibiotic treatment for cross transmission. Infection. 2018;46(1):103–112. | ||
Borgmann S, Rieß B, von Wernitz-Keibel T, Matthias Buehler M, Strommenger B, Layer F. Recovery of a 10-year-old girl from methicillin-resistant Staphylococcus aureus sepsis in response to low-dose ceftaroline treatment. TCRM. 2016;12:749–753. | ||
Steger S, Demetz F, Schmidt C, Borgmann S. Low percentage of asylum seekers colonized with Multi-Resistant bacteria treated at a German hospital. Jacobs J Epidemiol Prev Med. 2016;2(1):21. | ||
Ryan L, O’Mahony E, Wrenn C, et al. Epidemiology and molecular typing of VRE bloodstream isolates in an Irish tertiary care hospital. J Antimicrob Chemother. 2015;70(10):2718–2724. | ||
D’Agata EM, Gautam S, Green WK, Tang YW, Tang Y. High rate of false-negative results of the rectal swab culture method in detection of gastrointestinal colonization with vancomycin-resistant enterococci. Clin Infect Dis. 2002;34(2):167–172. | ||
Brasg I, Elligsen M, MacFadden D, Daneman N. Predictive utility of swab screening for vancomycin-resistant enterococcus in selection of empiric antibiotics for Enterococcus sterile-site infections: a retrospective cohort study. CMAJ Open. 2017;5(3):E632–E637. | ||
Remschmidt C, Schröder C, Behnke M, Gastmeier P, Geffers C, Kramer TS. Continuous increase of vancomycin resistance in enterococci causing nosocomial infections in Germany - 10 years of surveillance. Antimicrob Resist Infect Control. 2018;7(1):217. | ||
van Loon K, Voor In ‘t Holt AF, Vos MC. A systematic review and meta-analyses of the clinical epidemiology of carbapenem-resistant Enterobacteriaceae. Antimicrob Agents Chemother. 2018;62(1):e01730-17. | ||
Vidal M, Forestier C, Charbonnel N, Henard S, Rabaud C, Lesens O. Probiotics and intestinal colonization by vancomycin-resistant enterococci in mice and humans. J Clin Microbiol. 2010;48(7):2595–2598. | ||
Crouzet L, Rigottier-Gois L, Serror P. Potential use of probiotic and commensal bacteria as non-antibiotic strategies against vancomycin-resistant enterococci. FEMS Microbiol Lett. 2015;362(8):fnv012. | ||
Ubeda C, Bucci V, Caballero S, et al. Intestinal microbiota containing Barnesiella species cures vancomycin-resistant Enterococcus faecium colonization. Infect Immun. 2013;81(3):965–973. | ||
Stiefel U, Nerandzic MM, Pultz MJ, Donskey CJ. Gastrointestinal colonization with a cephalosporinase-producing bacteroides species preserves colonization resistance against vancomycin-resistant enterococcus and Clostridium difficile in cephalosporin-treated mice. Antimicrob Agents Chemother. 2014;58(8):4535–4542. | ||
Crouzet L, Derrien M, Cherbuy C, et al. Lactobacillus paracasei CNCM I-3689 reduces vancomycin-resistant Enterococcus persistence and promotes Bacteroidetes resilience in the gut following antibiotic challenge. Sci Rep. 2018;8(1):5098. | ||
Tytgat HL, Douillard FP, Reunanen J, et al. Lactobacillus rhamnosus GG Outcompetes Enterococcus faecium via Mucus-Binding Pili: Evidence for a Novel and Heterospecific Probiotic Mechanism. Appl Environ Microbiol. 2016;82(19):5756–5762. | ||
Li M, Lee K, Hsu M, Nau G, Mylonakis E, Ramratnam B. Lactobacillus-derived extracellular vesicles enhance host immune responses against vancomycin-resistant enterococci. BMC Microbiol. 2017;17(1):66. | ||
Topcuoglu S, Gursoy T, Ovalı F, Serce O, Karatekin G. A new risk factor for neonatal vancomycin-resistant Enterococcus colonisation: bacterial probiotics. J Matern Fetal Neonatal Med. 2015;28(12):1491–1494. | ||
Drug Commission of the German Medical Association. Neue Kontraindikation von Saccharomyces boulardii (Saccharomyces cerevisiae HANSEN CBS 5926) bei schwerkranken oder immunsupprimierten Patienten. [German]. Available from: https://www.akdae.de/Arzneimittelsicherheit/RHB/Archiv/2018/20180122.pdf. Accessed July 11, 2018. |
Supplementary materials
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.