Back to Journals » Biologics: Targets and Therapy » Volume 15
Treatment with Ixekizumab Following Secukinumab Failure in Patients with Psoriatic Arthritis: Real-Life Experience from a Resistant Population
Authors Berman J , Furer V, Berman M, Isakov O, Zisman D, Haddad A, Elkayam O
Received 15 July 2021
Accepted for publication 18 September 2021
Published 18 November 2021 Volume 2021:15 Pages 463—470
DOI https://doi.org/10.2147/BTT.S326792
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Shein-Chung Chow
Julia Berman,1,2,* Victoria Furer,2,3,* Mark Berman,2,3 Ofer Isakov,1,2 Devy Zisman,4,5 Amir Haddad,4 Ori Elkayam2,3
1Department of Medicine ‘T’, Sourasky Medical Center, Tel Aviv, Israel; 2Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel; 3Department of Rheumatology, Sourasky Medical Center, Tel Aviv, Israel; 4Rheumatology Unit, Carmel Medical Center, Haifa, Israel; 5The Ruth and Bruce Rappaport Faculty of Medicine,Technion, Haifa, Israel
*These authors contributed equally to this work
Correspondence: Julia Berman Email [email protected]
Objective: To assess the clinical response to ixekizumab following secukinumab failure in patients with psoriatic arthritis.
Methods: A retrospective multi-center observational study included psoriatic arthritis (PsA) patients with a history of treatment with secukinumab, further treated with ixekizumab. Primary endpoint was primary response to treatment (drug survival > 6 months); secondary endpoints were changes in disease activity indices from initiation of ixekizumab to 6 and 12 months later and overall drug survival.
Results: Of 23 PsA patients, 86% (n = 20) received more than two TNF inhibitors (TNFi). Median secukinumab treatment time was 15 months (IQR 10– 21.5 months). Subsequently, 19 patients (83%) had a primary response to ixekizumab. Overall treatment duration during follow-up period for primary responders was 14 months (IQR 10– 20.5). Reasons for ixekizumab cessation were worsening psoriasis (27%), peripheral arthritis (27%), both (47%), worsening of axial disease (13%), and adverse events (6%). Articular disease indices including Disease Activity Index for Psoriatic Arthritis (DAPSA), tender joints count (TJC) and Simplified Disease Activity Index (SDAI) were significantly lower at 6 and 12 months (DAPSA 1.5– 2 levels reduction; p = 0.018 and 1– 1.5 levels reduction; p = 0.031, respectively; TJC − 2.16 [− 4.0, − 0.3]; p = 0.025 and − 1.69 [− 3.09, − 0.28]; p = 0.022, respectively; SDAI − 10.13 [− 16.4, − 3.8], p = 0.003 and − 12.2 [− 17.1, − 7.2], p = 0.0002, respectively). PASI75 at 6 and 12 months was achieved by 63% and 57%, respectively, and PASI100 at 6 and 12 months by 31% and 21%, respectively.
Conclusion: Patients with resistant PsA, including inadequate response to secukinumab, demonstrated a good response to ixekizumab, albeit limited on time. Within class switch from secukinumab to ixekizumab may be a plausible therapeutic option in PsA patients following secukinumab failure.
Keywords: secukinumab, ixekizumab, arthritis, psoriatic, duration of therapy, antibodies, monoclonal, humanized, psoriasis
Introduction
Psoriasis is a common, chronic systemic disease with a relapsing-remitting behavior that affects 0.9% to 8.5% of the population worldwide. Psoriatic arthritis (PsA) affects a subset of 20–30% of psoriasis patients.1 Pathogenesis of psoriasis has evolved in recent years. The previous concept of a “TH1 pathway disease” driven mainly by a cell-mediated adaptive immune response has shifted to the TH17 disease mediated by interleukin 17 (IL-17), as mainly responsible for the inflammatory response.2 This, in turn, has led the way to the clinical use of IL-17 inhibitors for the treatment of psoriasis and PsA.3
Secukinumab, a fully human IgG1κ anti-interleukin-17A monoclonal antibody represents an efficacious the treatment of psoriasis and PsA, FDA approved for both indications in 2015 and 2016, respectively. A subsequent anti-IL-17 agent, ixekizumab, a humanized IgG4 anti-17A monoclonal antibody, was approved by the FDA for the treatment of psoriasis and PsA in 2016 and 2017, respectively.
In the field of psoriasis, there is growing evidence of a successful switching between the two anti-IL-17 agents in case of an insufficient response to one of the treatments.4,5 In a 12‐week, retrospective study of 31 patients with psoriasis who failed treatment with secukinumab, a positive response was seen in 71% of patients switched to ixekizumab.6 In the field of PsA, data on switching of biologics is mainly limited to TNF inhibitors (TNFi).7,8 An effective switching from TNFi to ixekizumab was demonstrated in a Phase 3 clinical trial.9,10 However, there is currently no data regarding the efficacy of ixekizumab in patients with PsA who failed secukinumab. In this study, we report a real-life clinic experience regarding the efficacy of ixekizumab in patients with PsA following secukinumab failure.
Methods
A retrospective observational study was conducted in two rheumatology centers in Israel, including PsA patients (Classification Criteria for Psoriatic Arthritis (CASPAR criteria11)) with a history of treatment with secukinumab, further treated with ixekizumab for a minimum of 3 months. The baseline characteristics of the cohort, treatment details, and disease activity indices were reviewed. Primary endpoint was primary response to treatment, defined as treatment duration more than 6 months. Lack of efficacy or loss of efficacy in terms of articular and/or skin disease and side effects over time were reported as a reason for switching to another anti-IL17 agent. Lack of efficacy (primary failure) was defined by initially not responding to treatment within 6 months. Loss of efficacy (secondary failure) was defined as loss of clinical response after 6 months of treatment in patients who had initially demonstrated clinical response.
Secondary endpoints were changes in disease activity indices from initiation of ixekizumab to 6 and 12 months. Skin disease severity was assessed using the Psoriasis Area and Severity Index (PASI)12 and Body Surface Area (BSA); peripheral joint disease was assessed using 68-tender and 66-swollen joints counts (TJC and SJC accordingly); the presence of enthesitis was assessed by Leeds Enthesitis Index (LEI),13 disease activity was assessed by Clinical Disease Activity Index (CDAI),14 Simplified Disease Activity Index (SDAI),15 and Disease Activity Index for Psoriatic Arthritis (DAPSA). DAPSA levels were categorized into four levels: remission (0–4), low (5–14), moderate (15–28) and high (>28).16 Axial disease was defined as clinical evidence of inflammatory back pain with or without radiographic evidence or a magnetic resonance imaging (MRI)-based sacroiliitis.
Statistical Analysis
The mean change in various disease parameters between the beginning of the follow-up, with the initiation of ixekizumab, and the different follow-up points (6 and 12 months) were tested using a one-sample t-test. Time until treatment failure was estimated using Kaplan–Meier curves and compared using the Log rank test. Hazard ratios (HRs) and corresponding 95% confidence intervals (CIs) were calculated using the Cox proportional-hazards model to test the association between each variable and the time to treatment failure. DAPSA levels at the beginning of treatment were compared to the levels at 6 and 12 months of therapy using the paired Wilcoxon signed rank test. A 95% confidence interval was calculated for the change in levels. All statistical analysis was performed using R Statistical Software (version 3.6.3; R Foundation for Statistical Computing, Vienna, Austria).
Results
Clinical and Demographic Variables
A total of 23 PsA patients were enrolled in the study. The demographics and clinical characteristics of the study sample are detailed in Table 1. There were 12 male (52%) and 11 female (48%) patients. The mean age was 58.7 years (13.4 SD) and the mean BMI was 31.2 (4.8 SD). The mean duration of psoriasis was 22 years (standard deviation (SD) 13.9) and the mean duration of PsA was 14 years (SD 9.3). Most patients (n = 20, 86%) received two or more TNFi and ten patients (43%) received both TNFi and ustekinumab. The median number of biologics prior to secukinumab was 3 (IQR 2–4).
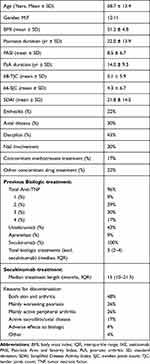 |
Table 1 PsA Cohort Characteristics at the Initiation of the IXE Treatment (N = 23) |
Of the 23 patients, only one patient (4.0%) was defined as a primary non-responder to secukinumab, whilst others experienced a secondary treatment failure (n = 22, 96%). The median duration of treatment with secukinumab was 15 months (IQR 10–21.5 months). Loss of efficacy related to skin and joint disease were the two major causes for a switch of treatments – 11 patients (48%) suffered both from active peripheral arthritis and worsening skin disease, while six patients (26%) had either active skin disease or peripheral arthritis. Four patients (17%) had active axial disease and one patient discontinued treatment due to adverse events (dermatitis).
Ixekizumab Response and Drug Survival
Of the 23 patients treated with ixekizumab after secukinumab, during a median follow-up time of 20 months (IQR 13.5–26.5), 19 patients (83%) had an initial response to treatment. Four patients (17%) had experienced primary treatment failure, with a median treatment of 4 months (IQR 3.75–4.5). Of the initial responders, subsequently 15 patients (65%) had experienced treatment failure during the follow-up period, with a median treatment duration of 8 months (IQR 6.5–13.5). Overall treatment duration during follow-up period for primary responders was 14 months (IQR 10–20.5). Overall, drug survival was 83% at 6 months and 50% at 12 months. Of the 15 patients who discontinued the treatment, 4 patients (27%) have done so mainly because of worsening psoriasis; 4 patients (27%) mainly because of worsening peripheral arthritis, seven patients (47%) because of worsening of both skin disease and arthritis, two patients (13%) due to worsening axial disease, and one patient (6%) due to adverse events (dermatitis). A survival curve (Kaplan-Meier) on ixekizumab is presented in Figure 1A.
 |
Figure 1 Survival on ixekizumab. (A) survival on ixekizumab; (B) a comparison of survival on ixekizumab dependent on survival on secukinumab. Abbreviations: IXE, ixekizumab; COS, Secukinumab. |
Factors Affecting Ixekizumab Response
Of the 9 patients treated with secukinumab for 6–12 months, 3 patients (33%) then experienced primary failure of ixekizumab, compared to only one patient (8%) of the 13 patients that received secukinumab for over a year. Most of the patients treated with secukinumab for over a year (8 out of 13) were still receiving ixekizumab after 12 months. However, in a univariate analysis, the duration of treatment with secukinumab was not found to be significantly associated with time interval until treatment failure with ixekizumab (p = 0.336) (Figure 1B).
Secondary Endpoints – Arthritis and Skin Disease Parameters
Data regarding disease indices response to ixekizumab treatment is presented in Figure 2. Data availability is described in Supplementary Table 1. The change in various disease parameters was evaluated after 6 and 12 months of ixekizumab treatment. There was a significant improvement in TJC at 6 and 12 months (−2.16 [−4.0, −0.3]; p = 0.025 and-1.69 [−3.09, −0.28]; p = 0.022, respectively). SJC was significantly improved at 6 months but not at 12 months (−2.68 [−5.3, −0.04]; p = 0.046 and −1.50 [−4.25,1.25]; p = 0.26, respectively). CDAI score was significantly improved at 6 months (−10.19, [−16.26, −4.1], p = 0.002) and at 12 months (−9.29 [−14.8, −3.71], p = 0.003) as was SDAI score (−10.13 [−16.4, −3.8], p = 0.003 and −12.2 [−17.1, −7.2], p = 0.0002). DAPSA levels at the beginning of treatment were compared to levels at 6 and 12 months of therapy. Of the 21 patients for which DAPSA score was available at the beginning of treatment, 16 had a moderate/high DAPSA score. At 6 months, 8 patients (50%) with a previous moderate/high disease activity improved to low disease activity/remission. At 12 months, of 11 patients with data available, 5 (31% of patient with moderate/high disease activity at beginning) remained at low disease activity/remission. A paired Wilcoxon signed-rank test showed that ixekizumab treatment was associated with significantly lower DAPSA levels at 6 and 12 months (1.5–2 levels reduction; p = 0.0115 and 1–1.5 levels reduction; p = 0.0192, respectively) (Supplementary Figure 1).
Measures of skin disease also demonstrated improvement – BSA was significantly reduced after 6 months (−7.16 [−2.81, −12.5], p = 0.01) but not after 12 months (−5.6 [−1.40, −14.4], p = 0.189) and PASI was significantly lower after 6 months (−5.08, [−3.59, −8.06], p = 0.002) and 12 months (−6.9, [−10.54, −3.34], p = 0.001). Overall, of the available data of patients with skin disease, at six months, PASI50 was achieved by 81% (13 patients), PASI75 was achieved by 63% (10 patients), PASI90 was achieved by 50% (8 patients) and PASI100 by 31% (5 patients). At 12 months, PASI50 and PASI75 were achieved by 57% (8 patients), PASI90 was achieved by 43% (6 patients) and PASI100 by 21% (3 patients) (Supplementary Figure 2).
Discussion
This is the first study to report a real-life clinic experience of IL-17 switch in patients with resistant PsA, as an advanced line of treatment after multiple biologics failure. In this study, patients after failure of multiple biologic treatments experienced a median survival of 14 months (10–20.5 IQR) on ixekizumab after they had previously failed secukinumab. Most of the patients demonstrated primary response to ixekizumab after the failure of secukinumab, with a significant reduction of disease activity after 6 and 12 months. Subsequently, overtime, most patients had experienced treatment failure, due to worsening peripheral disease, worsening dermatologic disease, or both; only a few experienced adverse events causing treatment cessation. There was a trend towards better drug survival for patients previously treated with secukinumab for more than a year.
Data on the efficacy of treatment switches for joint disease in PsA are limited and mostly focused on switching between TNFi.7 Current data suggest that TNFi response rates and drug survival diminish after switching, although patients treated with advanced treatment lines still experience an improvement in disease activity. In those trials, the second-line drug survival after one year was around 60%–74%, and closer to 50% for the third treatment line,17,18 comparable to the 50% survival rate in our study. Of note, these results were published before the approval of non-TNFi agents for PsA, thus fewer drug-switching options were available, which could have led to longer drug survival on advanced treatment lines.
Trials examining the switching between the TNFi class to drugs with a different mechanism of action include the PSUMMIT2 trial for ustekinumab,19 the FUTURE2 trial for secukinumab,20 and the SPIRIT-P2 trial, which demonstrated the efficacy of ixekizumab for patients after one or two TNFi failures.9 Currently, there are no clinical trials examining the optimal treatment strategy for patients exhausting multiple treatment options in different drug classes. Since no head-to-head trial data are available within mechanisms of action, the current guidelines by the European League Against Rheumatism (EULAR)21 and by the Group for Research and Assessment of Psoriasis and PsA (GRAPPA)22 recommend switching within a class as a viable option, based on expert opinion and some observational data.
In the treatment of plaque psoriasis, however, there is a growing body of evidence supporting the switching within class between secukinumab and ixekizumab. In a meta-analysis of 14 publications comprising 655 patients, about half the patients previously treated with secukinumab reached PASI100 after short-term treatment with ixekizumab, and a higher proportion (75% of patients) reached PASI75 in a similar timeframe,23 similar to the dermatologic response rate seen in our study. In a retrospective study of 50 patients previously treated with secukinumab, the mean drug survival of ixekizumab was 16 months, similar to the 14 months survival in our study, with a similar proportion of patients remaining on ixekizumab at the study end.24 In a multicenter study, 69 patients, of which 20 were also affected by psoriatic arthritis, were treated with ixekizumab following a loss of efficacy to secukinumab. The majority of patients (72.4%) reached 24 weeks of treatment, and most of them (40 patients) achieved PASI75.25 These and several other trials support the notion that ixekizumab might represent a valid treatment option in patients with psoriasis who failed secukinumab.4,5,26
As for treatment safety, in patients treated for ixekizumab for plaque psoriasis, PsA, and axial spondyloarthritis, the incidence of AEs leading to discontinuation was generally low (5.1 per 100 patient years).27 There are currently no data regarding the safety of within-class switch in relation to adverse events (AE). Specifically, it is currently unknown whether AEs on one anti-IL17 tend to recur with a second agent. Our data show a high safety profile for secukinumab and ixekizumab, as highlighted by the low rate of drug discontinuation due to AEs, although it is possible that patients with severe AEs on one anti-IL17 agents were less likely to be treated with a second agent in the first place. There was only one patient who discontinued secukinumab due to adverse events and later developed another AE with ixekizumab treatment. However, the low incidence of both events does not allow for the inference of causality.
The potential mechanism for efficacy in switching from one anti-IL-17A to another remains unclear. The in vitro affinity for IL-17A for ixekizumab is about 50–100 times higher than for secukinumab.28 This could be attributed to it being a humanized high‐affinity monoclonal antibody of the subclass IgG4, compared to secukinumab being a fully human monoclonal IgG1κ antibody; or to a lower equilibrium dissociation constants (Kd) for ixekizumab, allowing a higher potential for IL-17A blockade.29 This in vitro data is further supported by clinical evidence of matching-adjusted indirect comparisons showing a slightly higher (10–12%) response rate for psoriasis treatment with ixekizumab compared to secukinumab.30
To our knowledge, this is the first study to demonstrate the efficacy of within-class switch between secukinumab and ixekizumab for the treatment of psoriatic arthritis. The main strength of this study is the inclusion of a resistant and pre-treated patient population which is usually excluded from clinical trials. Limitations of this study include the relatively small sample size, retrospective analysis, and lack of complete data on disease activity measures. A potential bias is the relatively high response rate to secukinumab in our cohort, which is higher than previously demonstrated in clinical trials,31 hence our population might represent a subset of patients more susceptible to IL-17A pathway inhibition.
In conclusion, our study suggests that PsA patients after failure of multiple biologic treatments experienced a significant response in terms of peripheral arthritis and dermatologic disease on ixekizumab following the previous failure with secukinumab. However, the treatment effect was limited on time with 65% failure after a median time of 8 months. Within class switch from secukinumab to ixekizumab may be considered as a plausible therapeutic option in PsA patients following secukinumab failure.
Ethics Approval and Informed Consent
The study was conducted in accordance with the principles of the Declaration of Helsinki and approved by the Institutional Review Board, approval number 0332-10 (Tel Aviv Sourasky Medical Center) and 0055-16 CMC (Haifa Carmel Medical Center). Informed consent was waived owing to the retrospective study design.
Acknowledgment
The abstract for this paper was presented as an abstract at the Annual European Congress of Rheumatology EULAR 2021 Virtual, 2–5 June 2021 (https://ard.bmj.com/content/80/Suppl_1/1309), and also presented as a poster in the GRAPPA Annual Meeting and Trainee Symposium July 8, 2021.
Funding
There is no funding to report.
Disclosure
Dr Victoria Furer report personal fees from Novartis, personal fees from Eli Lilly, during the conduct of the study. Dr Devy Zisman report grants from Pfizer, personal fees from Eli Lilly, personal fees from Novartis, personal fees from AbbVie, outside the submitted work. Professor Ori Elkayam reports has received honorary fees as a speaker and as consultant in advisory board from Lilly and Novartis. The authors report no other conflicts of interest in this work.
References
1. Lloyd P, Ryan C, Menter A. Psoriatic arthritis: an update. Arthritis. 2012;2012:1–6. doi:10.1155/2012/176298
2. Martin DA, Towne JE, Kricorian G, et al. The Emerging Role of Interleukin-17 in the Pathogenesis of Psoriasis: preclinical and Clinical Findings. J Invest Dermatol. 2013;133(1):17–26. doi:10.1038/jid.2012.194
3. Kirkham BW, Kavanaugh A, Reich K. Interleukin-17A: a unique pathway in immune-mediated diseases: psoriasis, psoriatic arthritis and rheumatoid arthritis. Immunology. 2014;141(2):133–142. doi:10.1111/imm.12142
4. Bokor-Billmann T, Schäkel K. No need to change the drug class: ixekizumab- following secukinumab-therapy in psoriasis. J Dermatol Treat. 2019;30(3):216–220. doi:10.1080/09546634.2018.1506081
5. Deza G, Notario J, Lopez‐Ferrer A, et al. Initial results of ixekizumab efficacy and safety in real-world plaque psoriasis patients: a multicentre retrospective study. J Eur Acad Dermatol Venereol. 2019;33(3):553–559. doi:10.1111/jdv.15288
6. Georgakopoulos JR, Phung M, Ighani A, Lam K, Yeung J. Biologic switching between interleukin 17A antagonists secukinumab and ixekizumab: a 12-week, multicenter, retrospective study. J Eur Acad Dermatol Venereol. 2019;33(1):e7–e8. doi:10.1111/jdv.15100
7. Merola JF, Lockshin B, Mody EA. Switching biologics in the treatment of psoriatic arthritis. Semin Arthritis Rheum. 2017;47(1):29–37. doi:10.1016/j.semarthrit.2017.02.001
8. Damiani G, Conic RRZ, Vita V, et al. When IL-17 inhibitors fail: real-life evidence to switch from secukinumab to Adalimumab or ustekinumab. Dermatol Ther. 2019;32(2):e12793. doi:10.1111/dth.12793
9. Nash P, Kirkham B, Okada M, et al. Ixekizumab for the treatment of patients with active psoriatic arthritis and an inadequate response to tumour necrosis factor inhibitors: results from the 24-week randomised, double-blind, placebo-controlled period of the SPIRIT-P2 phase 3 trial. Lancet Lond Engl. 2017;389(10086):2317–2327. doi:10.1016/S0140-6736(17)31429-0
10. Genovese MC, Combe B, Kremer JM, et al. Safety and efficacy of ixekizumab in patients with PsA and previous inadequate response to TNF inhibitors: week 52 results from SPIRIT-P2. Rheumatol Oxf Engl. 2018;57(11):2001–2011. doi:10.1093/rheumatology/key182
11. Taylor W, Gladman D, Helliwell P, et al. Classification criteria for psoriatic arthritis: development of new criteria from a large international study. Arthritis Rheum. 2006;54(8):2665–2673. doi:10.1002/art.21972
12. Fredriksson T, Pettersson U. Severe psoriasis–oral therapy with a new retinoid. Dermatologica. 1978;157(4):238–244. doi:10.1159/000250839
13. Healy PJ, Helliwell PS. Measuring clinical enthesitis in psoriatic arthritis: assessment of existing measures and development of an instrument specific to psoriatic arthritis. Arthritis Rheum. 2008;59(5):686–691. doi:10.1002/art.23568
14. Aletaha D, Nell VPK, Stamm T, et al. Acute phase reactants add little to composite disease activity indices for rheumatoid arthritis: validation of a clinical activity score. Arthritis Res Ther. 2005;7(4):R796–806. doi:10.1186/ar1740
15. Smolen JS, Breedveld FC, Schiff MH, et al. A simplified disease activity index for rheumatoid arthritis for use in clinical practice. Rheumatol Oxf Engl. 2003;42(2):244–257. doi:10.1093/rheumatology/keg072
16. Schoels MM, Aletaha D, Alasti F, Smolen JS. Disease activity in psoriatic arthritis (PsA): defining remission and treatment success using the DAPSA score. Ann Rheum Dis. 2016;75(5):811–818. doi:10.1136/annrheumdis-2015-207507
17. Glintborg B, Østergaard M, Krogh NS, et al. Clinical Response, Drug Survival, and Predictors Thereof Among 548 Patients With Psoriatic Arthritis Who Switched Tumor Necrosis Factor α Inhibitor Therapy: results from the Danish Nationwide DANBIO Registry. Arthritis Rheum. 2013;65(5):1213–1223. doi:10.1002/art.37876
18. Saad AA, Ashcroft DM, Watson KD, et al. Persistence with anti-tumour necrosis factor therapies in patients with psoriatic arthritis: observational study from the British Society of Rheumatology Biologics Register. Arthritis Res Ther. 2009;11(2):R52. doi:10.1186/ar2670
19. Ritchlin C, Rahman P, Kavanaugh A, et al. Efficacy and safety of the anti-IL-12/23 p40 monoclonal antibody, ustekinumab, in patients with active psoriatic arthritis despite conventional non-biological and biological anti-tumour necrosis factor therapy: 6-month and 1-year results of the phase 3, multicentre, double-blind, placebo-controlled, randomised PSUMMIT 2 trial. Ann Rheum Dis. 2014;73(6):990–999. doi:10.1136/annrheumdis-2013-204655
20. McInnes IB, Mease PJ, Kirkham B, et al. Secukinumab, a human anti-interleukin-17A monoclonal antibody, in patients with psoriatic arthritis (FUTURE 2): a randomised, double-blind, placebo-controlled, phase 3 trial. The Lancet. 2015;386(9999):1137–1146. doi:10.1016/S0140-6736(15)61134-5
21. Gossec L, Baraliakos X, Kerschbaumer A, et al. EULAR recommendations for the management of psoriatic arthritis with pharmacological therapies: 2019 update. Ann Rheum Dis. 2020;79(6):700–712. doi:10.1136/annrheumdis-2020-217159
22. Coates LC, Kavanaugh A, Mease PJ, et al. Group for Research and Assessment of Psoriasis and Psoriatic Arthritis 2015 Treatment Recommendations for Psoriatic Arthritis. Arthritis Rheumatol Hoboken NJ. 2016;68(5):1060–1071. doi:10.1002/art.39573
23. Loft N, Halling A-S, Egeberg A, Skov L. Efficacy of a second interleukin 17 inhibitor in patients with psoriasis: a systematic review and meta-analysis. J Am Acad Dermatol. 2021;84(1):130–138. doi:10.1016/j.jaad.2020.07.085
24. Sherman S, Zloczower O, Noyman Y, Amitay-Laish I, Hodak E, Pavlovsky L. Ixekizumab Survival in Heavily Pretreated Patients with Psoriasis: a Two-year Single-centre Retrospective Study. Acta Derm Venereol. 2020;100(19):adv00349. doi:10.2340/00015555-3714
25. Conti A, Peccerillo F, Amerio P, et al. Efficacy and safety of switching to ixekizumab in secukinumab nonresponder patients with psoriasis: results from a multicentre experience. Br J Dermatol. 2019;180(6):1547–1548. doi:10.1111/bjd.17580
26. Gasslitter I, Kirsten N, Augustin M, et al. Successful intra-class switching among IL-17 antagonists: a multicentre, multinational, retrospective study. Arch Dermatol Res. 2019;311(5):421–424. doi:10.1007/s00403-019-01907-y
27. Genovese MC, Mysler E, Tomita T, et al. Safety of ixekizumab in adult patients with plaque psoriasis, psoriatic arthritis and axial spondyloarthritis: data from 21 clinical trials. Rheumatology. 2020;59(12):3834–3844. doi:10.1093/rheumatology/keaa189
28. Paul C. Ixekizumab or secukinumab in psoriasis: what difference does it make? Br J Dermatol. 2018;178(5):1003–1005. doi:10.1111/bjd.16497
29. Liu L, Lu J, Allan BW, et al. Generation and characterization of ixekizumab, a humanized monoclonal antibody that neutralizes interleukin-17A. J Inflamm Res. 2016;9:39–50. doi:10.2147/JIR.S100940
30. Warren RB, Brnabic A, Saure D, et al. Matching-adjusted indirect comparison of efficacy in patients with moderate-to-severe plaque psoriasis treated with ixekizumab vs. secukinumab. Br J Dermatol. 2018;178(5):1064–1071. doi:10.1111/bjd.16140
31. Mease PJ, McInnes IB, Kirkham B, et al. Secukinumab Inhibition of Interleukin-17A in Patients with Psoriatic Arthritis. N Engl J Med. 2015;373(14):1329–1339. doi:10.1056/NEJMoa1412679
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

