Back to Journals » OncoTargets and Therapy » Volume 12
Treatment Response To Osimertinib In EGFR-Mutated Leptomeningeal Metastases From Non-Small Cell Lung Cancer: A Case Series
Authors Li H, Yu T, Huang M, Guo A, Qian X, Yin Z
Received 25 December 2018
Accepted for publication 10 September 2019
Published 20 September 2019 Volume 2019:12 Pages 7785—7790
DOI https://doi.org/10.2147/OTT.S199452
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Faris Farassati

Huiying Li,1,* Tingting Yu,1,* Mingmin Huang,1 Aibin Guo,1 Xiaoping Qian,2 Zhenyu Yin1
1Department of Geriatric Oncology, Affiliated Nanjing Drum Tower Hospital of Nanjing University Medical School, Nanjing, People’s Republic of China; 2The Comprehensive Cancer Center of Drum Tower Hospital, Clinical Cancer Institute of Nanjing University, Nanjing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Zhenyu Yin
Department of Geriatric Oncology, Affiliated Nanjing Drum Tower Hospital of Nanjing University Medical School, No 321 Zhongshan Road, Nanjing, Jiangsu 210008, People’s Republic of China
Tel +86 139 1390 4579
Email [email protected]
Xiaoping Qian
The Comprehensive Cancer Center of Drum Tower Hospital, Clinical Cancer Institute of Nanjing University, No 321 Zhongshan Road, Nanjing, Jiangsu 210008, People’s Republic of China
Tel +86 151 5067 1158
Email [email protected]
Abstract: Therapy for leptomeningeal metastases (LM) from non-small cell lung cancer (NSCLC) is challenging, and conventional treatments have little impact on the disease course. We report three cases that were definitively diagnosed as LM from NSCLC with a mutation of epidermal growth factor receptor (EGFR) L858R. The systemic therapies of chemotherapy, local radiotherapy, and early generation tyrosine kinase inhibitors (TKIs) were implemented but ineffective. Three patients were treated with the third-generation TKI osimertinib at 80 mg daily, despite their different detection levels of T790M in the cerebrospinal fluid (CSF) and plasma, and achieved symptomatic remission, a decline of carcinoembryonic antigen (CEA) levels, and stable lesions. After the progression of LM, osimertinib at 160 mg daily further lengthened the quality of life and survival time of patients without any notable side effects during treatment. Recent related studies and our cases indicate that osimertinib has a positive effect on LM from EGFR-mutant NSCLC, regardless of T790M status.
Keywords: non-small cell lung cancer, leptomeningeal metastases, osimertinib, EGFR mutation, T790M
Introduction
Leptomeningeal metastases (LM) refer to the spread of cancerous cells to the leptomeninges, arachnoid, cavum subarachnoidale, and other cerebrospinal fluid compartments.1 The morbidity of LM is approximately 3–5% among patients with non-small cell lung cancer (NSCLC) and is higher in patients with epidermal growth factor receptor (EGFR) mutation when compared to those with EGFR wildtype (9.4% vs 1.7%) according to the latest report.2,3
The therapy of LM from NSCLC is challenging due to the obstruction of the blood brain barrier (BBB) to early generation tyrosine kinase inhibitors (TKIs), which have a ratio of cerebrospinal fluid (CSF) to blood concentration only approximately 1%.4–6 Recently, third-generation TKI osimertinib (AZD9291) attracted extensive attention owing to its remarkable central nervous system (CNS) permeability and reliable antitumour activity.7 Here, we report three cases that were confirmed to be LM from EGFR mutation-positive NSCLC and benefited from osimertinib.
Materials And Methods
The sequencing technologies utilized for EGFR sequencing in tumor biopsies, blood and CSF are the same across the three cases. The droplet digital polymerase chain reaction (ddPCR) is used to analyse EGFR mutation of tumour tissue, and standard next-generation sequencing (NGS) is adopted to analyse EGFR status of plasma and CSF.
Case Reports
Case 1
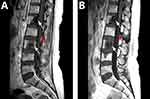
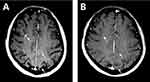
A 51-year-old Asian female non-smoker was diagnosed with right upper lobe lung adenocarcinoma with multiple osseous metastases in July 2015 after appearing with severe back pain. EGFR testing revealed an exon 21 L858R point mutation and c-Met overexpression. Treatment with gefitinib at 250 mg daily was started in August 2015. Meanwhile, the patient received palliative radiation of 30 Gy in 10 fractions from T5-T7 spine. A positive clinical response was achieved after treatment. However, multiple metastases were diagnosed in October 2015 with the help of magnetic resonance imaging (MRI) of the brain. In consideration of the overexpression of c-Met, a combination treatment of gefitinib and crizotinib was applied, and the disease stabilized for 5 months. Thereafter, imaging studies suggested disease progression in the brain, right lung and bone. Re-biopsy of the right upper lobe primary lesion revealed mutations of both EGFR L858R and T790M. The systemic therapy was changed to osimertinib at 80 mg daily, and a course of cyber-knife radiotherapy was implemented for the primary lesion in the right lung. The treatment was tolerable for the patient and obtained stable clinical remission for 9 months. In December 2017, the patient started to display severe headaches, weakness of the lower extremities, neck ankyloses, and gradually aggravated back pain. Plasma carcinoembryonic antigen (CEA) levels were markedly increased, and MRI discovered LMs (Figures 1A and 2A), which were further confirmed by positive CSF cytology. The T790M mutation was absent in the CSF. To enhance the efficacy, the dose of osimertinib was increased to 160 mg daily. The clinical symptoms of headaches, neck ankyloses, and back pain gradually alleviated. Her CEA level and MRI findings remained stable (Figures 1B and 2B) for 8 months without any notable side effects during treatment. Unfortunately, she gradually became emaciated and died of a severe lung infection in November 2018.
Case 2
A 60-year-old Asian female non-smoker presented with a dry cough in April 2009. Chest computed tomography (CT) suggested left lower lobe lung cancer. The clinical stage was pT3N0M0 IIB according to the postsurgical pathology. Adjuvant chemotherapy with docetaxel and oxaliplatin was applied for 6 cycles. The disease was controlled for 6 years until May 2015, when the patient showed symptoms of dizziness, headache, nausea, and dysuria. Based on MRI and positive CSF cytology, she was diagnosed with LM. The EGFR test showed a point mutation of exon 21 L858R. Gefitinib treatment was adopted, which stabilized the disease for 1 year. In May 2016, the symptoms of headache and nausea returned and were confirmed by MRI to be a result of the progression of LM. Plasma EGFR L858R and T790M mutation testing produced positive results. Osimertinib at 80 mg daily was implemented as a second-line treatment, which led to symptomatic remission and a decline in CEA levels. In September 2017, the patient presented with epileptic seizures and urinary retention. MRI revealed the enhancement of the cerebrospinal membrane. Osimertinib was increased to 160 mg daily and maintained the remission for 6 months. Although the CEA level decreased, the patient became lethargic and mute and passed away in March 2018.
Case 3
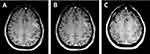
A 56-year-old Asian female was diagnosed with right upper lobe lung cancer by CT scan and positron emission tomography CT (PET-CT). Thoracoscopic resection of the lung cancer was carried out, the pathological examination displayed pleural metastasis, and the presence of the EGFR L858R mutation was confirmed. Gefitinib was started in August 2015. In December 2017, she presented with hemifacial spasms and short-term aphasia. Although the CSF cytology was negative, LM was suspected based on PET-CT and MRI (Figure 3A). Plasma EGFR L858R mutation testing produced positive results, while the test for T790M was negative. Osimertinib at 80 mg daily was administered, which decreased the CEA levels, alleviated neurological signs and symptoms, and reduced the lesion size (Figure 3B). Five months after beginning the osimertinib treatment, she presented neck stiffness and lower limb fatigue. The EGFR test of the CSF demonstrated L858R but without a T790M mutation. Osimertinib at 160 mg daily was started in May 2018, which resulted in stable clinical improvement. At the last follow-up in May 2019, a repeat MRI revealed a shrunken lesion of LM (Figure 3C).
Discussion
LM development from NSCLC is normally accompanied by poor prognosis, with a median overall survival time of 8.7 months if relying on conventional treatments.2,3 When lung cancer patients show symptoms of headaches, nausea and vomiting, dysuria, fatigue, mental abnormalities, epileptic seizures and other symptoms, clinicians should be vigilant of the occurrence of LM. The diagnosis of LM is mainly dependent on the clinical manifestations, cytologic examinations of CSF, and imaging examinations. The specificity of LM diagnosis can reach 75–85% by CSF analysis, which is widely recognized as the gold standard and is advised to be repeated twice on account of its low sensitivity.8 Heteromorphic cells and EGFR-mutated deoxyribonucleic acid (DNA) were discovered in the first and second cases presented here, except for the third case. However, LM was deduced to exist in the third case based on the typical symptoms and MRI, which was verified by the significant treatment effect.
The patients with LM are recommended to be grouped according to graded prognostic assessment (GPA), including Karnofsky performance status (KPS), age, and the degree of intracranial and extracranial metastasis.9 For the group with low GPA scores, the main purposes of treatment are to relieve neurological symptoms, improve the quality of life, and extend the maximum overall survival time. For the group with high scores and relatively good prognoses, more aggressive treatments are recommended, generally including chemotherapy, radiotherapy, immunotherapy and molecular targeted therapy. Systemic chemotherapy is a prior consideration for patients with NSCLC with no targetable mutations.2 Intrathecal chemotherapy, especially with methotrexate, has a notable effect in the treatment of LM,10 but it should be executed prudently to avoid conspicuous neurological toxicity and infection. Radiotherapy could effectively control limited lesions in the brain; however, there is no valid evidence that whole brain and craniospinal radiotherapy are effective for diffuse LM, which may lead to severe marrow suppression and increase mortality.10 In the area of immunotherapy, extraordinary strides have been made, most notably with the programmed death-1 (PD-1)/PD-ligand 1 antibodies, which prove to be potent for many kinds of tumour. However, only a few relevant cases have been reported, and no major breakthroughs have been made in the therapy of LM.11,12
Generations of EGFR-TKIs have varying degrees of efficacy in treating LM from EGFR-mutant NSCLC, and one of the most commonly used is osimertinib. In the five TKIs that are recommended prior to first-line systemic therapy (osimertinib, erlotinib, afatinib, gefitinib, dacomitinib), osimertinib is the preferred choice due to its outstanding antineoplastic efficacy.13 Moreover, osimertinib is confirmed to have a several-fold higher permeability compared to some other EGFR-TKIs.7 These advantages demonstrate the vital role of osimertinib in controlling LM.
Regarding the dose of osimertinib, several studies and cases indicate that remarkable efficacy can be achieved by using osimertinib at 80 mg daily.14–17 At the same time, high-dose osimertinib has also been proven to be very effective by the BLOOM study. Twenty-one patients with CNS-positive LM from EGFR-mutant NSCLC were selected for the study. After treatment, 7 of the patients had radiological improvement, 5 showed improvement in neurological function, and 2 had confirmed CSF cytology clearance.18 All of the cases we reported gained remarkable clinical improvement at the standard dose, and after the disease progressed, osimertinib at 160 mg daily further prolonged the survival of the patients without increasing the occurrence of toxicity. It could be that high-dose osimertinib ensures a high local concentration in the CNS and has marked anti-tumour effects. Further studies are required to elucidate the optimal dose of osimertinib.
The dissimilar detection of the T790M mutation in the CNS and extra-CNS sites also deserves special attention, as in case 1 in this paper, who had a T790M mutation in all plasma and lung tissue except the CNS. The circulating tumour DNA in CSF can express gene mutations of central LM more accurately than plasma can.19 Some studies suggest that patients who are resistant to gefitinib have a lower T790M mutation rate in cerebrospinal fluid compared to in extracranial lesions.20,21 Taken together, it appears that the low CNS permeability gives rise to the lower acquired T790M mutation in CNS.
Recently, in a prospective study of patients with NSCLC after the loss of prior TKI therapy, the patients with T790M mutations in both CNS and extra-CNS lesions showed an excellent response, and the patients with a T790M mutation only in the extra-CNS site rendered the disease stable.14 In addition, some cases report and phase 1 of the BLOOM study showed that osimertinib could be very effective despite T790M status.18,22,23 Most recently, Haiying Cheng and Roman Perez-Soler recommend osimertinib as the best choice for EGFR T790M-positive LM patients. When T790M is negative, osimertinib is also an important choice.24
Conclusion
In summary, osimertinib has a positive effect on LM from EGFR-mutant NSCLC regardless of T790M status. Furthermore, when standard-dose osimertinib (80 mg daily) is ineffective, high-dose osimertinib (160 mg daily) can further prolong progression-free survival. The optimal dose and the ideal combination of other treatments need more clinical cases and further prospective studies to be determined.
Statement Of Ethics
This is a retrospective case series and institutional approval was not needed.
Consent For Publication
Written informed consent was obtained from the patients or their next-of-kin regarding the publication of the case details and associated images.
Acknowledgments
This study was supported by grants from the Natural Science Foundation of Jiangsu Province (Grant No Bk20161107), the Health Planning Commission Development Project of Jiangsu Province (Grant No H2817042), and the Key Project of Nanjing Public Health Bureau, China (Grant No ZKX17012).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Gleissner B, Chamberlain MC. Neoplastic meningitis. Lancet Neurol. 2006;5:443–452. doi:10.1016/S1474-4422(06)70443-4
2. Remon J, Le Rhun E, Besse B. Leptomeningeal carcinomatosis in non-small cell lung cancer patients: a continuing challenge in the personalized treatment era. Cancer Treat Rev. 2017;53:128–137. doi:10.1016/j.ctrv.2016.12.006
3. Li YS, Jiang BY, Yang JJ, et al. Leptomeningeal metastases in patients with NSCLC with EGFR mutations. J Thorac Oncol. 2016;11:1962–1969. doi:10.1016/j.jtho.2016.06.029
4. Deng Y, Feng W, Wu J, et al. The concentration of erlotinib in the cerebrospinal fluid of patients with brain metastasis from non-small-cell lung cancer. Mol Clin Oncol. 2014;2:116–120. doi:10.3892/mco.2013.190
5. Togashi Y, Masago K, Masuda S, et al. Cerebrospinal fluid concentration of gefitinib and erlotinib in patients with non-small cell lung cancer. Cancer Chemother Pharmacol. 2012;70:399–405. doi:10.1007/s00280-012-1929-4
6. Hoffknecht P, Tufman A, Wehler T, et al. Efficacy of the irreversible ErbB family blocker afatinib in epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI)-pretreated non-small-cell lung cancer patients with brain metastases or leptomeningeal disease. J Thorac Oncol. 2015;10:156–163. doi:10.1097/JTO.0000000000000380
7. Ballard P, Yates JW, Yang Z, et al. Preclinical comparison of osimertinib with other EGFR-TKIs in EGFR-mutant NSCLC brain metastases models, and early evidence of clinical brain metastases activity. Clin Cancer Res. 2016;22:5130–5140. doi:10.1158/1078-0432.CCR-16-0399
8. Grossman SA, Krabak MJ. Leptomeningeal carcinomatosis. Cancer Treat Rev. 1999;25:103–119. doi:10.1053/ctrv.1999.0119
9. NCCN Clinical Practice Guideline in Oncology (NCCN Guidelines®). Central nervous system cancers. Leptomeningeal metastases. Version 2. 2018. Available from: http://www.nccn.org.
10. Chamberlain MC. Leptomeningeal metastasis. Curr Opin Oncol. 2010;22:627–635. doi:10.1097/CCO.0b013e32833de986
11. Gion M, Remon J, Caramella C, et al. Symptomatic leptomeningeal metastasis improvement with nivolumab in advanced non-small cell lung cancer patient. Lung Cancer. 2017;108:72–74. doi:10.1016/j.lungcan.2017.02.022
12. Dudnik E, Yust-Katz S, Nechushtan H, et al. Intracranial response to nivolumab in NSCLC patients with untreated or progressing CNS metastases. Lung Cancer. 2016;98:114–117. doi:10.1016/j.lungcan.2016.05.031
13. NCCN Clinical Practice Guideline in Oncology (NCCN Guidelines®). Non-small cell lung cancer version 2. 2019. Available from: http://www.nccn.org/patients/guidelines/cancers.aspx#nsclc.
14. Nanjo S, Hata A, Okuda C, et al. Standard-dose osimertinib for refractory leptomeningeal metastases in T790M-positive EGFR-mutant non-small cell lung cancer. Br J Cancer. 2018;118:32–37. doi:10.1038/bjc.2017.394
15. Chalmers A, Jensen L, Akerley W. Durable response to osimertinib in EGFR mutated T790M wildtype nonsmall cell lung cancer with leptomeningeal metastases: a case report. Lung Cancer. 2017;114:68–69. doi:10.1016/j.lungcan.2017.10.009
16. Chan OS, Leung WK, Yeung RM. Sustained response to standard dose osimertinib in a patient with plasma T790M-positive leptomeningeal metastases from primary lung adenocarcinoma. Asia-Pac J Clin Oncol. 2017;13:428–430. doi:10.1111/ajco.12673
17. Niu H, Zhou J, Maan H, et al. Treatment of Leptomeningeal Metastases in a patient with non-small cell lung cancer Harboring EGFR T790M. Case Rep Oncol. 2017;10:840–845. doi:10.1159/000480452
18. Yang JC, Cho BC, Kim D, et al. Osimertinib for patients (pts) with leptomeningeal metastases (LM) from EGFR-mutant non-small cell lung cancer (NSCLC): updated results from the BLOOM study. Proc Am Soc Clin Oncol. 2017;35:2020. doi:10.1200/JCO.2017.35.15_suppl.2020
19. De Mattos-Arruda L, Mayor R, Ng CK, et al. Cerebrospinal fluid-derived circulating tumour DNA better represents the genomic alterations of brain tumours than plasma. Nat Commun. 2015;6:8839. doi:10.1038/ncomms9839
20. Nanjo S, Arai S, Wang W, et al. MET copy number gain is associated with gefitinib resistance in leptomeningeal carcinomatosis of EGFR-mutant lung cancer. Mol Cancer Ther. 2017;16:506–515. doi:10.1158/1535-7163.MCT-16-0522
21. Balak MN, Gong Y, Riely GJ, et al. Novel D761Y and common secondary T790M mutations in epidermal growth factor receptor-mutant lung adenocarcinomas with acquired resistance to kinase inhibitors. Clin Cancer Res. 2006;12:6494–6501. doi:10.1158/1078-0432.CCR-06-1570
22. Facchinetti F, Bozzetti F, Minari R, et al. Meeting with triumph and disaster: osimertinib in T790M-unknown CNS progression in EGFR-mutated non-small cell lung cancer. Tumori. 2018;2018:300891618809826. doi:10.1177/0300891618809826
23. Jänne PA, Yang JC, Kim DW, et al. AZD9291 in EGFR inhibitor-resistant nonsmall-cell lung cancer. N Engl J Med. 2015;372:1689–1699. doi:10.1056/NEJMoa1411817
24. Cheng H, Perez-Soler R. Leptomeningeal metastases in non-small-cell lung cancer. Lancet Oncol. 2018;19:43–55. doi:10.1016/S1470-2045(17)30689-7
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.