Back to Journals » Cancer Management and Research » Volume 10
Treatment-related adverse effects with pazopanib, sorafenib and sunitinib in patients with advanced soft tissue sarcoma: a pooled analysis
Authors Que Y , Liang Y , Zhao JJ, Ding Y, Peng RQ, Guan YX, Zhang X
Received 3 February 2018
Accepted for publication 4 April 2018
Published 19 July 2018 Volume 2018:10 Pages 2141—2150
DOI https://doi.org/10.2147/CMAR.S164535
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Antonella D'Anneo
Yi Que,1,* Yao Liang,2,* Jingjing Zhao,1 Ya Ding,1 Ruiqing Peng,1 Yuanxiang Guan,2 Xing Zhang1
1Department of Medical Melanoma and Sarcoma, State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Sun Yat-Sen University Cancer Center, Guangzhou, People’s Republic of China; 2Department of Gastric and Pancreatic Surgery, State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Sun Yat-Sen University Cancer Center, Guangzhou, People’s Republic of China
*These authors contributed equally to this work
Objective: Research efforts have investigated therapies targeting tyrosine kinase signaling pathways. We performed a pooled analysis to determine the frequency of severe adverse effects in patients with soft tissue sarcoma treated with pazopanib, sorafenib and sunitinib.
Materials and methods: We performed a comprehensive search of PubMed, Web of Science, Ovid, the Cochrane Library and Embase databases from the drugs’ inception to May 2017 to identify clinical trials. All-grade and severe adverse events (AEs; grade≥3) were analyzed.
Results: A total of 10 trials published between 2009 and 2016, including 843 patients, were eligible for analysis. We included 424 patients (three studies) who received pazopanib 800 mg daily, 353 patients (five studies) who received sorafenib 400 mg twice daily and 66 patients (two studies) who received sunitinib 37.5 mg daily. The incidence of AEs is different among the three VEGFR-tyrosine kinase inhibitors (TKIs). Pazopanib showed higher incidence of all-grade nausea, diarrhea and hypertension compared with sorafenib and sunitinib. However, patients in the sorafenib group experienced a significantly higher frequency of all-grade rash (26.1%), hand–foot syndrome (33.4%) and mucositis (38.5%). The difference was highly significant for sorafenib vs. pazopanib in the incidence of all-grade rash (odds ratio [OR] 1.649, 95% CI 1.086–2.505, P=0.023), hand–foot syndrome (OR 3.096, 95% CI 1.271–7.544, P=0.009) and mucositis (OR 4.562, 95% CI 2.132–9.609, P<0.001). Moreover, the frequency of grade ≥3 mucositis was significantly higher in the sunitinib group compared with the pazopanib or sorafenib group (7.6% vs. 1.3%, OR 6.448, 95% CI 1.499–27.731, P=0.013).
Conclusion: Statistically significant differences in certain common adverse effects, such as all-grade and severe AEs, were detected among pazopanib, sorafenib and sunitinib in the current study. Early and prompt management is critically needed to avoid unnecessary dose reductions and treatment-related discontinuations.
Keywords: pazopanib, sorafenib, sunitinib, soft tissue sarcoma
Introduction
Soft tissue sarcomas (STSs) are a group of rare mesenchymal cancers that include approximately 50 histological subtypes and comprise approximately 1% of all adult cancers.1,2 Surgery with or without radiotherapy is the primary treatment for early-stage localized STS.3,4 The conventional treatment for patients with inoperable or metastatic STSs is an anthracycline (usually doxorubicin), either as a monotherapy or in combination with ifofamide.5 However, the prognosis of metastatic or advanced STS is poor.
Given the need for improved therapies, investigations into novel treatments for advanced STS are continuing. Recently, studies have been performed to test anti-angiogenic treatment, and efforts have been focused on therapies targeting tyrosine kinase signaling pathways.6–8 The small molecule vascular endothelial growth factor inhibitor, pazopanib, is a multi-targeted tyrosine kinase inhibitor (TKI) with high affinity against VEGFR-1/2/3 and with a lower affinity against PDGFR-α/β, FGFR-1/2 and stem cell factor receptor (c-KitR).9 Based on the clinical trials, pazopanib was determined to be well tolerated in metastatic or advanced STS and demonstrated antitumor activity in non-liposarcoma.10,11 Therefore, the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) have approved pazopanib for the treatment of advanced STS in those who have received prior chemotherapy.12
Sorafenib, also a small molecule B-raf and VEGFR inhibitor, is potentially useful in several specific sarcoma subtypes, such as angiosarcomas.13 Moreover, sorafenib in patients with solid tumors indicated a promising 30% PFR12 in patients with metastatic sarcomas.14 Furthermore, in malignant peripheral nerve sheath tumours (MPNSTs) with the loss of NF1 and the activation of the ras–raf signaling pathway,15–17 sorafenib demonstrated activity. Based on preclinical data, the level of tumor growth inhibition for pazopanib was similar to that for sorafenib.18–20
Sunitinib is also a multi-targeted TKI with activity against VEGFRs1/2/3, PDGFR-α/β, KIT, FLT3, RET and CSF-1.21 The role of sunitinib has also been extensively studied in STS. Furthermore, clinical efficacy has been indicated in patients with advanced STS, predominantly in liposarcomas, leimyosarcomas,22 solitary fibrous tumors23 and alveolar STSs.24
In the past decade, three TKIs have been developed as angiogenesis inhibitors and showed their antitumor response in several solid tumors.10,25–29 However, the use of VEGFR-TKIs is limited by their different side effects, in which the precise underlying mechanism often remains unclear. The difference in the toxicity pattern among pazopanib, sunitinib and sorafenib remains unclear.30 Although pazopanib is the only one which has been approved by the FDA for patients with sarcoma, sunitinib and sorafenib have shown definite efficacy in some specific types of sarcomas. There is a great potential for TKIs in the treatment of patients with sarcoma. Notably, the three TKIs show a promising response rate in STS. Compliance with anticancer therapy is determined by their tolerability. Therefore, it is important to choose optimal TKIs by performing a pooled analysis of the occurrence of adverse events (AEs) based on data extracted from clinical trials of patients with STS.
Materials and methods
Study identification
The following computerized databases were used to search the relevant literature for clinical trials: PubMed, Web of Science, Ovid, the Cochrane Library and Embase, encompassing the period from the drugs’ inception to May 2017. The search keywords were “pazopanib,” “sunitinib,” “sorafenib,” and “sarcoma.” Abstracts from American Society of Clinical Oncology (ASCO) meetings were hand searched to scan for updated data and to identify new studies. In addition, duplicate data were removed, and the articles were screened to determine whether the article was relevant. Duplicate research or irrelevant articles were discarded after retrieval and review. Reference lists were also hand searched to identify any additional articles.
The study’s inclusion criteria were as follows: 1) patients ≥18 years or older with metastatic or locally advanced STS (non-GISTs); 2) intervention: pazopanib, sunitinib or sorafenib, not combined with other therapies; 3) sufficient data presented on treatment-related AEs (TRAEs), including all information about all-grade and grade ≥3 toxicity and 4) written in English. All case reports, letters, commentaries and reviews were excluded.
Data extraction and quality control
The first author’s name, publication year, therapeutic drug (pazopanib, sunitinib or sorafenib), number of patients evaluable for all-grade and grade ≥3 toxicity (nausea, diarrhea, fatigue, vomiting, hypertension, hand–foot syndrome, rash, elevated ALT, neutropenia, leukopenia and anemia, mucositis, number of patients experiencing treatment-related death [TRD] and withdrawal resulting from severe toxicity) were evaluated. Clinical trials were collected for patients receiving pazopanib 800 mg daily, sorafenib 400 mg twice daily and sunitinib 37.5 mg daily according to the FDA-recommended dose. Two studies including patients receiving sunitinib 50 mg daily were excluded.22,31 Studies were independently selected by two authors based on the aforementioned inclusion criteria.
The full texts of nonrandomized clinical trials were assessed using the 9-point Newcastle–Ottawa Scale (NOS). Two investigators evaluated the studies independently. Studies were categorized into three broad perspectives, including selection, comparability and outcome for cohort studies or exposure for case–control studies. A score of 7 or greater was considered to be high quality. The risk of bias in the included studies was independently assessed by two investigators using the Cochrane Collaboration’s tool for assessing the risk of bias in randomized controlled trials (RCTs).32 Two authors independently assessed each study under five main headings for the risk of bias.
Statistical analyses
All statistical analyses were performed using GraphPad Prism software (version 6.0; GraphPad Software, Inc., La Jolla, CA, USA). Fisher’s exact or chi-square tests were used to compare the frequencies of AEs among three multiple receptor tyrosine kinases. All tests were two tailed, and statistical significance was considered at P<0.05.
Results
Characteristics of the original selected studies
Based on our inclusion criteria, 10 clinical trials were identified to address multiple receptor tyrosine kinase-treated STSs (Figure 1). Among the 10 trials published between 2009 and 2016, 843 patients with STS were eligible for the current study. The sample size of the eligible trials ranged from 14 to 239. We included 424 patients (three studies)10,11,33 who received pazopanib, 353 patients (five studies)13,34–37 who received sorafenib and 66 patients (two studies)38,39 who received sunitinib. All the patients received the multiple TKI alone. The primary characteristics of the included trials are listed in Table 1.
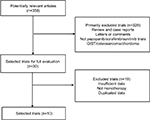  | Figure 1 Flow diagram of selection of trials included in the pooled analysis. |
  | Table 1 Baseline characteristics of trials included in the pooled analysis Abbreviation: NA, not available. |
Study quality assessment and risk of bias
The methodological quality of all non-RCTs (NRCTs; excluding abstracts and conference abstracts) is listed in Table S1. No major flaws of the included RCTs were detected in assessing their risk of bias (Table S2). However, the expected absence of a blinded intervention was a common caveat.
Frequency of all-grade TRAEs between different VEGFR-TKI types
We analyzed the incidence and odds ratio (OR) of TRAEs by VEGFR-TKI in patients with STS. The incidence of all-grade nausea was highest with pazopanib (47.2%) followed by sunitinib (24.2%) and sorafenib (7.6%). The difference between incidence was highly significant for pazopanib vs. sunitinib (OR 2.799, 95% CI 1.539–5.088, P<0.001), sunitinib vs. sorafenib (OR 3.865, 95% CI 1.740–8.584, P=0.001) and pazopanib vs. sorafenib (OR 10.815, 95% CI 5.933–19.713, P<0.001; Figure 2A).
A similar pattern was observed in the frequency of all-grade hypertension. The incidence of all-grade hypertension was highest with pazopanib (40.9%) followed by sunitinib (18.2%) and sorafenib (9.3%). The difference between the incidences was highly significant for pazopanib vs. sorafenib (OR 6.723, 95% CI 4.451–10.155, P<0.001), sunitinib vs. sorafenib (OR 2.155, 95% CI 1.048–4.431, P=0.049) and pazopanib vs. sunitinib (OR 3.120, 95% CI 1.616–6.024, P<0.001; Figure 2B).
For all-grade fatigue, the frequency was 54.3% for pazopanib, 45.5% for sunitinib and 30.0% for sorafenib. Significant differences were found between pazopanib vs. sorafenib (OR 2.772, 95% CI 2.045–3.757, P<0.001). Meanwhile, patients in the sunitinib group experienced significantly higher frequency of all-grade fatigue than patients in the sorafenib group (OR 1.942, 95% CI 1.137–3.317, P=0.021), whereas the difference between pazopanib vs. sunitinib was not significant (OR 1.428, 95% CI 0.845–2.413, P=0.185; Figure 2C).
Similarly, the frequency of all-grade diarrhea was significantly greater in patients treated with pazopanib than in those treated with sorafenib (43.2% vs. 23.5%, OR 2.470, 95% CI 1.808–3.375, P<0.001). Moreover, statistical significance was observed between the sunitinib and sorafenib groups (42.4% vs. 23.5% OR 2.397, 95% CI 1.388–4.141, P=0.002), Nonetheless, there was no significant difference between the pazopanib and sunitinib cohort (43.2% vs. 42.4%, OR 1.031, 95% CI 0.610–1.741, P=1.000; Figure 2D).
All-grade vomiting was significantly more common for pazopanib compared with sorafenib (27.4% vs. 5.4%, OR 6.183, 95% CI 3.324–11.501, P<0.001). However, statistical significance was not observed between the sorafenib and sunitinib groups (5.4% vs. 7.1%, OR 0.792, 95% CI 0.095–6.570, P=0.580). In addition, there was no significant difference between pazopanib and sunitinib (27.4% vs. 7.1%, OR 4.896, 95% CI 0.633–37.848, P=0.126; Figure 2E).
The frequency of all-grade neutropenia differed significantly between pazopanib and sorafenib (25.4% vs. 0.0%, OR 1.283, 95% CI 1.186–1.387, P<0.001). Statistical significance was also observed between sunitinib and sorafenib (21.2% vs. 0.0%, OR 1.750, 95% CI 1.465–2.091, P=0.002). However, there was no significant difference between pazopanib and sunitinib (25.4% vs. 21.2%, OR 1.265, 95% CI 0.643–2.489, P=0.616; Figure 2F). For all-grade leukopenia, patients in the pazopanib group also experienced significantly higher frequency than patients in the sorafenib group (42.3% vs. 10.4%, OR 6.293, 95% CI 3.352–11.814, P<0.001) and sunitinib group (42.3% vs. 14.3%, OR 4.390, 95% CI 0.947–20.347, P=0.048), whereas there was no significant difference between sunitinib and sorafenib (14.3% vs. 10.4%, OR 1.433, 95% CI 0.292–7.026, P=0.649; Figure 2G). A similar pattern was observed in the frequency of all-grade anemia between pazopanib and sorafenib (74.6% vs. 12.8%, OR 20.022, 95% CI 7.278–55.085, P<0.001) and pazopanib and sunitinib (74.6% vs. 15.2%, OR 16.489, 95% CI 7.621–35.677, P<0.001), Nonetheless, there was no significant difference between the sunitinib and sorafenib groups (15.2% vs. 12.8%, OR 1.214, 95% CI 0.383–3.854, P=1.000; Figure 2H).
Another common adverse effect, elevated all-grade ALT, was more common for pazopanib compared with sorafenib (24.7% vs. 7.7%, OR 3.907, 95% CI 2.159–7.069, P<0.001) and sunitinib vs. sorafenib (21.2% vs. 7.7%, OR 3.200, 95% CI 1.354–7.566, P=0.010). Statistical significance was not observed between pazopanib and sunitinib (24.7% vs. 21.2%, OR 1.221, 95% CI 0.603–2.471, P=0.730; Figure 2I).
A further analysis of common skin and mucosa dysfunctions (rash, hand–foot syndrome and mucositis) was conducted among the three VEGFR-TKI types. Patients in the sorafenib group experienced a significantly higher frequency of all-grade rash (26.1%), hand–foot syndrome (33.4%) and mucositis (38.5%). The difference was highly significant for sorafenib vs. pazopanib for the incidence of all-grade rash (OR 1.649, 95% CI 1.086–2.505, P=0.023; Figure 2J), hand–foot syndrome (OR 3.096, 95% CI 1.271–7.544, P=0.009; Figure 2K) and mucositis (OR 4.562, 95% CI 2.132–9.609, P<0.001; Figure 2L).
Frequency of severe TRAEs (grade ≥3) between different VEGFR-TKI types
Grade ≥3 fatigue was significantly more common for pazopanib compared with sorafenib (11.0% vs. 6.5%, OR 1.778, 95% CI 1.046–3.022, P=0.037) and sunitinib (11.0% vs. 1.5%, OR 8.053, 95% CI 1.089–59.555, P=0.012). Nonetheless, there was no significant difference between the sorafenib and sunitinib cohorts (6.5% vs. 1.5%, OR 4.530, 95% CI 0.601–34.141, P=0.149; Figure 3A).
A similar pattern was observed in the frequency of grade ≥3 vomiting in the pazopanib vs. sorafenib groups (3.5% vs. 0.5%, OR 7.647, 95% CI 0.971–60.211, P=0.028), whereas there was no significant difference between pazopanib and sunitinib (3.5% vs. 0.0%, OR 1.051, 95% CI 1.024–1.079, P=1.000) or sorafenib and sunitinib (0.5% vs. 0.0%, OR 1.067, 95% CI 1.031–1.104, P=1.000; Figure 3B).
Patients in the pazopanib group experienced a significantly higher frequency of severe hypertension than patients in the sorafenib group (7.1% vs. 3.4%, OR 2.143, 95% CI 1.069–4.298, P=0.032). However, the frequency of grade ≥3 hypertension did not differ significantly between the pazopanib and sunitinib groups (7.1% vs. 1.5%, OR 4.902, 95% CI 0.655–36.710, P=0.101). Moreover, statistical significance was not observed between the sorafenib and sunitinib groups (3.4% vs. 1.5%, OR 2.287, 95% CI 0.292–17.896, P=0.702; Figure 3C).
Another common adverse effect, grade ≥3 rash, was less common for pazopanib compared with sorafenib (0.4% vs. 8.3%, OR 0.047, 95% CI 0.006–0.346, P<0.001). Statistical significance was not observed between pazopanib and sunitinib (0.4% vs. 0.0%, OR 1.059, 95% CI 1.028–1.091, P=1.000); likewise, it was not observed between sorafenib and sunitinib (8.3% vs. 0.0%, OR 1.049, 95% CI 1.023–1.075, P=0.613; Figure 3D).
For grade ≥3 mucositis, the frequency was highest with sunitinib (7.6%), followed by sorafenib (5.1%) and pazopanib (1.3%). Statistical significance was observed in investigating the frequency of mucositis grade ≥3 for sunitinib vs. pazopanib (7.6% vs. 1.3%, OR 6.448, 95% CI 1.499–27.731, P=0.013; Figure 3E).
As for grade ≥3 nausea, neutropenia, leukopenia, anemia, elevated ALT and hand–foot syndrome, no statistical significance was detected among all three cohorts (data not shown).
By pooled analysis, complete responses and partial responses were observed in 39 patients in the pazopanib group, 27 in the sorafenib group and five in the sunitinib group. Objective response rates were similar among the three groups (Table S3).
Identification of withdrawal toxicity and TRD for pazopanib vs. sorafenib
The overall frequency of AEs that resulted in treatment withdrawal for pazopanib and sorafenib was 11.1% (47 of 424 evaluable patients) and 11.0% (39 of 353 evaluable patients), respectively. No significant difference was observed among the two groups. However, a significant difference in AEs that resulted in TRD was observed between pazopanib and sorafenib (3.3% vs. 0.0, OR 1.620, 95% CI 1.480–1.733, P=0.029). The frequency of the overall TRD for pazopanib and sorafenib was 3.3% (eight of 239 evaluable patients) and 0.0% (zero of 139 evaluable patients), respectively.
Discussion
In the past decade, several small molecule TKIs, including pazopanib, sunitinib and sorafenib, have demonstrated clinical efficacy in STS.10,11,13,36,38 However, the use of these inhibitors is limited by the occurrence of severe adverse effects, such as hypertension, rash and fatigue.40,41 Treatments that alleviate and prevent side effects ultimately lead to enhanced health-related quality of life (HRQoL) of patients. The determination of frequency of TRAEs of different TKIs in STS may enable the early management of most susceptible patients. In this regard, there is a critical need to determine the frequency of AEs to reduce the risk of treatment-related withdrawal or death.
To the best of our knowledge, the current study is the first pooled analysis in STS, focusing on the differences in TRAEs among pazopanib, sunitinib and sorafenib. In our analysis, pazopanib had the highest incidence of all-grade nausea, fatigue, vomiting, diarrhea, hypertension, elevated alanine aminotransferase (ALT), neutropenia, leukopenia and anemia compared with sorafenib and sunitinib; likewise, the frequency of grade ≥3 fatigue, vomiting and hypertension was also the highest in pazopanib-treated patients. We further observed that the frequency of AEs that resulted in TRD was significantly different between the pazopanib and sorafenib groups. Conversely, the frequency of rash, hand–foot syndrome and mucositis was highest in the sorafenib group compared with the pazopanib and sunitinib groups, and sorafenib-treated patients had the highest incidence of grade ≥3 rash among the three groups. Moreover, the frequency of grade ≥3 mucositis was significantly higher in the sunitinib group compared with the pazopanib or sorafenib groups.
A double-blind, randomized crossover study sought to assess the preference between sunitinib and pazopanib for patients with metastatic renal cell carcinoma (RCC).42 This study observed that, for most of the AEs, especially fatigue, patients preferred pazopanib over sunitinib. In a meta-analysis, Santoni et al43 demonstrated that sorafenib had a lower incidence of risk ratio (RR) of all-grade gastrointestinal (GI) events compared with pazopanib and sunitinib. However, these results have not focused on the STS subgroup. In addition, the differences in some common adverse effects of anti-angiogenesis, including rash, hand–foot syndrome and hypertension, among pazopanib, sunitinib and sorafenib, have not been determined. The different evaluation time points of adverse effects and different doses of TKIs in the studies may drive the different results. For example, in our article, we included patients who received pazopanib 800 mg daily and sunitinib 37.5 mg daily. However, Escudier et al42 reported that patients with metastatic RCC were randomly assigned to pazopanib 800 mg/day for 10 weeks, a 2-week washout, and then sunitinib 50 mg/day (4 weeks on, 2 weeks off, 4 weeks on).
Imatinib mesylate is also a competitive inhibitor of tyrosine kinases selectively associated with c-Kit and platelet derived growth factor receptors.44 Besides GIST, it has been registered for advanced/metastatic dermatofibrosarcoma protuberans (DFSPs). Two Phase II trials formally proved the activity of the drug in this disease, with a 50% overall response rate leading to the registration for this disease.45,46 Similar activity has been shown in the rare fibrosarcomatous variant although with a more limited duration.47,48 Imatinib is therefore currently used in classical locally advanced DFSP and in metastatic fibrosarcomatous DFSP. Given that the good clinical response and limited dose-related effect of imatinib in DFSP may to some extent influence the result, we did not include imatinib in the pooled analysis.
Our study has several limitations. First, this study analyzed all published clinical trials and determined the statistical significance of severe AEs among VEGFR-TKIs. However, the small number of patients receiving sunitinib compared with those receiving pazopanib or sorafenib may to some extent be a limitation. Thus, a large sample size of prospective RCTs is required. Second, a portion of AE information was not obtained, even though we contacted the corresponding authors. Third, the association between treatment-related toxicities and clinical outcome in patients with VEGFR-TKI still remains to be elucidated. Further studies are urgently needed to understand the underlying mechanism of this association.
Conclusion
Our study has shown that different VEGFR-TKIs are associated with a significantly increased risk of treatment-related toxicities. The prompt and early management of these events is critically needed to reduce their impact on patient outcome and quality of life (QoL) to optimize medical resource utilization. Therefore, physicians and patients should be aware of these risks when managing the use of these VEGFR-TKIs in STS.
Acknowledgment
This study was supported by the National Scientific Foundation of China (No. 81372887), the National Basic Research Program of China (Grant No. 2013CB910500) and the National Scientific Foundation of China (No. 81572403).
Disclosure
The authors report no conflicts of interest in this work.
References
Clark MA, Fisher C, Judson I, Thomas JM. Soft-tissue sarcomas in adults. N Engl J Med. 2005;353(7):701–711. | ||
Casali PG, Blay JY; Experts ECECPo. Soft tissue sarcomas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21(suppl 5):v198–v203. | ||
Daigeler A, Zmarsly I, Hirsch T, et al. Long-term outcome after local recurrence of soft tissue sarcoma: a retrospective analysis of factors predictive of survival in 135 patients with locally recurrent soft tissue sarcoma. Br J Cancer. 2014;110(6):1456–1464. | ||
Weitz J, Antonescu CR, Brennan MF. Localized extremity soft tissue sarcoma: improved knowledge with unchanged survival over time. J Clin Oncol. 2003;21(14):2719–2725. | ||
Sleijfer S, Ouali M, van Glabbeke M, et al. Prognostic and predictive factors for outcome to first-line ifosfamide-containing chemotherapy for adult patients with advanced soft tissue sarcomas: an exploratory, retrospective analysis on large series from the European Organization for Research and Treatment of Cancer-Soft Tissue and Bone Sarcoma Group (EORTC-STBSG). Eur J Cancer. 2010;46(1):72–83. | ||
Cudmore MJ, Hewett PW, Ahmad S, et al. The role of heterodimerization between VEGFR-1 and VEGFR-2 in the regulation of endothelial cell homeostasis. Nat Commun. 2012;3:972. | ||
Ivy SP, Wick JY, Kaufman BM. An overview of small-molecule inhibitors of VEGFR signaling. Nat Rev Clin Oncol. 2009;6(10):569–579. | ||
HDAC Inhibition Overcomes Resistance to VEGFR inhibitors in solid tumors. Cancer Discov. 2017;7(4):350. | ||
Schutz FA, Choueiri TK, Sternberg CN. Pazopanib: clinical development of a potent anti-angiogenic drug. Crit Rev Oncol Hematol. 2011;77(3):163–171. | ||
van der Graaf WT, Blay JY, Chawla SP, et al. Pazopanib for metastatic soft-tissue sarcoma (PALETTE): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2012;379(9829):1879–1886. | ||
Sleijfer S, Ray-Coquard I, Papai Z, et al. Pazopanib, a multikinase angiogenesis inhibitor, in patients with relapsed or refractory advanced soft tissue sarcoma: a phase II study from the European organisation for research and treatment of cancer-soft tissue and bone sarcoma group (EORTC study 62043). J Clin Oncol. 2009;27(19):3126–3132. | ||
Medscape [homepage on the Internet]. FDA Approves Votrient for Advanced Soft Tissue Sarcoma. 2018. Available from: www.fda.gov. Accessed April 24, 2018. | ||
Maki RG, D’Adamo DR, Keohan ML, et al. Phase II study of sorafenib in patients with metastatic or recurrent sarcomas. J Clin Oncol. 2009;27(19):3133–3140. | ||
Strumberg D, Richly H, Hilger RA, et al. Phase I clinical and pharmacokinetic study of the Novel Raf kinase and vascular endothelial growth factor receptor inhibitor BAY 43-9006 in patients with advanced refractory solid tumors. J Clin Oncol. 2005;23(5):965–972. | ||
Jhanwar SC, Chen Q, Li FP, Brennan MF, Woodruff JM. Cytogenetic analysis of soft tissue sarcomas. Recurrent chromosome abnormalities in malignant peripheral nerve sheath tumors (MPNST). Cancer Genet Cytogenet. 1994;78(2):138–144. | ||
Johnson MR, DeClue JE, Felzmann S, et al. Neurofibromin can inhibit Ras-dependent growth by a mechanism independent of its GTPase-accelerating function. Mol Cell Biol. 1994;14(1):641–645. | ||
Mori S, Satoh T, Koide H, Nakafuku M, Villafranca E, Kaziro Y. Inhibition of Ras/Raf interaction by anti-oncogenic mutants of neurofibromin, the neurofibromatosis type 1 (NF1) gene product, in cell-free systems. J Biol Chem. 1995;270(48):28834–28838. | ||
Keir ST, Maris JM, Lock R, et al. Initial testing (stage 1) of the multi-targeted kinase inhibitor sorafenib by the pediatric preclinical testing program. Pediatr Blood Cancer. 2010;55(6):1126–1133. | ||
Maris JM, Courtright J, Houghton PJ, et al. Initial testing (stage 1) of sunitinib by the pediatric preclinical testing program. Pediatr Blood Cancer. 2008;51(1):42–48. | ||
Keir ST, Morton CL, Wu J, Kurmasheva RT, Houghton PJ, Smith MA. Initial testing of the multitargeted kinase inhibitor pazopanib by the Pediatric Preclinical Testing Program. Pediatr Blood Cancer. 2012;59(3):586–588. | ||
Chow LQ, Eckhardt SG. Sunitinib: from rational design to clinical efficacy. J Clin Oncol. 2007;25(7):884–896. | ||
Mahmood ST, Agresta S, Vigil CE, et al. Phase II study of sunitinib malate, a multitargeted tyrosine kinase inhibitor in patients with relapsed or refractory soft tissue sarcomas. Focus on three prevalent histologies: leiomyosarcoma, liposarcoma and malignant fibrous histiocytoma. Int J Cancer. 2011;129(8):1963–1969. | ||
Stacchiotti S, Negri T, Libertini M, et al. Sunitinib malate in solitary fibrous tumor (SFT). Ann Oncol. 2012;23(12):3171–3179. | ||
Stacchiotti S, Negri T, Zaffaroni N, et al. Sunitinib in advanced alveolar soft part sarcoma: evidence of a direct antitumor effect. Ann Oncol. 2011;22(7):1682–1690. | ||
Demetri GD, van Oosterom AT, Garrett CR, et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomised controlled trial. Lancet. 2006;368(9544):1329–1338. | ||
Escudier B, Eisen T, Stadler WM, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356(2):125–134. | ||
Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378–390. | ||
Sternberg CN, Davis ID, Mardiak J, et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. J Clin Oncol. 2010;28(6):1061–1068. | ||
Raymond E, Dahan L, Raoul JL, et al. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med. 2011;364(6):501–513. | ||
Ryan JL, Carroll JK, Ryan EP, Mustian KM, Fiscella K, Morrow GR. Mechanisms of cancer-related fatigue. Oncologist. 2007;12(suppl 1):22–34. | ||
Hensley ML, Sill MW, Scribner DR Jr, et al. Sunitinib malate in the treatment of recurrent or persistent uterine leiomyosarcoma: a Gynecologic Oncology Group phase II study. Gynecol Oncol. 2009;115(3):460–465. | ||
Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. | ||
Yoo KH, Kim HS, Lee SJ, et al. Efficacy of pazopanib monotherapy in patients who had been heavily pretreated for metastatic soft tissue sarcoma: a retrospective case series. BMC Cancer. 2015;15:154. | ||
von Mehren M, Rankin C, Goldblum JR, et al. Phase 2 Southwest Oncology Group-directed intergroup trial (S0505) of sorafenib in advanced soft tissue sarcomas. Cancer. 2012;118(3):770–776. | ||
Ray-Coquard I, Italiano A, Bompas E, et al. Sorafenib for patients with advanced angiosarcoma: a phase II Trial from the French Sarcoma Group (GSF/GETO). Oncologist. 2012;17(2):260–266. | ||
Santoro A, Comandone A, Basso U, et al. Phase II prospective study with sorafenib in advanced soft tissue sarcomas after anthracycline-based therapy. Ann Oncol. 2013;24(4):1093–1098. | ||
Bramswig K, Ploner F, Martel A, et al. Sorafenib in advanced, heavily pretreated patients with soft tissue sarcomas. Anticancer Drugs. 2014;25(7):848–853. | ||
George S, Merriam P, Maki RG, et al. Multicenter phase II trial of sunitinib in the treatment of nongastrointestinal stromal tumor sarcomas. J Clin Oncol. 2009;27(19):3154–3160. | ||
Li T, Wang L, Wang H, et al. A retrospective analysis of 14 consecutive Chinese patients with unresectable or metastatic alveolar soft part sarcoma treated with sunitinib. Invest New Drugs. 2016;34(6):701–706. | ||
Liu B, Ding F, Zhang D, Wei GH. Risk of venous and arterial thromboembolic events associated with VEGFR-TKIs: a meta-analysis. Cancer Chemother Pharmacol. 2017;80(3):487–495. | ||
Massey PR, Okman JS, Wilkerson J, Cowen EW. Tyrosine kinase inhibitors directed against the vascular endothelial growth factor receptor (VEGFR) have distinct cutaneous toxicity profiles: a meta-analysis and review of the literature. Support Care Cancer. 2015;23(6):1827–1835. | ||
Escudier B, Porta C, Bono P, et al. Randomized, controlled, double-blind, cross-over trial assessing treatment preference for pazopanib versus sunitinib in patients with metastatic renal cell carcinoma: PISCES Study. J Clin Oncol. 2014;32(14):1412–1418. | ||
Santoni M, Conti A, Massari F, et al. Treatment-related fatigue with sorafenib, sunitinib and pazopanib in patients with advanced solid tumors: an up-to-date review and meta-analysis of clinical trials. Int J Cancer. 2015;136(1):1–10. | ||
Croom KF, Perry CM. Imatinib mesylate: in the treatment of gastrointestinal stromal tumours. Drugs. 2003;63(5):513–522. discussion 523–514. | ||
Rutkowski P, Wozniak A, Switaj T. Advances in molecular characterization and targeted therapy in dermatofibrosarcoma protuberans. Sarcoma. 2011;2011:959132. | ||
Rutkowski P, Van Glabbeke M, Rankin CJ, et al. Imatinib mesylate in advanced dermatofibrosarcoma protuberans: pooled analysis of two phase II clinical trials. J Clin Oncol. 2010;28(10):1772–1779. | ||
Kerob D, Porcher R, Verola O, et al. Imatinib mesylate as a preoperative therapy in dermatofibrosarcoma: results of a multicenter phase II study on 25 patients. Clin Cancer Res. 2010;16(12):3288–3295. | ||
Stacchiotti S, Pedeutour F, Negri T, et al. Dermatofibrosarcoma protuberans-derived fibrosarcoma: clinical history, biological profile and sensitivity to imatinib. Int J Cancer. 2011;129(7):1761–1772. |
Supplementary materials
  | Table S2 Risk of bias in RCTs Abbreviation: RCTs, randomized controlled trials. |
  | Table S3 The clinical therapeutic effects of the included studies Abbreviations: NA, not available; PR, partial response; CR, complete response; SD, stable disease; PD, progressive disease. |
References
Sleijfer S, Ray-Coquard I, Papai Z, et al. Pazopanib, a multikinase angiogenesis inhibitor, in patients with relapsed or refractory advanced soft tissue sarcoma: a phase II study from the European organisation for research and treatment of cancer-soft tissue and bone sarcoma group (EORTC study 62043). J Clin Oncol. 2009;27(19):3126–3132. | ||
Yoo KH, Kim HS, Lee SJ, et al. Efficacy of pazopanib monotherapy in patients who had been heavily pretreated for metastatic soft tissue sarcoma: a retrospective case series. BMC Cancer. 2015;15:154. | ||
Maki RG, D’Adamo DR, Keohan ML, et al. Phase II study of sorafenib in patients with metastatic or recurrent sarcomas. J Clin Oncol. 2009;27(19):3133–3140. | ||
von Mehren M, Rankin C, Goldblum JR, et al. Phase 2 Southwest Oncology Group-directed intergroup trial (S0505) of sorafenib in advanced soft tissue sarcomas. Cancer. 2012;118(3):770–776. | ||
Ray-Coquard I, Italiano A, Bompas E, et al. Sorafenib for patients with advanced angiosarcoma: a phase II Trial from the French Sarcoma Group (GSF/GETO). Oncologist. 2012;17(2):260–266. | ||
Santoro A, Comandone A, Basso U, et al. Phase II prospective study with sorafenib in advanced soft tissue sarcomas after anthracycline-based therapy. Ann Oncol. 2013;24(4):1093–1098. | ||
Bramswig K, Ploner F, Martel A, et al. Sorafenib in advanced, heavily pretreated patients with soft tissue sarcomas. Anticancer Drugs. 2014;25(7):848–853. | ||
George S, Merriam P, Maki RG, et al. Multicenter phase II trial of sunitinib in the treatment of nongastrointestinal stromal tumor sarcomas. J Clin Oncol. 2009;27(19):3154–3160. | ||
Li T, Wang L, Wang H, et al. A retrospective analysis of 14 consecutive Chinese patients with unresectable or metastatic alveolar soft part sarcoma treated with sunitinib. Invest New Drugs. 2016;34(6):701–706. | ||
van der Graaf WT, Blay JY, Chawla SP, et al. Pazopanib for metastatic soft-tissue sarcoma (PALETTE): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2012;379(9829):1879–1886. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



