Back to Journals » Therapeutics and Clinical Risk Management » Volume 16
Treatment Pattern Analysis and Health-care Resource Consumption on Patients with Psoriatic Arthritis or Ankylosing Spondylitis Treated with Biological Drugs in a Northern Italian Region
Authors Perrone V, Giacomini E, Sangiorgi D , Andretta M, Menti AM , Naclerio M , Ritrovato D , Degli Esposti L
Received 5 February 2020
Accepted for publication 13 May 2020
Published 9 June 2020 Volume 2020:16 Pages 509—521
DOI https://doi.org/10.2147/TCRM.S248390
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Garry Walsh
Valentina Perrone,1 Elisa Giacomini,1 Diego Sangiorgi,1 Margherita Andretta,2 Anna Michela Menti,2 Mariantonietta Naclerio,3 Daniela Ritrovato,3 Luca Degli Esposti1
1CliCon S.r.l. Health, Economics & Outcomes Research, Ravenna, Italy; 2Health Technology Assessment Unit, Azienda Zero, Padova, Italy; 3Novartis Farma Italy, Origgio, VA, Italy
Correspondence: Valentina Perrone
Clicon Srl, Health, Economics and Outcomes Research, Via Salara, 36, 48100, Ravenna, Italy
Tel +39 544 38393
Fax +39 544 212699
Email [email protected]
Purpose: To analyze the treatment patterns of psoriatic arthritis (PSA) or ankylosing spondylitis (AS) patients under biological therapies and to evaluate in this population the health-care resource consumption and related costs.
Patients and Methods: A retrospective analysis was performed on administrative databases of the Veneto region. Patients ≥ 18 years with at least one prescription of biological drugs and a diagnosis at any level for PSA or AS from January 1, 2011 to December 31, 2016 (inclusion period) were included. Index date (ID) was defined as date of first biological drug prescription during inclusion period. Patients were characterized the year before ID and followed-up for one year after ID. The drug utilization profile in terms of adherence, persistence and therapeutic regimen changes, and the health-care resource consumption was analyzed during follow-up.
Results: A total of 2602 patients were included: 1857 with PSA and 745 with AS. In the PSA cohort, 40.3% of patients were prescribed adalimumab, 35.6% etanercept, 8.0% golimumab, 7.5% infliximab, 5.6% ustekinumab and 3.0% certolizumab. Percentage of PSA patients adherent to treatment was higher among ustekinumab patients (91.3%) and lower among etanercept users (54.3%). Persistence ranged from 53.2% (infliximab) to 70.3% (etanercept). Regarding AS cohort, 45.5% of patients were prescribed adalimumab, 26% etanercept, 17.3% infliximab, 9.7% golimumab and 1.5% certolizumab. Adherence ranged from 46.9% (etanercept) to 90.9% (certolizumab) and persistence from 62.8% (adalimumab) to 81.8% (certolizumab). Mean annual health-care costs (including costs for drug treatment, diagnostic services, specialist visits and hospital admissions) ranged from € 9727 (certolizumab) to € 14,994 (ustekinumab) among PSA patients and from € 9875 (infliximab) to € 12,991 (golimumab) among AS patients.
Conclusion: This study in Veneto region gave a picture of biological treatment patterns among PSA and AS patients in a real-world setting. Our findings showed the high degree of variability concerning utilization of each biological drug and provided insight on the economic burden of both diseases.
Keywords: psoriatic arthritis, ankylosing spondylitis, biologic drugs, drug utilization, real-world
Introduction
Psoriatic arthritis (PSA) and ankylosing spondylitis (AS) are chronic inflammatory rheumatologic diseases belonging to the spondyloarthritis family.1 Both PSA and AS have a negative impact on quality of life of affected patients. PSA is a potentially disabling heterogeneous condition characterized by multiple clinical features that vary considerably among patients, ranging from musculoskeletal as arthritis, enthesitis, dactylitis, to dermatological manifestations as psoriasis (up to 47.1% of psoriatic patients reported PSA)2 and nail disease.3,4 PSA onset is usually between 30 and 50 years old, with an equal distribution among genders.5 To date, there is not an estimation of PSA prevalence for the Italian general population, and available data ranging from 0.09% to 0.42% are referred to specific geographic areas2,6,7 and collected in different years.
The hallmark of AS is represented by inflammatory back pain since AS affects mainly the spine, sacroiliac joints and axial skeleton.1,8 Less frequently, peripheral arthritis or extraarticular features involving eye, gastrointestinal tract, skin, heart, and vascular system are observed.9 The protracted inflammation can ultimately lead to immobilization due to the fusion of vertebrae.8 AS is two/three times more common in men than in women, and onset is generally in the age-range of 20–40 years, typically in the third decade of life.8 As for PSA, the prevalence of AS in Italy was estimated in a regional community-based study, and it was found to be 0.37%.7
Over the past 20 years, biological agents changed the management of pharmacologic treatment for chronic inflammatory autoimmune diseases, thus revolutionizing the field of rheumatology.10 These drugs are genetically engineered proteins targeting the immune system to abrogate the inflammatory response.11 According to the latest European League Against Rheumatism (EULAR) recommendation,12,13 PSA and AS patients that do not benefit from conventional synthetic disease-modifying drugs (csDMARDs) should be treated with biological agents. There is no evidence supporting a better efficacy of a biological drug over another, and choice is usually made according to the clinical characteristics of patients and adverse effects, interactions and contraindications of pharmacological treatment.14 In case of failure of a biological agent, switching to another one occurs frequently in clinical practice for PSA and AS patients, and is considered a successful strategy for improving the outcomes.15 Switching from one TNF inhibitor to another proved to be effective for many patients, and recently also switching to biological agents based on a different mechanism of action represents a valid alternative.16 The chronic nature of PSA and AS requires lifelong treatment. Ensuring long-term medication adherence is the key for successful treatment with biological drugs, as adherence and persistence to medication are determining factors to achieve the most clinical benefit from these therapies.17 Nonadherence and nonpersistence are related to higher risk of disease progression and treatment failure and place a substantial burden on the health-care system.17 Adherence and persistence to treatment in patients affected by rheumatic diseases vary widely among these diseases.18
Biological treatments are expensive, and the economic burden of these drugs must be taken into account to guarantee an equal access to preferred treatments for all patients.10 However, the specific drug costs could be compensated by the significant efficacy of biological therapy to reduce symptoms, to slow disease progression and to improve patients functional status as well as quality of life.14,19,20 A recent systematic review focusing on cost-illness and cost-effectiveness of PSA showed that biological treatments are more cost-effective than conventional therapies, as the increased direct costs related to biological drugs were offset by reduction of indirect costs thanks to the improvement of treatment efficacy.21 Few real-world studies are reported on the economic burden of PSA and AS in Italy.22 During 2005 Olivieri et al evaluated costs, efficacy and cost-effectiveness of antiTNF agents in PSA patients not responding to conventional treatment,23 while a more updated study was conducted by us on health resources utilization for PSA and psoriatic patients before and after prescription for biological treatments.24 For AS, the only economic burden evaluation reported for Italy regarded the impact of delay in spondyloarthritis diagnosis.25 The aim of the present study was to analyze therapeutic pathways and adherence and persistence to treatment of patients affected by PSA or AS treated with biological agents, and to evaluate health-care resources consumption and related costs for the Regional Health System.
Patients and Methods
Data Source
An observational retrospective study based on administrative databases of the Veneto region was conducted on two distinct cohorts of PSA and AS patients, respectively. To perform the analysis, the following databases were accessed: demographic database to retrieve patients characteristics; payment exemption database that includes disease exemption code and date of exemption; pharmaceutical database providing data on prescription of drugs reimbursed by the Italian National Health Service (INHS), drug name, Anatomical-Therapeutic Chemical (ATC) code, dispensing date, number and cost of packages; hospitalization database including primary and secondary diagnoses at hospital discharge and procedures; diagnostic test and specialist visit databases to get data on outpatient specialist services in terms of type, description, date and charge of laboratory test or specialist visit. Each patient is identified by an anonymous code, which permitted electronic linkage between these databases. Data were extracted by the staff of the region and their databases were anonymized in full compliance with the European General Data Protection Regulation (GDPR) (2016/679). No identifiers related to patients were provided to the researchers. All the results of the analyses were produced as aggregated summaries, which are not possible to assign, either directly or indirectly, to individual patients. Informed consent was not required for using encrypted retrospective information. According to the Italian law,26 this study has been notified to the local ethics committee of the region involved in the study.
Study Population
All adult patients (≥18 years old) in the Veneto region were included if they presented at least one prescription of biological drugs (ATC L04A) and a diagnosis for PSA or AS confirmed by at least one hospital discharge—at any diagnosis level—with an ICD-9CM code (696.0 for PSA, 720.0 for AS) or an exemption code (045.696.0 for PSA, 054.720.0 for AS) from January 1, 2011 to December 31, 2016 (inclusion period). Index date (ID) assigned for each patient included corresponded to the date of first prescription for biological drug. Patients with concomitant PSA and AS diagnosis or that were transferred to another region after the ID were excluded from the analysis. Patients were characterized during the year before ID (characterization period) and followed-up for 12 months after ID (follow-up period). Patients were defined “naïve” if they did not have a prescription for biological drugs during the characterization period, otherwise they were defined “established”.
Pharmacoutilization Analysis
Therapeutic patterns for the treatment of PSA/AS were assessed at discharge and over time, to estimate pharmacoutilization profile. Therapeutic variations have been assessed comparing treatments during the follow-up period. Biological treatments analyzed were: etanercept (ATC code: L04AB01), infliximab (ATC code: L04AB02), adalimumab (ATC code: L04AB04), certolizumab (ATC code: L04AB05), golimumab (ATC code: L04AB06), ustekinumab (ATC code: L04AC05). csDMARDs analysed were methotrexate (-MTX- ATC codes: L01BA01, L04AX03), cyclosporine (ATC codes: L04AD01, S01XA18), leflunomide (ATC code: L04AA13), sulfasalazine (ATC code: A07EC01), acitretin (ATC code: D05BB02), azathioprine (ATC code: L04AX01), hydroxychloroquine (ATC code: P01BA02).
Comorbidities were assessed during the characterization period and identified according to ICD-9-CM codes: psoriasis (PSO) (ICD-9-CM code: 696.1, ATC code: D05A), rheumatoid arthritis (RA) (ICD-9-CM code: 714), gastrointestinal diseases—Crohn’s disease (CD) (ICD-9-CM code: 555), ulcerative colitis (UC) (ICD-9-CM code: 556).
Patients having at least one prescription for csDMARD during follow-up period were considered with a concomitant csDMARD treatment. Switch was defined as the presence of a biological therapy during the follow-up period different from that administered at the ID. Adherence to therapy was determined by calculating the proportion of days covered (PDC). Patients were considered as adherent to therapy if, according to defined daily dose, they had been covered by the drug for at least 80% of days of follow-up (PDC ≥80%). To observe the proportion of patients still in treatment with biological drugs at the end of follow-up, persistence was defined as presence of biological drugs during the last trimester of follow-up period, while treatment interruption was defined as the absence of biological drugs during the last trimester of the follow-up period.
Cost and Health-care Consumption Analysis
The mean annual health-care costs per patient for the management of PSA/AS patients, based on total related resource consumption, were assessed during the follow-up period. Health-care resource consumption was analyzed in terms of biological drugs and csDMARDs, hospitalization related to PSA/AS, patient’s access and mean number of specialist visits and laboratory tests related to the diseases. The health-care cost analysis was conducted with the perspective of the regional health-care service (RHS). The costs are reported in euros (€). Drug costs were evaluated using the RHS purchase price. Hospitalization costs were determined using the DRG (diagnosis-related groups) tariffs, that represent the reimbursement levels of the RHS to health-care providers. The cost of instrumental and laboratory tests was defined according to the tariffs applied by the region.
Statistical Analysis
The analyses were descriptive, no formal statistical comparisons were performed. Continuous variables were reported as mean and standard deviation (±SD), whereas categorical variables were expressed as numbers and percentages. Following the “Opinion 05/2014 on Anonymization Techniques” drafted by the “European Commission Article 29 Working Party”, the analyses involving fewer than three patients were not reported, as potentially reconductable to single individuals. Therefore, results referred to ≤3 patients were reported as NI (not issuable).
All analyses were performed using STATA SE, version 12.0 (StataCorp LLC, College Station, TX, USA).
Results
Two thousand, six hundred and two patients of the Veneto region were included in the study. Of them, 1857 had a diagnosis of PSA, and 745 of AS (Figure 1). Patients were stratified according to biological drug used. Distribution according type of treatment and baseline characteristics are reported in Table 1 for the PSA cohort and in Table 2 for the AS cohort. Adalimumab, etanercept, golimumab, infliximab, and certolizumab belonged to the class of TNF inhibitors, and were prescribed in both cohorts, while IL12/23 inhibitor ustekinumab was analyzed in the PSA cohort only, as it was not indicated for AS.
 |
Table 1 Baseline Characteristics of PSA Patients Stratified for Biological Treatments |
 |
Table 2 Baseline Characteristics of as Patients Stratified for Biological Treatments |
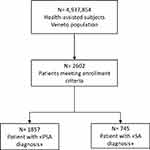 |
Figure 1 Flowchart of included patients. |
PSA Cohort
Among the PSA cohort, patients were treated as follows: 749 (40.3%) with adalimumab, 661 (35.6%) with etanercept, 149 (8.0%) with golimumab, 139 (7.5%) with infliximab, 104 (5.6%) with ustekinumab and 55 (3.0%) with certolizumab. Mean age ±SD ranged from 50.9±9.9 (golimumab) to 54.7±12.0 (etanercept); male proportion ranged from 43.6% (certolizumab) to 68.3% (infliximab). 51% of PSA patients has a co-diagnosis of psoriasis. Patients that presented a concomitant treatment with csDMARD were 58.4% in golimumab, 54.5% in certolizumab, 52.5% in infliximab, 46.2% in the adalimumab, 33.1% in etanercept, and 23.1% in ustekinumab groups (Figure 2A). The proportion of patients for each concomitant csDMARD treatment in the PSA cohort is reported in Supplementary Table S1. Switch to another biological drugs interested 27.3% certolizumab, 16.5% infliximab, 12.8% golimumab, 12.7% of adalimumab,10.6% ustekinumab and 9.4% etanercept treated patients. Days to switch ranged from 163 (etanercept) to 245 (ustekinumab). Among all groups, established patients had a higher percentage of switch than naïve (Figure 3A). Adherence to biological treatment (Figure 4A) was higher among ustekinumab patients (91.3%), followed by certolizumab (80.0%), infliximab (77.0%), golimumab (76.5%), adalimumab (66.4%) and etanercept (54.3%). Percentage of patients persistent to therapy was 70.3% (etanercept), 67.3% (adalimumab), 65.4% (ustekinumab), 64.4% (golimumab), 61.8% (certolizumab), and 53.2% (infliximab) (Figure 4A). Patients that interrupted biological therapy during the follow-up period ranged from 18.2% (certolizumab) to 32.4% (infliximab) (Figure 5A). No hospitalization related to PSA was observed in the certolizumab group, but this result must be interpreted taking into account the low number of patients treated with this biological drug, while the higher percentage (5.8%) was observed in the ustekinumab group (Figure 6A). Regarding health-care resources during follow-up, patients with a specialist visit or laboratory test related to PSA are reported in Figure 7A. The proportion of patients with specialist visits ranged from 7.3% (certolizumab) to 13.6% (etanercept), and the mean number of visits (Figure 8A) ranged from 0.1 (certolizumab and golimumab) to 0.4 (ustekinumab), while the percentage of patients with a laboratory test for PSA ranged from 93.3% (ustekinumab) to 97.3% (golimumab), and the mean number of tests ranged from 4.1 (adalimumab) to 6.0 (golimumab) (Figure 8A). Annual total cost according to type of treatment was: €14,994 (ustekinumab), €12,192 (golimumab), €12,115 (adalimumab), €10,888 (etanercept), €10,178 (infliximab) and €9727 (certolizumab), (Figure 9).
 |
Figure 5 Biological treatment interruptions in PSA (A) and AS (B) cohorts. Abbreviations: Adal, aalimumab; Cert, certolizumab; Etan, etanercept; Goli, golimumab; Infl, infliximab; Ustek, ustekinumab. |
AS Cohort
Among the 745 AS patients included, 339 (45.5%) were treated with adalimumab, 194 (26.0%) with etanercept, 129 (17.3%) with infliximab, 72 (9.7%) with golimumab and 11 (1.5%) with certolizumab. Mean age ranged from 44.9 (adalimumab) to 49.7 (certolizumab). Male percentage ranged from 57.8% (adalimumab) to 72.7% (certolizumab). Co-diagnosis of RA was more frequent among etanercept patients (17.0%), while infliximab group has the higher percentage of gastrointestinal diseases co-diagnosis (35.7%). Proportion of patients with a concomitant csDMARDs treatment (Figure 2B) was: 34.9% (infliximab), 27.3% (certolizumab), 26.5% (adalimumab), 26.4% (golimumab), and 20.1% (etanercept). Proportion of patients for each concomitant csDMARD treatment in AS cohort is reported in Supplementary Table S1. In Figure 3B percentage of patients switching to another biological agents are reported: 27.3% among certolizumab, 9.7% golimumab, 9.3% etanercept, 7.0% infliximab and 5.6% adalimumab groups. Switch for naïve and established patients is also shown. Days to switch ranged from 159 (etanercept) to 271 (certolizumab). Certolizumab and infliximab patients were the most adherent to biological treatment (90.9% and 90.7%, respectively), followed by golimumab (83.3%) and adalimumab (58.7%), while etanercept patients were the less adherent (46.9%) (Figure 4B). Percentage of patients persistent to therapy was 81.8% (certolizumab), 80.6% (golimumab), 76.0% (infliximab), 66.0% (etanercept) and 62.8% (adalimumab), (Figure 4B). Patients that interrupted biological therapy during follow-up period ranged from 12.5% (golimumab) to 33.3% (adalimumab), as displayed in Figure 5B. Similarly to the PSA cohort, the low number of certolizumab patients must be considered to contextualize the absence of hospitalization related to AS observed in this treatment group, while in the other groups it ranged from 2.3% (infliximab) to 2.8% (golimumab) (Figure 6B). Percentage of patients with specialist visits related to AS (Figure 7B) were lower in the certolizumab group (9.1%) and higher among adalimumab patients (12.7%), while the mean number of visits (Figure 8B) was 0.2 for all treatment, except certolizumab (0.1). Concerning laboratory tests (Figure 7B), all patients (100%) treated with certolizumab had a laboratory test, while among all treatments considered, the percentage was lower in the etanercept group (85.1%). Mean number of laboratory tests for AS ranged from 3.8 (etanercept) to 6.0 (infliximab) (Figure 8B). Annual total cost was €12,991 for golimumab, €11,338 for adalimumab, €10,774 for certolizumab, €10,251 for etanercept and €9875 for infliximab treated patients (Figure 10).
Discussion
Over the past decades, biological drugs have outlined a new scenario for the treatment of chronic rheumatic diseases. In this study, the distribution of biological therapies among PSA and AS patients was evaluated in an Italian real-world setting of clinical practice. To the best of our knowledge, no similar analysis was reported before in Italy considering treatment patterns of biological drugs prescribed for PSA and AS patients.
In both cohorts, adalimumab and etanercept were the most prescribed drugs, while certolizumab was the less prescribed. A similar trend was observed in a Sweden study on naïve patients treated with subcutaneous TNF inhibitors, in which adalimumab and etanercept were respectively the first and the second drug most used for both PSA and AS patients, while certolizumab was the latter.27 This profile could be explained by the different years of approval, as while etanercept and adalimumab were approved in the late 1990s and the early 2000s, certolizumab was the latest among TNF inhibitors to be approved for PSA and AS treatment in EU.27,28 Moreover, a study conducted on PSA patients in Germany showed that among biological users (N=1835), the majority of them were treated with adalimumab (N=775) followed by etanercept (N=600), ustekinumab (N=155), golimumab (N=152) and certolizumab (N=32).29 Regarding AS, a similar pattern of use was observed in a US study: patients were treated with adalimumab (44.1%), etanercept (40.9%), infliximab (10.6%), golimumab (4.3%) and certolizumab (0.1%).30
Concomitant csDMARDs treatments were more frequent in PSA than AS patients in all treatment groups considered. According to Ankylosing Spondylitis International Society (ASAS) and EULAR recommendations, there are no evidence to support the obligatory use of csDMARDs for AS management,31 while concomitant csDMARD could be beneficial for PSA patients.12
PSA and AS can share similarities with RA in clinical presentation and manifestation challenging the differential diagnosis;32,33 this could be reflected in the rate of patients with RA diagnosis in both cohorts. Switching among biological drugs is considered a common therapeutic strategy for PSA34 and AS35 patients experiencing treatment failure. Our findings showed that both PSA and AS naïve patients had the lower percentage of switch in all treatment groups. A retrospective US study based on administrative databases was performed considering naïve PSA and AS patients during the first year after initiating TNF inhibitors:36 percentage of switchers among naïve PSA and AS patients was 10% and 8% (etanercept), 10% and 13% (infliximab), respectively and 11% for both (adalimumab). In our cohorts of PSA and AS naïve patients, switchers were 4.7% and 5% for etanercept, 6.4% and 4.8% for adalimumab and 13.3% and 3.8% for infliximab, respectively. Moreover, a different switch pattern was also observed in another US study by Hunter et al,30 in which the percentage of switchers among AS patients newly prescribed TNF inhibitors was >25% for all the treatments considered. In this case, difference of percentages could be due to different lengths of follow-up, as the authors evaluated treatment patterns during the two years after ID while in our study follow-up lasted one year.
Adherence to treatment is of pivotal importance in the context of therapies for rheumatic chronic diseases as PSA and AS, and poor adherence is reported to negatively influence the effectiveness of treatments.18 Adherence rate ranged from 54.3% (etanercept) to 91.3% (ustekinumab) in PSA and between 46.9% (etanercept) and 90.9% (certolizumab) in AS. Anghel et al18 observed a wide variability of adherence rate calculated for PSA and AS, ranging from 38.3% observed in a Czech Republic study37 to 85.2% in a French study,38 both based on self-reported questionnaire of patients.
Persistence to therapy in the PSA cohort was between 53.2% (infliximab) and 70.3% (etanercept). These data were in line with what was reported in a systematic review17 that estimated the overall persistence for PSA patients among all considered study to be 61%. As for AS, persistence to therapy ranged from 62.8% (adalimumab) to 81.8% (certolizumab). A high persistence rate of 80% was also reported by Machado et al during the first year of follow up in AS patients treated with TNF inhibitors with or without concomitant csDMARDs treatment.39 Within each treatment group, the difference observed between the rates of adherence and persistence could be seen also as a consequence of the de-escalation of biological dosages; indeed, evidence of dose tapering strategies for PSA and AS are reported in literature either as dose reduction or increasing in spacing.40 Dose tapering is also reported in AS guidelines,13 while recommendations for dose tapering in PSA patients are awaited with the new guidelines.12
Among treatment groups of PSA and AS cohorts, as expected, costs related to biological drugs have the major impact. However, cost-effectiveness of biological drugs was also demonstrated in Italian studies. In 2008, Olivieri et al23 carried out a cost evaluation study on PSA patients naïve to TNF inhibitors not responding to conventional therapies, comparing costs six months before and after onset of biological drugs, proving that antiTNF therapy was cost-effective in the short-term. We recently evaluated, in a real-world study, the health resources consumption before and after biological treatments in PSA patients: a reduction of costs for hospitalization, specialist outpatient care, and diagnostic procedure related to the disease was observed when biological treatment started.24
Cost for csDMARDs was higher for PSA than for AS patients in all treatment groups, reflecting the lower percentage of concomitant csDMARDs therapy in the AS cohort, while costs for the other health-care resources analyzed were similar.
This study has some limitations mainly due to the type of data sources used and the descriptive nature of the analysis. Administrative database analyses do indeed limit the interpretation of results as they lack data on clinical outcome measures, such as effectiveness of treatment and disease severity, data on comorbidities and other potential confounders that could have influenced our results. Moreover, the mean number of specialist visits could be underestimated as administrative databases contain data on health-care resources reimbursed by INHS and do not track specialist visits in private health settings. The results are retrieved from a single Italian region, therefore further analyses on a larger sample size and on a large sample of Italian regions are needed to generalize our findings.
Conclusion
In this real-world study we reported treatment patterns for PSA and AS patients in Italy, providing detailed analyses on pharmacoutilization and health-care resources consumption for each biological treatment. Our results brought up the high variability between the different available biological therapies and the economic burden of both diseases, therefore they could help to increase knowledge on the management of patients affected by PSA or AS in a real clinical practice setting.
Acknowledgments
The abstract of this paper was presented at the 22nd ISPOR Annual European Congress 2019 in Copenhagen, Denmark as a poster presentation with interim findings. The poster’s abstract was published in Value in Health, 2019; 22: S432. https://doi.org/10.1016/j.jval.2019.09.186.
Funding
This study was funded by Novartis. The views expressed here are those of the authors and not necessarily those of the sponsor.
Disclosure
VP, DS, EG, and LDE report no conflicts of interest in relation to this work and are employees of Clicon S.r.l. Clicon S.r.l. is an independent company. The agreement signed by Clicon S.r.l. and Novartis does not create any entityship, joint venture or any similar relationship between parties. Neither CliCon S.r.l. nor any of their representatives are employees of Novartis for any purpose. DR and MN are employees of Novartis Pharma, Origgio, Italy. AMM and MA received scholarship from Novartis.
References
1. Reveille JD. 57 - Spondyloarthritis. In: Rich RR, Fleisher TA, Shearer WT, Schroeder HW, Frew AJ, Weyand CM, editors. Clinical Immunology (Fifth Edition).
2. Prignano F, Rogai V, Cavallucci E, Bitossi A, Hammen V, Cantini F. Epidemiology of psoriasis and psoriatic arthritis in Italy—a systematic review. Curr Rheumatol Rep. 2018;20(43):1–12. doi:10.1007/s11926-018-0753-1
3. Belasco J, Wei N. Psoriatic arthritis: what is happening at the joint? Rheumatol Ther. 2019;6(3):305–315. doi:10.1007/s40744-019-0159-1
4. Coates LC, Helliwell PS. Psoriatic arthritis: state of the art review. Clin Med. 2017;17(1):65–70. doi:10.7861/clinmedicine.17-1-65
5. Hoover KB. Chapter 33. Psoriathic Arthritis. In: Musculoskeletal Imaging. Volume 1, Rotations in Radiology; Oxford University Press 2019.
6. Cimmino MA, Zampogna A, Murroni S, et al. Methodology of an epidemiologic prevalence study in rheumatology: the chiavari study. Reumatismo. 2002;54(1):40–47. doi:10.4081/reumatismo.2002.40
7. De Angelis R, Salaffi F, Grassi W. Prevalence of spondyloarthropathies in an Italian population sample: a regional community-based study. Scand J Rheumatol. 2007;36(1):14–21. doi:10.1080/03009740600904243
8. Shahinyan A, Margaryan L, Samuel S. Ankylosing spondylitis. In: Abd-Elsayed A, editor. Pain. Cham: Springer International Publishing; 2019:1239–1241.
9. Łosińska K, Korkosz M, Kwaśny-Krochin B. Endothelial dysfunction in patients with ankylosing spondylitis. Reumatologia. 2019;57(2):100–105. doi:10.5114/reum.2019.84815
10. Sarzi-Puttini P, Ceribelli A, Marotto D, Batticciotto A, Atzeni F. Systemic rheumatic diseases: from biological agents to small molecules. Autoimmun Rev. 2019;18(6):583–592. doi:10.1016/j.autrev.2018.12.009
11. So A, Inman RD. An overview of biologic disease-modifying antirheumatic drugs in axial spondyloarthritis and psoriatic arthritis. Best Pract Res Clin Rheumatol. 2018;32(3):453–471. doi:10.1016/j.berh.2018.12.002
12. Gossec L, Smolen JS, Ramiro S, et al. European League Against Rheumatism (EULAR) recommendations for the management of psoriatic arthritis with pharmacological therapies: 2015 update. Ann Rheum Dis. 2016;75(3):499–510. doi:10.1136/annrheumdis-2015-208337
13. van der Heijde D, Ramiro S, Landewé R, et al. 2016 update of the ASAS-EULAR management recommendations for axial spondyloarthritis. Ann Rheum Dis. 2017;76(6):978–991. doi:10.1136/annrheumdis-2016-210770
14. D’Angelo S, Tramontano G, Gilio M, Leccese P, Olivieri I. Review of the treatment of psoriatic arthritis with biological agents: choice of drug for initial therapy and switch therapy for non-responders. Open Access Rheumatol. 2017;9:21–28. doi:10.2147/OARRR.S56073
15. Conti F, Ceccarelli F, Marocchi E, et al. Switching tumour necrosis factor antagonists in patients with ankylosing spondylitis and psoriatic arthritis: an observational study over a 5-year period. Ann Rheum Dis. 2007;66(10):1393–1397. doi:10.1136/ard.2007.073569
16. Merola JF, Lockshin B, Mody EA. Switching biologics in the treatment of psoriatic arthritis. Semin Arthritis Rheum. 2017;47(1):29–37. doi:10.1016/j.semarthrit.2017.02.001
17. Murage M, Tongbram V, Feldman S, et al. Medication adherence and persistence in patients with rheumatoid arthritis, psoriasis, and psoriatic arthritis: a systematic literature review. PPA. 2018;12:1483–1503. doi:10.2147/PPA.S167508
18. Anghel L-A, Farcaş A, Oprean R. Medication adherence and persistence in patients with autoimmune rheumatic diseases: a narrative review. PPA. 2018;12:1151–1166. doi:10.2147/PPA.S165101
19. Clegg DO. Treatment of ankylosing spondylitis. J Rheumatol Suppl. 2006;78:24–31.
20. Chen M-H, Lee M-H, Liao H-T, Chen W-S, Lai -C-C, Tsai C-Y. Health-related quality of life outcomes in patients with rheumatoid arthritis and ankylosing spondylitis after tapering biologic treatment. Clin Rheumatol. 2018;37(2):429–438. doi:10.1007/s10067-017-3965-2
21. D’Angiolella LS, Cortesi PA, Lafranconi A, et al. Cost and cost effectiveness of treatments for psoriatic arthritis: a systematic literature review. PharmacoEconomics. 2018;36(5):567–589. doi:10.1007/s40273-018-0618-5
22. Lubrano E, Spadaro A. Pharmacoeconomic burden in the treatment of psoriatic arthritis: from systematic reviews to real clinical practice studies. BMC Musculoskelet Disord. 2014;15:25. doi:10.1186/1471-2474-15-25
23. Olivieri I, de Portu S, Salvarani C, et al. The psoriatic arthritis cost evaluation study: a cost-of-illness study on tumour necrosis factor inhibitors in psoriatic arthritis patients with inadequate response to conventional therapy. Rheumatology. 2008;47(11):1664–1670. doi:10.1093/rheumatology/ken320
24. Degli Esposti L, Perrone V, Sangiorgi D, et al. Analysis of drug utilization and health care resource consumption in patients with psoriasis and psoriatic arthritis before and after treatment with biological therapies. BTT. 2018;12:151–158. doi:10.2147/BTT.S168691
25. Mennini FS, Viti R, Marcellusi A, Sciattella P, Viapiana O, Rossini M. Economic evaluation of spondyloarthritis: economic impact of diagnostic delay in Italy. Clinicoecon Outcomes Res. 2018;10:45–51. doi:10.2147/CEOR.S144209
26. Agenzia Italiana del Farmaco (AIFA). Guideline for the classification and conduction of the observational studies on medicines; 2010. Available from: https://www.agenziafarmaco.gov.it/ricclin/sites/default/files/files_ wysiwyg/files/CIRCULARS/Circular%2031st%20May%202010.pdf.
27. Dalén J, Svedbom A, Black CM, et al. Treatment persistence among patients with immune-mediated rheumatic disease newly treated with subcutaneous TNF-alpha inhibitors and costs associated with non-persistence. Rheumatol Int. 2016;36(7):987–995. doi:10.1007/s00296-016-3423-5
28. Marin J, Acosta Felquer ML, Soriano ER. Spotlight on certolizumab pegol in the treatment of axial spondyloarthritis: efficacy, safety and place in therapy. Open Access Rheumatol. 2018;10:33–41. doi:10.2147/OARRR.S116654
29. Sondermann W, Ventzke J, Matusiewicz D, Körber A. Analysis of pharmaceutical care in patients with psoriatic arthritis using statutory health insurance data: health care study of psoriatic arthritis using statutory health insurance data. J Dtsch Dermatol Ges. 2018;16(3):285–294.
30. Hunter T, Schroeder K, Sandoval D, Deodhar A. Persistence, discontinuation, and switching patterns of newly initiated TNF inhibitor therapy in ankylosing spondylitis patients in the United States. Rheumatol Ther. 2019;6(2):207–215. doi:10.1007/s40744-019-0148-4
31. Braun J, van den Berg R, Baraliakos X, et al. 2010 update of the ASAS/EULAR recommendations for the management of ankylosing spondylitis. Ann Rheum Dis. 2011;70(6):896–904. doi:10.1136/ard.2011.151027
32. Merola JF, Espinoza LR, Fleischmann R. Distinguishing rheumatoid arthritis from psoriatic arthritis. RMD Open. 2018;4(2):e000656. doi:10.1136/rmdopen-2018-000656
33. Barczyńska TA, Węgierska M, Żuchowski P, et al. Coexistence of rheumatoid arthritis and ankylosing spondylitis. Reumatologia. 2015;53(5):279–285. doi:10.5114/reum.2015.55832
34. Costa L, Perricone C, Chimenti MS, et al. Switching between biological treatments in psoriatic arthritis: a review of the evidence. Drugs R D. 2017;17(4):509–522. doi:10.1007/s40268-017-0215-7
35. Navarro-Compán V, Plasencia-Rodríguez C, de Miguel E, Diaz Del Campo P, Balsa A, Gratacós J. Switching biological disease-modifying antirheumatic drugs in patients with axial spondyloarthritis: results from a systematic literature review. RMD Open. 2017;3(2):e000524. doi:10.1136/rmdopen-2017-000524
36. Bonafede M, Fox KM, Watson C, Princic N, Gandra SR. Treatment patterns in the first year after initiating tumor necrosis factor blockers in real-world settings. Adv Ther. 2012;29(8):664–674. doi:10.1007/s12325-012-0037-5
37. Hromadkova L, Soukup T, Vlcek J. Quality of life and drug compliance: their interrelationship in rheumatic patients: qoL and drug compliance in rheumatic patients. J Eval Clin Pract. 2015;21(5):919–924. doi:10.1111/jep.12399
38. Betegnie A-L, Gauchet A, Lehmann A, et al. Why do patients with chronic inflammatory rheumatic diseases discontinue their biologics? An assessment of patients’ adherence using a self-report questionnaire. J Rheumatol. 2016;43(4):724–730.
39. de Á Machado MA, de Moura CS, Ferré F, Bernatsky S, Rahme E, de a Acurcio F. Treatment persistence in patients with rheumatoid arthritis and ankylosing spondylitis. Rev Saude Publica. 2016;50:50.
40. Edwards CJ, Fautrel B, Schulze-Koops H, Huizinga TWJ, Kruger K. Dosing down with biologic therapies: a systematic review and clinicians’ perspective. Rheumatology. 2017;56(11):1847–1856. doi:10.1093/rheumatology/kew464
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.








