Back to Journals » Research and Reports in Urology » Volume 13
Treatment of Urethral Stricture Disease in Women: Nonsystematic Review of Surgical Techniques and Intraoperative Considerations
Authors Chua KJ, Mikhail M , Patel HV, Tabakin AL, Doppalapudi SK, Sterling J, Tunuguntla HSGR
Received 11 March 2021
Accepted for publication 2 May 2021
Published 21 June 2021 Volume 2021:13 Pages 381—406
DOI https://doi.org/10.2147/RRU.S282651
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Jan Colli
Kevin J Chua, Mark Mikhail, Hiren V Patel, Alexandra L Tabakin, Sai Krishnaraya Doppalapudi, Joshua Sterling, Hari SGR Tunuguntla
Division of Urology, Department of Surgery, Rutgers, The State University of New Jersey, Robert Wood Johnson Medical School, New Brunswick, NJ, 08901, USA
Correspondence: Hari SGR Tunuguntla
Rutgers, The State University of New Jersey, Robert Wood Johnson Medical School, Division of Urology, Department of Surgery, Medical Education Building, Suite 584-B, 1, RWJ Place, New Brunswick, NJ, 08901, USA
Email [email protected]
Abstract: Female urethral strictures are rare, but underdiagnosed pathologies that can cause voiding dysfunction. These strictures are best managed with open reconstruction, as endoscopic treatments have high rates of failure. A flap urethroplasty can be performed with vaginal, labial or bladder tissue. Meanwhile, graft urethroplasties can utilize vaginal, labial, buccal or lingual tissue. It is important to consider the etiology and type of stricture, local vascularity, and prior attempts at repair when selecting the type of repair. Multiple different techniques have been described with theoretical advantages to each one. While some studies have reviewed a few of the reconstructive techniques to treat female urethral strictures, no single study has accounted for each individual technique. In this review, we discuss techniques captured by a number of systematic reviews and other articles. We will herein focus on reviewing and describing each unique technique of reconstruction in the setting of female urethral stricture.
Keywords: urethral stricture, urethral obstruction
Introduction
Urethral stricture refers to scarring of urethral epithelium with or without spongiofibrosis resulting in a narrowed urethral lumen.1 They can result from any process that causes injury to urethral epithelium which can then lead to scarring during the healing process of the injury and subsequent formation of a stricture.1 Urethral strictures predominantly occur in males causing obstructive voiding symptoms. However, while female urethral strictures (FUS) are rare, they can lead to equally bothersome symptoms. 2.7% to 8% of women with lower urinary tract symptoms are found to have bladder outlet obstruction, and 4 to 13% of these cases have been attributed to FUS.2,3 Taken together, FUS affect 0.1% to 1% of women with voiding complaints.2,3
There are multiple causes for these strictures in women. In a systematic review of FUS, idiopathic cause was the most common, accounting for 51.3% of cases, while iatrogenic accounted for 32.8% of cases. 8% were related to infection and inflammation and 6.6% related to trauma.3 Iatrogenic injury includes anterior vaginal or urethral surgery, urethral catheterization and irradiation for pelvic malignancies.2 Surgeries can include transurethral operations and open procedures such as excision of a synthetic midurethral sling or a diverticulectomy.4 Traumatic urethral injuries often result from obstetric complications such as an obstructed labor or from pelvic fractures which are more common in younger females.
Symptoms of urethral stricture are variable and include urinary hesitancy, poor flow, increased frequency, urgency, dysuria, recurrent urinary tract infections and urinary retention.5 Given the rarity and nonspecific presentation, FUS can often be overlooked. While a comprehensive history and physical exam can raise one’s suspicion, more definitive tests include urethroscopic evaluation, VCUG and video urodynamics. Meanwhile other supplementary tests include maximum urinary flow rate, post void residual, MRI, CT, micturating cystourethrogram, video cystometrogram and retrograde urethrogram.3
Treatment options include endoscopic treatment or open reconstruction. While urethral dilation and internal urethrotomy are less invasive, they have a much higher failure rate compared to open reconstruction. Urethroplasties can be done with a flap or graft. Flaps can utilize vaginal, labial or bladder mucosa. Meanwhile, grafts can use vaginal, labial, buccal, or lingual mucosa. In a retrospective study using the National Inpatient Sample, 10.2% of male urethral strictures were repaired with urethral reconstruction while only 3.4% of strictures in women were repaired with reconstruction, suggesting that urethral reconstruction of female urethras may be underperformed.6
It is important to consider the etiology and type of stricture, local vascularity, and prior attempts at repair in selecting the type of repair. Multiple different techniques have been described with theoretical advantages to each one. While some studies have reviewed a few of the reconstructive techniques described to treat FUS, no single study has accounted for each individual technique.2,7,8 This article will focus on reviewing and describing the different technique for FUS repair.
Methods
A literature search using PubMed and Google Scholar search was done to perform a comprehensive non-systematic review of articles published between January 1995 and December 2020 using the search terms female urethral stricture, female urethral surgery, female urethroplasty, female urethral reconstruction, urethral reconstruction in women, and urethroplasty in women. Articles selected were required to be original articles written in English. Systematic reviews, original articles, and case reports/series were included. Commentaries and news articles were excluded. The studies were independently reviewed. References of papers were reviewed for potential missed studies. All articles identified in previous systematic reviews (Osman 2013 et al and Sarin 2020 et al) were included.3,9
Studies reviewed are included in (Table 1, Table 2, Table 3) along with techniques used, success rates, location of strictures, follow up times, rate of new onset incontinence and of concomitant procedures. Each individual technique identified is described. A FUS treatment flowchart based on studies reviewed is in included in Figure 1A and B.
 |
Table 1 Summary of Studies Utilizing Endoscopic Procedures to Treat Female Urethral Strictures |
 |
Figure 1 (A) Algorithm for FUS repair. (B) Special considerations of FUS repair. |
Surgical Anatomy of Female Urethra
The female urethra is approximately 4 cm long and 6 mm in diameter.10 It is lined by an outer circular muscle layer and an inner longitudinal mucosal layer lining the lumen, which is also continuous with the bladder mucosa and the internal urethral sphincter.11 The external urethral sphincter is located at the distal two thirds of the urethra and is composed of striated muscle. It has an omega-shape where the skeletal muscle is most deficient at the ventral, 6 o’clock position.8,12
The urethra is embedded in the anterior vaginal wall.10 It courses anteroinferiorly behind the pubic symphysis, crosses the perineal membrane and terminates at the external urethral orifice in the vestibule as an anteroposterior slit. It is supported by the pubourethral ligament and suspensory ligaments of the clitoris. Vasculature primarily is supplied by the vaginal artery, but the urethra also has arterial supply from the inferior vesical artery and the internal pudendal artery. It is also important to note nearby structures such as clitoral tissue lying along the dorsal aspect of the urethra and the neurovascular bundles which traverse along the ischiopubic ramus, under the pubic symphysis, and along the cephalad surface of the clitoral body toward the glans.13,14
Treatment of Female Urethral Stricture
FUS may be treated endoscopically or with open reconstruction of the urethra. Greater success is associated with open reconstruction. A summary of reviewed studies, their associated techniques and success rates can be seen in Tables 1–4. A flowchart of FUS treatment decision making based on the articles reviewed can be seen in Figure 1A and B.
 |
Table 2 Summary of Studies Utilizing Flap Urethroplasty to Treat Female Urethral Strictures |
 |
Table 3 Summary of Studies Utilizing Vaginal/Labial Graft Urethroplasty to Treat Female Urethral Strictures |
 |
Table 4 Summary of Studies Utilizing Oral Graft Urethroplasty to Treat Female Urethral Strictures |
Endoscopic (Dilation and Internal Urethrotomy)
Endoscopic procedures provide a minimally invasive method of treatment with a few serious complications.15 Long term success rates are generally lower for endoscopic management than urethral reconstruction for FUS. Endoscopically, strictures may be treated with serial urethral dilation or internal urethrotomy. One recent systematic review by Sarin et al reports endoscopic treatment success rate as 49%.3,9,16 There is a lack of data of urethrotomy for FUS, which is likely due to the increased risk of sphincter compromise and new onset incontinence.17,18 One contemporary retrospective analysis in 2019 by Sharifian et al reviewed 23 patients who had an internal urethrotomy with a 66% success rate and a rate of new onset stress urinary incontinence (SUI) of 9.5%.19
In cases of completely occluded, yet short urethral strictures, a “cut to the light” procedure can be performed, which requires antegrade placement of a flexible cystoscope to aid in identifying the obliterated urethra.2,20 Meanwhile for urethral dilation, there is no specific recommendation for dilation calibration size as studies report on dilation from 24F to 41F with similar results. Romman et al performed a retrospective review of 93 patients who underwent sequential urethral dilation from 25F to 41F with a mean follow up of 46 months and a success rate of 51%.15 However, in patients with previous dilation, success decreased to 30.8%. Of note, tearing of the urethral meatus sometimes occurred and was repaired with interrupted 4–0 absorbable sutures placed transversely. Heidari et al performed a review of 86 patients without previous dilation at a single center demonstrating 100% success rate with dilation to 24 French; however the study had a short follow up time with a mean of 6 months.21 These results demonstrate that urethral dilation has potential to treat urethral strictures with low complication rates; however, repeat endoscopic treatment have higher failure rates.
Open Urethral Reconstruction Surgical Considerations
Open urethral reconstruction of FUS is more invasive and technically difficult than endoscopic treatment, but the success rate is much higher. In Sarin et al’s systematic review, the pooled success rate for flap urethroplasty of 108 patients was 92%. For graft urethroplasties, success rates ranged from 86 to 93%.3 There are a variety of techniques described each with theoretic advantages, but given that the sample sizes of studies have continued to be low, no single technique has proven to be superior.
Dorsal vs Ventral Approach
Most approaches are performed dorsally or ventrally. For the dorsal approach, advantages include: 1) increased vasculature and support, which can aid in healing, 2) allowing for a urethral meatus that will be directed upward to avoid an inward urinary stream facing the vagina, and 3) avoidance of anterior vaginal wall manipulation, which can hinder a future anti-incontinence procedure.4,14,22–24 Disadvantages include 1) increased blood loss given increased vasculature near the clitoris, 2) injury to the clitoris or neurovascular bundle which can lead to sexual dysfunction, and 3) increased risk of incontinence as there is risk to injuring supporting pubourethral ligaments and the dorsal muscular fibers of the external urethral sphincter.4,14,22–24 The converse is true of the ventral approach. Advantages include 1) decreased risk of incontinence, as the skeletal muscle of the external urethral sphincter is most deficient ventrally and 2) avoidance of the clitoris and neurovascular bundle, which therefore decreases risk of blood loss and sexual dysfunction.4,23,24 Disadvantages include 1) formation of a hypospadic urethra and 2) manipulation to the anterior vaginal wall, which can make an anti-incontinence procedure more difficult.4,25,26
While these complications are possible, they have not been well demonstrated across studies. Furthermore, techniques have been adjusted to account for them. For instance, an inward stream towards the vagina more often occurs during a ventral approach of a flap urethroplasty. Simonato et al and Romero-Maroto et al made modifications to the ventral technique to create an orthotopic meatus to avoid this issue.25,26 Additionally, Onol et al notes that the meatus can be reconstructed in the ventral approach so that the stream of urine can be directed away from the vagina.4 The risk of sexual dysfunction with the dorsal approach has not been well documented in studies.13 Given the course of the neurovascular bundle running on the cephalad surface of the clitoral bodies, dissection should be carried out away from this region.14 In a study by Manasa et al examining FSFI scores in patients before and after undergoing a dorsal onlay vaginal graft urethroplasty, 12 of 13 patients reported an improvement in sexual function with a mean follow up of 8.5 months.27
Tissue Type
Flaps used for FUS repair include vaginal, labial or bladder mucosa, whereas, graft repairs utilize vaginal, labial, buccal or lingual mucosa. Vaginal and labial tissue both are forms of hairless, elastic wet tissue with good vascular supply.28,29 Their proximity to the recipient site allows for easier healing and minimal scar formation. Using vaginal or labial tissue also avoids intra-oral complications that may occur with oral grafts.24 Additionally, labial tissue is advantageous as it can be used in the setting of vaginal scarring or injury.28,29 Bladder flaps are well vascularized and allow continence to be maintained given its detrusor tone and the presence of alpha-adrenergic receptors.20,30,31 However, repair with a bladder flap requires a more invasive suprapubic approach and has rarely been used in the setting of urethral stricture alone.20,31
Buccal mucosa and lingual mucosa are also useful given that they are wet, hairless tissue that can be used in the setting of scarring and destruction of vaginal or labial tissue.32 However, complications such as mental nerve injury, parotid gland injury, and other neurosensory complications of the oral cavity have been reported. Usage of lingual grafts helps avoid these injuries given that their location is away from the mental nerve and parotid gland.32
Martius, Gracilis, and Rectus Muscle Flaps
The use of Martius flaps for urethroplasty have been described by a few authors to reduce fistula formation, improve vasculature and provide healthy tissue between the urethra and vagina for an anti-incontinence procedure if the urethroplasty was performed ventrally.2,4,33–35 Martius flaps are particularly useful if there is inadequate periurethral tissue upon closure. Gracilis muscle flaps are useful if a wider area of coverage is needed. The use of rectus muscle flap has also been described by Xu et al via a transpubic approach.36
Concomitant Anti-Incontinence Procedure
Anti-incontinence procedures may be performed concomitantly for patients with SUI or in those deemed to have a high risk of postoperative incontinence.2,33 For instance, Blaivas et al performed a concomitant pubovaginal sling (PVS) with female urethral reconstruction for those with SUI symptoms, symptoms seen during stress cystogram, or if the sphincteric mechanism was believed to be abnormal at surgery.33 None of the 5 patients who had a PVS placed complained of incontinence post operatively. In a systematic review, 8 out of 108 patients who underwent a flap urethroplasty had a PVS placed concomitantly.3 Additionally, 1 out of 148 patients who underwent a dorsal buccal mucosal graft (BMG) had an anti-incontinence procedure performed. Furthermore, in a multi-institutional study by Lane et al, 4 out of 135 patients undergoing urethral reconstruction had an anti-incontinence procedure performed concomitantly with urethral reconstruction.37 Alternatively, a PVS can be placed post-operatively if symptoms occur. Lane et al reported that 8 out of their 135 patients who underwent urethral reconstruction had de novo SUI and 5 underwent subsequent anti-incontinence surgery.37 Having an informed discussion with the patient is important when deciding to simultaneously perform a PVS. It is also important to note that de novo SUI is rare as only 6% of urethral reconstructions in Lane’s study were complicated by de novo SUI.37
Inlay vs Onlay
Most urethroplasty techniques described in the literature were “onlays” as they were not performed intraurethrally.22 The first reported intraurethral procedures performed for FUS were described by Hoag et al in 2016, and subsequently by Nayak et al and Joshi et al.12,22,38 Advantages of the inlay approach include: 1) avoidance of dorsal urethral mobilization which can reduce postoperative pain, 2) avoidance of dissection and possible disruption to the nerve and vascular supply of the clitoris which can decrease blood loss and postop sexual dysfunction, 3) avoidance of a vaginal incision which can reduce both pain and risk of a urethrovaginal or vesicovaginal fistula, 4) avoidance of dissection of anterior vaginal wall which then mirrors the benefit of the dorsal onlay technique by protecting this tissue for future anti-incontinence procedures, and 5) avoidance of division of pubourethral ligaments.12,22
Choice of Repair According to the Length and Location of the Stricture
There are currently no guidelines on how to treat FUS in regards to length and location despite the multitude of techniques that have been described. Few suggestions have been made in the literature. Meatoplasties can be appropriate for strictures involving the distal few millimeters of the urethra where less complex reconstruction is required.5 Per Nitti, the Blandy ventral vaginal flap urethroplasty is also appropriate for distal urethral strictures up to 2cm from the meatus.11 Meanwhile for mid and proximal urethral strictures, options include the U-shaped ventral vaginal flap urethroplasty described by Palou, a tubularized vaginal flap urethroplasty and a graft urethroplasty.11 However, it is important to note that each reconstructive technique has had reported successes in each location of the urethra (Tables 1–4). Further research with larger sample sizes are needed to better identify if certain techniques have advantages depending on length and location of the stricture.
Open Urethral Reconstruction – Flap Urethroplasty Techniques
U-Shaped Ventral Vaginal Flap Urethroplasty (Blandy)
The U-Shaped Inlay Vaginal Flap Urethroplasty was first described by Blandy, but first reported by Schwender et al in 2006.39,40 It is one of the most common techniques in literature that has been used to treat FUS.3,4,33,40–42 A U-shaped vaginal flap is outlined on the anterior vaginal wall with the apex of the U at the urethral meatus and a plane is dissected between the vaginal mucosa and periurethral tissue (Figure 2). A nasal speculum is inserted into the urethral meatus and the strictured area is incised posteriorly at 6 o’clock. The vaginal flap is mobilized so that the apex of the U-shaped vaginal flap is approximated to the apex of the incision in the urethral mucosa and sutured into place. Authors have noted that this technique can lead to a hypospadic urethra leading to an inward, splaying urinary stream.4,25,26 Onol et al notes that 2 of his patients had an inward urinary stream which improved after 6 months, and that in subsequent cases he was able to reconstruct the urethra to direct the urinary stream away from the vagina successfully.
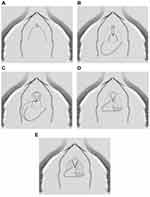 |
Figure 2 Anterior vaginal wall flap (“Blandy flap”). (A) Inverted U-incision. (B) Ventral stricturotomy; (C and D) Suturing the tip of the U-flap to the proximal part of the opened urethra. (E) Further suturing the edges of the flap to the urethral edges. Reprinted from Waterloos M, Verla W. Female Urethroplasty: A Practical Guide Emphasizing Diagnosis and Surgical Treatment of Female Urethral Stricture Disease. BioMed Research International. 2019;2019:6,715,257. Copyright © 2019 Marjan Waterloos and Wesley Verla. This is an open access article distributed under the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0/).8 |
U-Shaped Ventral Vaginal Flap Urethroplasty (Palou)
This method was first described by Palou in 1996 in a case report for a female patient with a distal urethral stricture. It was re-described by Rosenblum in 2011 and Faiena in 2016.2,7,11 While this specific technique has not been used in other case series for treatment of urethral strictures, the technique is similar to the Blandy technique. However, instead of the apex of the U-shaped vaginal flap being made at the urethral meatus, the apex of the U is carried out in the anterior vaginal mucosa while the base is at the urethral meatus (Figure 3). After incision of the stricture, the flap is then flipped upwards and sewn to the edges of the open posterior urethra using absorbable sutures. The anterior vaginal wall is closed primarily.
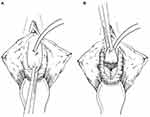 |
Figure 3 U-Shaped Ventral Vaginal Flap Urethroplasty (Palou). (A) Vaginal flap urethroplasty. A U-shaped incision of the anterior vaginal wall is performed. (B) The vaginal wall flap is transposed to the shortened or damaged urethra by tubularization or as a posterior plate of tissue. Reprinted from Rosenblum N, Nitti VW. Female urethral reconstruction. Urol Clin North Am. Feb 2011;38(1):55–64, vi. © 2011 Elsevier Inc. Published by Elsevier Inc. All rights reserved.7 |
C-Shaped Ventral Vaginal Flap Urethroplasty
Simonato et al performed a variation of the ventral vaginal flap urethroplasty.26 Instead of a U-shaped flap, a C-shaped flap is created. This technique was inspired by the Orandi technique performed in males. A C-shaped vaginal flap is outlined on the anterior vaginal wall, approximately 2 to 3 cm long and 3 to 4 cm wide (Figure 4). The strictured urethra is incised at the 6 o’clock position. The vaginal tissue flap is mobilized with a lateral vascular pedicle and it is partially de-epithelialized medially; doing so favors tissue scar formation where the sutures are placed and prevents fistula formation. The distal end of the flap is sutured to the open urethral edge on the left using 4–0 absorbable sutures. The proximal portion of the flap is sutured to the right side of the open urethra (Figures 4 and 5). The margins of the proximal epithelialized flap are approximated and sutured to the vaginal wall. Theoretical advantages include a vascular flap that is anatomical, improved prevention of fistula formation by staggering and protecting sutures, compression of the dorsal urethral wall which can aid in continence and decreasing infection, and a urethral meatus that remains in an orthotopic and collapsed position.
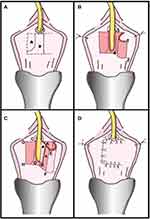 |
Figure 4 C-Shaped Ventral Vaginal Flap Urethroplasty. (A) Incision margin on anterior vaginal wall (dotted line), mucous membrane to be de-epithelialized (asterisk) and area that must remain intact (triangle). (B) Isolation of vaginal mucous flap of ventral urethral part and subsequent lengthwise incision of stenotic urethra. Most distal flap portion 1–2 is sutured to left side of open urethra 3–4. (C) Suture of distal flap margin to urethral edge closest to vascularized flap pedicle. De-epithelization of area on flap (asterisk) favors tissue cicatrix formation. More proximal portion of flap A–B is sutured to C–D on right side of open urethra. Flap distal end is folded over so that vaginal mucous membranes become urethral lumen (triangle). (D) Suture of edge of flap A–B to edge of urethra C–D and suture of E–F edge to G–H edge. Reprinted from Simonato A, Varca V, Esposito M, Carmignani G. Vaginal Flap Urethroplasty for Wide Female Stricture Disease. Journal of Urology. 2010;184(4):1381–1385. Copyright © 2010, Wolters Kluwer Health.26 |
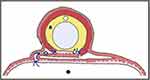 |
Figure 5 Suture placement of C-Shaped Ventral Vaginal Flap Urethroplasty. Urethroplasty cross-section shows how suture lines (blue lines) are well protected by at least 1 layer of vaginal lumen mucous membrane. Closure of wound in vaginal lumen (arrow) efficiently tautens urethroplasty sutures. Asterisk indicates de-epithelialized mucous membrane. Triangle indicates vaginal mucous membrane (pink) used to reconstruct urethra. Pound sign indicates bladder catheter. Black circle represents vaginal lumen. Reprinted from Simonato A, Varca V, Esposito M, Carmignani G. Vaginal Flap Urethroplasty for Wide Female Stricture Disease. Journal of Urology. 2010;184(4):1381–1385. Copyright © 2010, Wolters Kluwer Health.26 |
Ventral Lateral Based Anterior Vaginal Wall Flap Urethroplasty
Romero-Maroto et al described a ventral lateral-based anterior vaginal wall flap urethroplasty inspired by the Orandi technique.25 Romero et al highlights that this technique allows for a ventral approach while maintaining the orthotopic position of the meatus with no effect on the direction of the urinary stream. An anterior vaginal wall incision is made (Figure 6). The vaginal wall is then mobilized, and the side where the flap is chosen to be taken is dissected as to preserve as much vascular supply as possible. The urethra is incised ventrally from the meatus and across the full length of the stricture. A rectangular piece of vaginal wall, selected based on the stricture’s length and caliber, is mobilized with a wide axial vascular pedicle. The inner vaginal flap is sutured to the closest urethral margin while the outer vaginal edge is sutured to the contralateral edge with 3–0 absorbable suture. The mucosal surface should be facing the urethral lumen. The meatus is closed in the orthotopic position, and the vaginal mucosa is closed with 2–0 absorbable sutures.
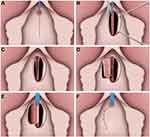 |
Figure 6 Ventral Lateral Based Anterior Vaginal Wall Flap Urethroplasty. (A) Midline anterior vaginal wall incision. (B) After mobilization of anterior vaginal wall, the urethra is incised ventrally from the meatus to the point where the stricture is completely open. (C) A rectangular-shaped piece of vaginal wall is then selected and the outer external flap border is mobilized with a wide vascular pedicle. (D) The vaginal flap is sutured to the margins of the urethrotomy defect. The inner vaginal flap edge to the closer urethral margin. (E) The outer vaginal flap edge is turned around and sutured to the contralateral edge. (F) The vaginal mucosa has been approximated. Reprinted from Romero-Maroto J, Verdú-Verdú L, Gómez-Pérez L, Pérez-Tomás C, Pacheco-Bru JJ, López-López A. Lateral-based Anterior Vaginal Wall Flap in the Treatment of Female Urethral Stricture: Efficacy and Safety. Eur Urol. 2018;73(1):123–128. © 2016 European Association of Urology. Published by Elsevier B.V. All rights reserved.25 |
Vaginal Wall Urethroplasty/Tubularized Vaginal Flap Urethroplasty
Tubularized vaginal flap urethroplasty or vaginal wall urethroplasty was described originally by Flisser.43 While this is not a technique commonly described in case series treating FUS, it was described in reviews by Rosenblum et al and Faiena et al Additionally, it is effective even in the setting of extensive scarring or destruction of the anterior vaginal wall.2,7,11,43
Vaginal flaps are created using local periurethral vaginal tissue (Figure 7).2,7 Two parallel incisions are made at the lateral vaginal walls.43 Lateral to medial mobilization of the vaginal tissue flaps allows for tubularization over the catheter. If lateral tissue is insufficient, a U-shaped anterior vaginal wall incision can be made and rotated anteriorly to create the posterior wall of the neourethra. If there is extensive scarring of the anterior vaginal wall, an oval shaped incision at the labia minora can be made as the base of a rotational flap which can be used as a neourethra (Figure 8). Any defects in the vaginal wall created by the flap can be closed primarily or using rotational flaps of adjacent vaginal wall or labia minora. Furthermore, a Martius fat pad flap or a gracilis muscle flap can be harvested if wider coverage is needed.2,43
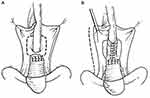 |
Figure 7 Vaginal Wall Urethroplasty/Tubularized Vaginal Flap Urethroplasty. Vaginal Flap Urethral Repair (A) 2 parallel incisions are made on either side of urethra and inverted “U” incision is made just cephalad to urethral defect. (B) Resulting flaps are sutured in place around catheter using 3 or 4-zero chromic catgut. Reprinted from Flisser AJ, Blaivas JG. Outcome of urethral reconstructive surgery in a series of 74 women. J Urol. 2003;169(6):2246–9. Copyright © 2018, Wolters Kluwer Health.43 |
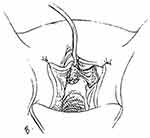 |
Figure 8 Labia Minora Flap as Base of Tubularized Flap Urethroplasty. Incision in labia minora frees flap of tissue that is rotated into neourethra. Reprinted from Flisser AJ, Blaivas JG. Outcome of urethral reconstructive surgery in a series of 74 women. J Urol. 2003;169(6):2246–9. Copyright © 2018, Wolters Kluwer Health.43 |
Lateral Vestibular Flap Urethroplasty
Romman et al described a lateral vestibular flap.44 This new technique was created to prevent the shorter hypospadic urethra associated with a ventral flap while avoiding injury at the 12 o’clock position which theoretically can lead to effects on sexual function. This repair also employs a broad based vascular flap to improve distal viability. In the technique, a broad based vestibular flap lateral to the meatus is marked out to create an approximately 15 mm to 20 mm wide flap (Figure 9). Absorbable sutures are placed on the medial edge of the flap for traction, and the flap is undermined over 3 to 4 cm. A 10 mm wedge of the distal lateral scarred urethral wall is excised. The superior portion of the flap is sutured to the upper urethral wall edge. A second suture is passed through the inferior portion of the flap and then through the inferior urethral wall edge, which rotates the flap inward and rebuilds the distal urethral wall. The flap is subsequently secured to the adjacent vestibular tissue.
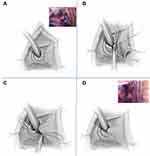 |
Figure 9 Lateral Vestibular Flap Urethroplasty. (A) Outline of the vestibular flap from the left labia minora. (B) Excision of left distal urethral wall between 3 and 5 o’clock. (C) The labia minora flap is rotated inward to prevent recurrence of the distal urethral scar. (D) Final appearance. Reprinted from Romman A, Takacs L, Gilleran J, Zimmern P. Vestibular flap urethroplasty in women with recurrent distal intramural urethral pathology. Neurourol Urodyn. 2015;34(3):213–8. © 2014 Wiley Periodicals, Inc.44 |
Dorsal Vestibular Flap Urethroplasty
Montorsi et al described a dorsal vestibular flap.45 In this approach, a suprameatal inverted Y-shaped incision is performed (Figure 10). The distal 3 cm of urethra is dissected free from surrounding tissue from the 9 o’clock to 3 o’clock position superiorly, after which the 12 o’clock position is cut with sharp scissors (Figure 11). A 1 to 3 cm long flap is developed from the lateral vaginal vestibule, the distal tip of which is anastomosed to the proximal end of the urethra using interrupted absorbable sutures, allowing the mucosal surface to face toward the lumen (Figure 12). Each lateral margin of the flap is sutured to the corresponding margin of the open urethra with a running 5–0 absorbable suture. The lateral periurethral incisions are closed with running 5–0 absorbable sutures.
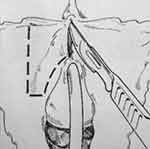 |
Figure 10 Dorsal Vestibular Flap Urethroplasty. With the Labia minora wide open, the skin incision is traced with a marker pen. Note the inverted-Y incision to dissect the urethra and the vestibular flap line of incision. Reprinted with permission from Montorsi F, Salonia A, Centemero A, et al Vestibular flap urethroplasty for strictures of the female urethra. Impact on symptoms and flow patterns. Urol Int. 2002;69(1):12–6. Copyright © 2002 Karger Publishers, Basel, Switzerland.45 |
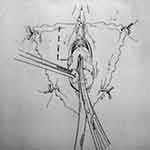 |
Figure 11 Dorsal Vestibular Flap Urethroplasty. The distal 3 cm of the urethra are dissected free from surrounding tissues from the 9- to the 3-o’clock positions and the dissected urethra is then incised longitudinally with scissors at the 12-o’clock position. Reprinted with permission from Montorsi F, Salonia A, Centemero A, et al Vestibular flap urethroplasty for strictures of the female urethra. Impact on symptoms and flow patterns. Urol Int. 2002;69(1):12–6. Copyright © 2002 Karger Publishers, Basel, Switzerland.45 |
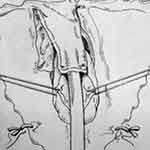 |
Figure 12 Dorsal Vestibular Flap Urethroplasty. The lateral periurethral incisions are closed with running sutures. Reprinted with permission from Montorsi F, Salonia A, Centemero A, et al Vestibular flap urethroplasty for strictures of the female urethra. Impact on symptoms and flow patterns. Urol Int. 2002;69(1):12–6. Copyright © 2002 Karger Publishers, Basel, Switzerland.45 |
Ventral Labial Flap Urethroplasty
Tanello et al described a ventral labial flap urethroplasty.29 After dissection of the vagina from periurethral tissue, an incision through the stricture is made in the ventral urethral wall until healthy mucosa is reached. A flap of labia is marked out based on the size of the stricture, mobilized on its vascular pedicle, and tunneled below the vulvovaginal wall (Figures 13 and 14). The epidermal surface of the flap is positioned over the urethral lumen and sutured to both sides of the open urethral margins in a continuous manner (Figure 15).
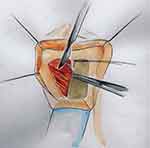 |
Figure 13 Ventral Labial Flap Urethroplasty. The flap is mobilized on its vascular pedicle. Reprinted with permission from Tanello M, Frego E, Simeone C, Cosciani Cunico S. Use of Pedicle Flap from the Labia minora for the Repair of Female Urethral Strictures. Urologia Internationalis. 2002;69(2):95–98. Copyright © 2002 Karger Publishers, Basel, Switzerland.29 |
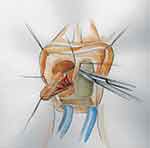 |
Figure 14 Ventral Labial Flap Urethroplasty. Transposition of the flap to the urethra. Reprinted with permission from Tanello M, Frego E, Simeone C, Cosciani Cunico S. Use of Pedicle Flap from the Labia minora for the Repair of Female Urethral Strictures. Urologia Internationalis. 2002;69(2):95–98. Copyright © 2002 Karger Publishers, Basel, Switzerland.29 |
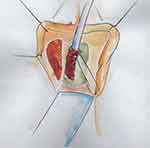 |
Figure 15 Ventral Labial Flap Urethroplasty. The flap is sutured to the margins of the open urethra and stented with a fenestrated catheter. Reprinted with permission from Tanello M, Frego E, Simeone C, Cosciani Cunico S. Use of Pedicle Flap from the Labia minora for the Repair of Female Urethral Strictures. Urologia Internationalis. 2002;69(2):95–98. Copyright © 2002 Karger Publishers, Basel, Switzerland.29 |
Dorsal Labial Flap Urethroplasty
Tao et al described their method of utilizing a dorsal labium flap urethroplasty.28 The urethra is incised at 12 o’clock until normal urethral tissue is reached. Surrounding tissue of the urethra is dissected away. A tongue shaped piece of labium is then selected depending on the length of the stricture, rotated, and sutured to the margins of the urethrotomy with interrupted 4–0 absorbable suture.
Tubularized Labial Flap Urethroplasty
Xu et al describes their technique of using transpubic access for a pedicled tubularized labial flap urethroplasty in the treatment of 8 patients with urethral obliterative strictures associated with urethrovaginal fistulas secondary to a pelvic fracture.36 The authors note that a transpubic approach is indicated if the patient has a complex urethral stricture and a urethrovaginal fistula, or if there is extensive scar tissue or anatomical defects present. The transpubic approach allows for good exposure of urethra while simultaneously allowing for repair of bladder neck incompetence and urethral fistulas. Additionally, it allows for the use of a pedicled omental flap of the rectus muscle to obliterate peri-anastomotic dead space, absorb inflammatory debris, and prevent fibrosis. Radwan et al also described a tubularized labial flap urethroplasty for patients with posttraumatic urethral loss rather than FUS.46 In their technique, they performed a transvaginal approach rather than a transpubic approach.
Xu et al describes a lower midline incision made above the pubic symphysis. A Gigli saw is used to remove part of the pubic bone and expose the urethra, adjacent fibrous tissue, and fracture fragments in the setting of a pelvic fracture. The anterior and posterior urethra is dissected. The fibrous tissue of the urethral stricture is completely excised and detached from the vagina to expose healthy urethra and the urethrovaginal fistula. The vaginal defect is closed with a continual 3–0 absorbable suture. A 3 cm x 3.5 cm flap is marked on the labia minora or majora and mobilized on its vascular pedicle and tubularized over a urethral catheter (Figure 16). If the labia minora flap is too small, a double face technique can be used where two flaps are approximated, sutured together, and then tubularized (Figure 17). Afterward, an anastomosis is performed with the tubularized flap and urethra near the excised pubic bone with interrupted 5–0 absorbable sutures. A pedicled rectus muscle flap is then dissected and wrapped around the anastomosis to obliterate the periurethral cavity, prevent fistula formation, provide blood supply and help preserve continence.
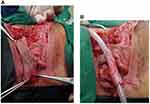 |
Figure 16 Tubularized Labial Flap. (A) A flap of appropriate size and shape was mobilized on its vascular pedicle. (B) The flap was tubularized over a fenestrated silicone stent with continual 5-zero polyglactin sutures. Reprinted from Xu Y-M, Sa Y-L, Fu Q, Zhang J, Xie H, Jin S-B. Transpubic Access Using Pedicle Tubularized Labial Urethroplasty for the Treatment of Female Urethral Strictures Associated with Urethrovaginal Fistulas Secondary to Pelvic Fracture. European Urology. 2009;56(1):193–200. Copyright © 2008 European Association of Urology. Published by Elsevier B.V. All rights reserved.36 |
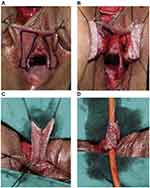 |
Figure 17 Tubularized Labial Flap. (A) The flaps were marked on the skin of the labia minora (B) The flap was mobilized on its vascular pedicle (C) Two flaps were pieced together with 5-zero polyglactin continual sutures (D) The flap was tubularized with 5-zero polyglactin continual sutures. Reprinted from Xu Y-M, Sa Y-L, Fu Q, Zhang J, Xie H, Jin S-B. Transpubic Access Using Pedicle Tubularized Labial Urethroplasty for the Treatment of Female Urethral Strictures Associated with Urethrovaginal Fistulas Secondary to Pelvic Fracture. European Urology. 2009;56(1):193–200. Copyright © 2008 European Association of Urology. Published by Elsevier B.V. All rights reserved.36 |
In Radwan et al.’s technique, the anterior vaginal wall is dissected to the level of the bladder. Two parallel incisions are made over the labia minora, and a 3 cm x 3 cm flap is mobilized. The flap is rotated through a tunnel created underneath the vaginal wall and tubularized over a urethral catheter. The flap is then anastomosed to the bladder. The lateral vaginal walls are closed over the the neourethra which is sutured to the labia distally.
Bladder Flap Urethroplasty
Tanagho first described the use of a bladder flap urethroplasty.31 It has also been reported in studies by Hemal et al and Radwan et al.20,46 While these studies did not necessarily use this technique for FUS alone, they are effective techniques for urethral reconstruction.
The technique described combines explanations given Tanagho and Hemal (Figure 18).2,7,20,30,31 The vagina and bladder neck are first dissected away from each other through a vaginal approach.31 Suprapubic exposure is then obtained to further mobilize the bladder.30,31 Stay sutures are placed on the anterior bladder wall to mark a square flap just above the internal sphincter which will be shaped into a 3.5 cm long tube.20,30 A transverse incision between the lower fixation sutures and two parallel upward incisions between the lower and upper fixation sutures are performed to develop the flap which can then be flipped upward.30 The transverse incision is extended to completely transect the bladder neck.30 The bladder flap can then be tubularized around a Foley catheter, brought behind the pubis, and anastomosed to the vestibule through a stab-wound incision of the vestibule.20,30,31 The apex of the trigone is sutured to the base of the reconstructed urethra, thereby completing a Y-shaped bladder closure – a straight limb upon closure of the tubularized flap and a V for the closure of the trigone at the bladder base.30 Hemal additionally suggests creation of an oblique bladder flap to avoid a vertical suture line which can about the anterior vaginal wall and increase the risk of a urethrovaginal fistula.20 However, this step is not always possible, as a vertical flap allows for a longer urethra, given that the oblique flap is limited by location of the ureteral orifice. Furthermore, Hemal performs a bladder neck suspension to ensure a proper urethrovesical angle to possibly improve continence.20
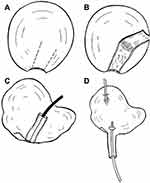 |
Figure 18 Bladder Flap Urethroplasty. Oblique anterior bladder flap urethral tube reconstruction (A) 2 parallel oblique incisions are made on anterior bladder wall. (B) oblique 2.5×4 cm anterior bladder flap is developed. (C) flap is tubularized over Foley catheter and bladder neck is closed at right angles to tube. (D) neourethra is brought behind pubis and anastomosed to vestibule. Reprinted from Hemal AK, Dorairajan LN, Gupta NP. Posttraumatic complete and partial loss of urethra with pelvic fracture in girls: an appraisal of management. The Journal of Urology. 2000;163(1):282–287. Copyright © 2018, Wolters Kluwer Health.20 |
Open Urethral Reconstruction – Free Graft Urethroplasty
Few additional consideration should be made when obtaining a graft. Primarily, the length must be approximately one third longer than the stricture, given postoperative shrinkage. The graft should then be carefully defatted.23 In some techniques, the graft is perforated with an 11-blade or needle to aid with capillary imbibition and inosculation of the graft.23,47 Additionally, quilting is important to minimize sliding of the graft, which also aids in the inosculation process.48
Dorsal Onlay
Dorsal onlay graft urethroplasties have been performed with vaginal, buccal and lingual mucosa.4,14,27,32,33,41,48–56 The dorsal part of the urethra is exposed by a reverse U-shaped incision suprameatally from the 3 o’clock to 9 o’clock position (Figure 19).57 The vulvar mucosa is separated from the urethra, and a plane is developed between the urethra and clitoral cavernous tissue. The striated urethral sphincter can be identified and displaced upward. A full thickness dorsal incision is made through the dorsal urethra until healthy mucosa is seen past the stricture.4,53 The free graft is then sutured on the lateral margins of the urethral plate in either a running or interrupted fashion with absorbable sutures. The augmented urethra is also quilted to the clitoral body in the midline, and the graft is approximated to the suprameatal incision distally. Of note, as reported by Mittal et al and Gomez et al, if the stricture is in the mid- or proximal portion of the urethra, the meatus can be spared, and a dorsal slit can be performed at the area of stricture.52,58
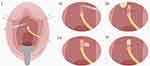 |
Figure 19 Dorsal Buccal Mucosal Graft Urethroplasty. (i) Periurethral incision and mobilization of the urethra. (ii) Dorsal incision of urethral stricture. (iii and iv) Edges of graft sutured to the urethral border and placed over the defect. (v) End result. Reprinted from Agochukwu-Mmonu N, Srirangapatanam S, Cohen A, Breyer B. Female Urethral Strictures: Review of Diagnosis, Etiology, and Management. Curr Urol Rep. 2019;20(11):74. Copyright © 2019, Springer Science Business Media, LLC, part of Springer Nature.57 |
Ventral Onlay
Ventral onlay graft urethroplasties have been performed with labial tissue and buccal mucosa.4,23,24,34,35,59,60 A longitudinal incision of the anterior vagina is made to expose the urethra. Periurethral tissue is dissected away, and the urethra is incised at the 6 o’clock position. The graft is then sutured proximally and distally with 5–0 or 6–0 absorbable sutures while a running or interrupted suture is used for the lateral anastomosis of the inlay. After suturing the graft, the previously strictured area should be widened and fixed to the anterior vaginal wall. The vaginal wall is closed in layers, and the vaginal skin closed with interrupted mattress sutures.4,23,24,34,35,59,60
Ventral Inlay
The vaginal sparing, ventral inlay graft urethroplasty is a recently described technique utilizing BMG. Hoag et al first described a vaginal sparing urethroplasty using a ventral inlay with BMG in 2016 in one patient with continued success at 10 months.12 Nayak et al described a similar approach with 11 out of 12 patients having a successful outcome at a mean follow up of 18 months.22 Given that this procedure is performed intraurethrally, Nayak et al describes this technique as a true “inlay” and remarks that all other female urethroplasties that have been described have been “onlays”.22
In the technique, the urethra is dilated. Labial retraction sutures are placed for exposure and a nasal speculum or tissue forceps is placed into the urethra (Figures 20 and 21). A ventral urethrotomy is performed endourethral at the 6 o’clock position. A ventral inlay technique is performed to place the graft from the bladder neck to the urethral meatus. Interrupted 4–0 absorbable sutures are used to circumferentially anastomose the graft to the edges of the urethrotomy followed by interrupted quilting sutures.
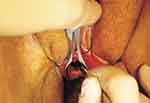 |
Figure 20 Ventral Inlay Urethroplasty. Intraoperative photograph demonstrating dilated urethra to accommodate nasal speculum, and ventral urethrotomy carried out sharply with 11-blade scalpel. Reprinted from Hoag N, Gani J, Chee J. Vaginal-sparing ventral buccal mucosal graft urethroplasty for female urethral stricture: A novel modification of surgical technique. Investigative and clinical urology. 2016;57(4):298–302 © The Korean Urological Association, 2016.12 |
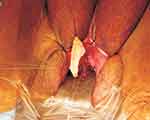 |
Figure 21 Ventral Inlay Urethroplasty. Intraoperative photograph demonstrating three apical/proximal urethral sutures, sutured to corresponding proximal end of harvested buccal mucosal graft before parachuting into position. Reprinted from Hoag N, Gani J, Chee J. Vaginal-sparing ventral buccal mucosal graft urethroplasty for female urethral stricture: A novel modification of surgical technique. Investigative and clinical urology. 2016;57(4):298–302. © The Korean Urological Association, 2016.12 |
Double Faced Inlay
The double-faced inlay graft urethroplasty is a recently described technique.38 In a case report by Joshi et al, the patient had a near obliterative mid and distal urethral stricture. The urethroplasty was performed intraurethrally to decrease the risk of incontinence. In the approach, the urethra is widened with forceps, and an incision is made intraurethrally through the mucosa on the posterior wall. The incision is continually widened until the stricture is completely incised. A BMG is harvested and inserted as an inlay. The same technique is then repeated on the anterior wall. The patient was asymptomatic at 6 months of follow up.
Tubularized Graft Urethroplasty
The tubularized BMG urethroplasty is a technique only described once. In the study by Onol et al, 2 patients had severe distal urethral strictures that almost completely obliterated the external meatus.4 In their technique, a circumferential incision is made to mobilize the urethral meatus and distal urethra from the periurethral tissue (Figure 22). The distal fibrotic urethra is amputated at a healthier proximal site. An incision is then made at the 6 o’clock position of the proximal mucosa. One corner of the 4×1.5 cm BMG is sutured to the apex of the ventral urethrotomy. The short edge of the rectangular graft is sewn to one side of the ventral mucosal incision. Then the BMG is rotated counterclockwise around a 22–24 Fr Foley catheter with the mucosa facing inward towards the lumen. The long edge of the graft is sutured circumferentially to the proximal urethral mucosa. The graft mucosa at the meatus is folded on itself and sutured to the initial circummeatal incision, creating a cosmetic and functional neomeatus.
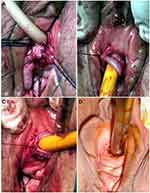 |
Figure 22 Tubularized Graft Urethroplasty. (Asterisk) Healthy proximal mucosa incised at 6-o’clock position. (A) One corner of buccal mucosa graft (G) sutured to apex of this ventral urethral defect (arrow) and short edge of rectangular graft sewn to left edge (asterisk) of ventral mucosal incision. (B) BMG then rotated counterclockwise on 22–24F Foley Catheter, with its mucosa facing inward (asterisk). (C) Completion of neomeatus with circumferential suturing of BMG to circummeatal incision. (D) Appearance of neomeatus 6 months later in same patient that could admit 26F sound without difficulty. Reprinted from Önol FF, Antar B, Köse O, Erdem MR, Önol ŞY. Techniques and Results of Urethroplasty for Female Urethral Strictures: Our Experience With 17 Patients. Urology. 2011;77(6):1318–1324. Copyright © 2011 Elsevier Inc. All rights reserved.4 |
Distal Urethrectomy with Meatoplasty
Distal urethral stricture within 5 to 10 mm of the meatus may be repaired with distal urethrectomy and advancement meatoplasty.2 Interrupted absorbable sutures are pre-placed in healthy proximal urethral mucosa 2 mm or less from the stricture to prevent inward retraction. Circumferential excision of the distal urethra and meatus is performed. Advancement of healthy mucosa to the vaginal epithelium is performed with circumferential interrupted sutures. A urethral catheter is kept in place for 1 to 3 days, and topical estrogen cream is used to promote healing and reduce recurrence.2 While this technique has been described, its efficacy is unclear as previous systematic reviews and large series have not identified its use.
Tissue Engineering
Tissue Engineering (TE) is a relatively new and developing alternative to traditional autologous grafting in urethral reconstruction. TE employs principles from materials science, cell biology, transplantation, and engineering to treat or replace damaged tissues.61 TE circumvents the obstacle of graft harvesting from the patient’s oral or vaginal mucosa, a process that can cause morbidity to the patient and limit reconstruction if the patient lacks adequate tissue.62–64 While current research is promising, more data is required to reveal what role TE will play in female urethroplasty.
Much of our current understanding regarding TE in urethroplasty comes from clinical trials involving male patients. A 2017 systematic review by Versteegeden et al identified 22 clinical studies that investigated male urethral TE, but only 1 study included female patients.65 Only 1 randomized trial was identified, which compared the use of BMG with an acellular bladder matrix scaffold in male patients with anterior urethral strictures.66 Success of the tissue engineered scaffold was highly dependent upon whether the patient had a healthy urethral bed, with only 2/6 (33.3%) patients with unhealthy urethral beds having positive long-term outcomes. In 2017, Ram-Liebig et al conducted a prospective, observational study consisting of 99 male patients with recurrent urethral stricture who were treated with tissue-engineered oral mucosa grafts.67 The study demonstrated a success rate of 70.8% (46/65) and 76.9% (30/39) at 12-month and 24-month follow-up, respectively.
Regarding TE for FUS, in 2002, Montovani et al described successful reconstructive urethroplasty utilizing porcine acellular matrix from small intestine mucosa for the repair of a 3 cm-long urethral stricture in 1 female patient.68 In 2017, Ansari & Karram described 2 female patients who underwent urethral reconstruction with acellular porcine urinary bladder matrix. Short term follow-up revealed recurrence of SUI in 1 patient.69 While some success has been shown with male urethroplasty utilizing TE, it is evident that more work must be done to explore the advantages and role of TE in female urethral reconstruction when compared to autologous grafting techniques.
Conclusion
While urethral strictures are rare in women, they can cause irritative and obstructive voiding symptoms, which can diminish their quality of life. Urethral dilation can be effective, but it is often associated with failure, especially upon multiple attempts. Female urethroplasty provides excellent cure rates and should be performed for recurrence after dilation. Further exploration is needed to determine whether certain techniques are superior in particular situations. The optimal management strategy in female urethral strictures requires clarification with larger and comparative series.
Abbreviation
FUS, female urethral stricture; BMG, buccal mucosal graft; PVS, pubovaginal sling; SUI, stress urinary incontinence; TE, tissue engineering.
Funding
There is no funding to report.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Aldamanhori R, Inman R. The treatment of complex female urethral pathology. Asian J Urol. 2018;5(3):160–163. doi:10.1016/j.ajur.2018.03.003
2. Faiena I, Koprowski C, Tunuguntla H. Female urethral reconstruction. J Urol. 2016;195(3):557–567. doi:10.1016/j.juro.2015.07.124
3. Sarin I, Narain TA, Panwar VK, Bhadoria AS, Goldman HB, Mittal A. Deciphering the enigma of female urethral strictures: a systematic review and meta-analysis of management modalities. Neurourol Urodyn. 2021;40(1):65–79. doi:10.1002/nau.24584
4. Önol FF, Antar B, Köse O, Erdem MR, Önol ŞY. Techniques and results of urethroplasty for female urethral strictures: our experience with 17 patients. Urology. 2011;77(6):1318–1324. doi:10.1016/j.urology.2011.01.017
5. Hoag N, Chee J. Surgical management of female urethral strictures. Transl Androl Urol. 2017;6(Suppl 2):S76–s80. doi:10.21037/tau.2017.01.20
6. Lazzeri M, Sansalone S, Guazzoni G, Barbagli G. Incidence, causes, and complications of urethral stricture disease. Eur Urol. 2016;15(1):2–6. doi:10.1016/j.eursup.2015.10.002
7. Rosenblum N, Nitti VW. Female urethral reconstruction. Urol Clin North Am. 2011;38(1):55–64, vi. doi:10.1016/j.ucl.2010.12.008
8. Waterloos M, Verla W. Female urethroplasty: a practical guide emphasizing diagnosis and surgical treatment of female urethral stricture disease. Biomed Res Int. 2019;2019:6715257. doi:10.1155/2019/6715257
9. Osman NI, Mangera A, Chapple CR. A systematic review of surgical techniques used in the treatment of female urethral stricture. Eur Urol. 2013;64(6):965–973. doi:10.1016/j.eururo.2013.07.038
10. Padmanabhan P. Surgical, radiographic and endoscopic anatomy of the female pelvis. In: Partin A, Dmochowski R, Kavoussi L, Peters C, editors. Campbell-Walsh-Wein Urology.
11. Nitti V. Female urethral reconstruction. In: Smith J, Howards S, Preminger G, Dmochowski R, editors. Hinman’s Atlas of Urologic Surgery.
12. Hoag N, Gani J, Chee J. Vaginal-sparing ventral buccal mucosal graft urethroplasty for female urethral stricture: a novel modification of surgical technique. Investig Clin Urol. 2016;57(4):298–302. doi:10.4111/icu.2016.57.4.298
13. West C, Lawrence A. Female urethroplasty: contemporary thinking. World J Urol. 2019;37(4):619–629. doi:10.1007/s00345-018-2564-4
14. Migliari R, Leone P, Berdondini E, De Angelis M, Barbagli G, Palminteri E. Dorsal buccal mucosa graft urethroplasty for female urethral strictures. J Urol. 2006;176(4):1473–1476. doi:10.1016/j.juro.2006.06.043
15. Romman AN, Alhalabi F, Zimmern PE. Distal intramural urethral pathology in women. J Urol. 2012;188(4):1218–1223. doi:10.1016/j.juro.2012.06.016
16. Smith AL, Ferlise VJ, Rovner ES. Female urethral strictures: successful management with long-term clean intermittent catheterization after urethral dilatation. BJU Int. 2006;98(1):96–99. doi:10.1111/j.1464-410X.2006.06206.x
17. Allen TD. Internal urethrotomy in female subjects. J Urol. 1986;136(6):1280. doi:10.1016/S0022-5347(17)45312-2
18. Popat S, Zimmern PE. Long-term management of luminal urethral stricture in women. Int Urogynecol J. 2016;27(11):1735–1741. doi:10.1007/s00192-016-3006-8
19. Sharifian H, Zargham M, Khorami MH, Mohamadi M, Mazdak H, Mozafarpour S. Internal urethrotomy in treatment of female with anatomical bladder outlet obstruction. Adv Biomed Res. 2019;8(1):36. doi:10.4103/abr.abr_200_18
20. Hemal AK, Dorairajan LN, Gupta NP. Posttraumatic complete and partial loss of urethra with pelvic fracture in girls: an appraisal of management. J Urol. 2000;163(1):282–287. doi:10.1016/S0022-5347(05)68037-8
21. Heidari F, Abbaszadeh S, Ghadian A, Tehrani Kia F. On demand urethral dilatation versus intermittent urethral dilatation: results and complications in women with urethral stricture. Nephrourol Mon. 2014;6(2):e15212–e15212. doi:10.5812/numonthly.15212
22. Nayak P, Mandal S, Das M. Ventral-inlay buccal mucosal graft urethroplasty for female urethral stricture. Indian J Urol. 2019;35(4):273–277. doi:10.4103/iju.IJU_57_19
23. Rehder P, Glodny B, Pichler R, Exeli L, Kerschbaumer A, Mitterberger MJ. Dorsal urethroplasty with labia minora skin graft for female urethral strictures. BJU Int. 2010;106(8):1211–1214. doi:10.1111/j.1464-410X.2010.09240.x
24. Gozzi C, Roosen A, Bastian PJ, Karl A, Stief C, Tritschler S. Volar onlay urethroplasty for reconstruction of female urethra in recurrent stricture disease. BJU Int. 2011;107(12):1964–1966. doi:10.1111/j.1464-410X.2010.09790.x
25. Romero-Maroto J, Verdú-Verdú L, Gómez-Pérez L, Pérez-Tomás C, Pacheco-Bru JJ, López-López A. Lateral-based anterior vaginal wall flap in the treatment of female urethral stricture: efficacy and safety. Eur Urol. 2018;73(1):123–128. doi:10.1016/j.eururo.2016.09.029
26. Simonato A, Varca V, Esposito M, Carmignani G. Vaginal flap urethroplasty for wide female stricture disease. J Urol. 2010;184(4):1381–1385. doi:10.1016/j.juro.2010.06.042
27. Manasa T, Khattar N, Tripathi M, Varshney A, Goel H, Sood R. Dorsal onlay graft urethroplasty for female urethral stricture improves sexual function: short-term results of a prospective study using vaginal graft. Indian J Urol. 2019;35(4):267–272. doi:10.4103/iju.IJU_134_19
28. Tao T, Xu Q-K, Hu Q, et al. Novel surgical technique for female distal urethral stricture disease: an evaluation of efficacy and safety compared with urethral dilatation. Int J Clin Exp Med. 2018;11:12002.
29. Tanello M, Frego E, Simeone C, Cosciani Cunico S. Use of pedicle flap from the labia minora for the repair of female urethral strictures. Urol Int. 2002;69(2):95–98. doi:10.1159/000065554
30. Tanagho EA, Smith DR. Clinical evaluation of a surgical technique for the correction of complete urinary incontinence. J Urol. 1972;107(3):402–411. doi:10.1016/S0022-5347(17)61040-1
31. Tanagho EA. Urethrosphincteric reconstruction for congenitally absent urethra. J Urol. 1976;116(2):237–242. doi:10.1016/S0022-5347(17)58762-5
32. Sharma GK, Pandey A, Bansal H, et al. Dorsal onlay lingual mucosal graft urethroplasty for urethral strictures in women. BJU Int. 2010;105(9):1309–1312. doi:10.1111/j.1464-410X.2009.08951.x
33. Blaivas JG, Santos JA, Tsui JF, et al. Management of urethral stricture in women. J Urol. 2012;188(5):1778–1782. doi:10.1016/j.juro.2012.07.042
34. Mukhtar BMB, Spilotros M, Malde S, Greenwell TJ. Ventral‐onlay buccal mucosa graft substitution urethroplasty for urethral stricture in women. BJU Int. 2017;120(5):710–716. doi:10.1111/bju.13970
35. Spilotros M, Malde S, Solomon E, et al. Female urethral stricture: a contemporary series. World J Urol. 2017;35(6):991–995. doi:10.1007/s00345-016-1947-7
36. Xu Y-M, Sa Y-L, Fu Q, Zhang J, Xie H, Jin S-B. Transpubic access using pedicle tubularized labial urethroplasty for the treatment of female urethral strictures associated with urethrovaginal fistulas secondary to pelvic fracture. Eur Urol. 2009;56(1):193–200. doi:10.1016/j.eururo.2008.04.046
37. Lane GI, Smith AL, Stambakio H, et al. Treatment of urethral stricture disease in women: a multi-institutional collaborative project from the SUFU research network. Neurourol Urodyn. 2020;39(8):2433–2441. doi:10.1002/nau.24507
38. Joshi PM, Kulkarni SB. A new technique of double-face buccal graft urethroplasty for female urethral strictures. Turk J Urol. 2019;46(2):165–168. doi:10.5152/tud.2019.19228
39. Schwender CE, Ng L, McGuire E, Gormley EA. Technique and results of urethroplasty for female stricture disease. J Urol. 2006;175(3 Pt 1):
40. Gormley EA. Vaginal flap urethroplasty for female urethral stricture disease. Neurourol Urodyn. 2010;29(S1):S42–S45. doi:10.1002/nau.20814
41. Kowalik C, Stoffel JT, Zinman L, Vanni AJ, Buckley JC. Intermediate outcomes after female urethral reconstruction: graft vs flap. Urology. 2014;83(5):1181–1185. doi:10.1016/j.urology.2013.12.052
42. Hajebrahimi S, Maroufi H, Mostafaei H, Salehi-Pourmehr H. Reconstruction of the urethra with an anterior vaginal mucosal flap in female urethral stricture. Int Urogynecol J. 2019;30(12):2055–2060. doi:10.1007/s00192-019-03910-3
43. Flisser AJ, Blaivas JG. Outcome of urethral reconstructive surgery in a series of 74 women. J Urol. 2003;169(6):2246–2249. doi:10.1097/01.ju.0000061763.88247.16
44. Romman A, Takacs L, Gilleran J, Zimmern P. Vestibular flap urethroplasty in women with recurrent distal intramural urethral pathology. Neurourol Urodyn. 2015;34(3):213–218. doi:10.1002/nau.22552
45. Montorsi F, Salonia A, Centemero A, et al. Vestibular flap urethroplasty for strictures of the female urethra. Impact on symptoms and flow patterns. Urol Int. 2002;69(1):12–16. doi:10.1159/000064353
46. Radwan MH, Abou Farha MO, Soliman MG, et al. Outcome of female urethral reconstruction: a 12-year experience. World J Urol. 2013;31(4):991–995. doi:10.1007/s00345-013-1087-2
47. Hampson LA, Myers JB, Vanni AJ, et al. Dorsal buccal graft urethroplasty in female urethral stricture disease: a multi-center experience. Transl Androl Urol. 2019;8(Suppl 1):S6–S12. doi:10.21037/tau.2019.03.02
48. Petrou SP, Rogers AE, Parker AS, Green KM, McRoberts JW. Dorsal vaginal graft urethroplasty for female urethral stricture disease: female dorsal vaginal graft urethroplasty. BJU Int. 2012;110(11c):E1090–E1095. doi:10.1111/j.1464-410X.2012.11233.x
49. Tsivian A, Sidi AA. Dorsal graft urethroplasty for female urethral stricture. J Urol. 2006;176(2):611–613. doi:10.1016/j.juro.2006.03.055
50. Singh M, Kapoor R, Kapoor D, Kapoor R, Srivastav A, Chipde S. Dorsal onlay vaginal graft urethroplasty for female urethral stricture. Indian J Urol. 2013;29(2):124–128. doi:10.4103/0970-1591.114034
51. Santosa KB, WisnuTirtayasa PM, Gde Oka A. Dorsal vaginal graft urethroplasty as a treatment for female urethral stricture: case reports of four patients. Biomed Pharmacol J. 2018;11(4):2163–2167. doi:10.13005/bpj/1597
52. Mittal A, Bahuguna G, Sarin I, Narain TA, Panwar VK, Singh G. Meatal-sparing dorsal onlay vaginal graft urethroplasty: widening the surgical horizons of female urethral stricture. International Urogynecology Journal. 2021;32(3):737–739. doi:10.1007/s00192-020-04524-w
53. Castillo OA, Sepúlveda F, Feria-Flores MA. Urethroplasty with dorsal oral mucosa graft in female urethral stenosis. Actas Urol Esp. 2011;35(4):246–249. doi:10.1016/S2173-5786(11)70058-0
54. Powell CR, Daniels D. Dorsal onlay buccal urethroplasty in the female is associated with high quality of life using validated lower urinary tract symptom instruments. Urol Pract. 2017;4(1):48–53. doi:10.1016/j.urpr.2016.02.002
55. Önol FF, Önol ŞY, Tahra A, Boylu U. Ventral inlay labia minora graft urethroplasty for the management of female urethral strictures. Urology. 2014;83(2):460–464. doi:10.1016/j.urology.2013.09.020
56. Goel A, Paul S, Dalela D, Sankhwar P, Sankhwar SN, Singh V. Dorsal onlay buccal mucosal graft urethroplasty in female urethral stricture disease: a single-center experience. Int Urogynecol J. 2014;25(4):525–530. doi:10.1007/s00192-013-2249-x
57. Agochukwu-Mmonu N, Srirangapatanam S, Cohen A, Breyer B. Female urethral strictures: review of diagnosis, etiology, and management. Curr Urol Rep. 2019;20(11):74. doi:10.1007/s11934-019-0933-1
58. Gomez RG, Segura FJ, Saavedra A, Campos RA. Female urethral reconstruction: dorsal buccal mucosa graft onlay. World J Urol. 2020;38(12):3047–3054. doi:10.1007/s00345-019-02958-6
59. Berglund RK, Vasavada S, Angermeier K, Rackley R. Buccal mucosa graft urethroplasty for recurrent stricture of female urethra. Urology. 2006;67(5):1069–1071. doi:10.1016/j.urology.2005.12.010
60. Ozlulerden Y, Celen S, Zumrutbas AE, Aybek Z. Female buccal mucosa graft urethroplasty: a new modified ventral onlay “AZ” technique. Int Urogynecol J. 2020;31(12):2543–2550. doi:10.1007/s00192-020-04354-w
61. Mangir N, Wilson KJ, Osman NI, Chapple CR. Current state of urethral tissue engineering. Curr Opin Urol. 2019;29(4):385–393. doi:10.1097/MOU.0000000000000637
62. Jang TL, Erickson B, Medendorp A, Gonzalez CM. Comparison of donor site intraoral morbidity after mucosal graft harvesting for urethral reconstruction. Urology. 2005;66(4):716–720. doi:10.1016/j.urology.2005.04.045
63. Ram-Liebig G, Bednarz J, Stuerzebecher B, et al. Regulatory challenges for autologous tissue engineered products on their way from bench to bedside in Europe. Adv Drug Deliv Rev. 2015;82–83:181–191. doi:10.1016/j.addr.2014.11.009
64. Bhargava S, Patterson JM, Inman RD, MacNeil S, Chapple CR. Tissue-engineered buccal mucosa urethroplasty—clinical outcomes. Eur Urol. 2008;53(6):1263–1271. doi:10.1016/j.eururo.2008.01.061
65. Versteegden LRM, de Jonge PKJD, IntHout J, et al. Tissue engineering of the urethra: a systematic review and meta-analysis of preclinical and clinical studies. Eur Urol. 2017;72(4):594–606. doi:10.1016/j.eururo.2017.03.026
66. El Kassaby A, AbouShwareb T, Atala A. Randomized Comparative Study between buccal mucosal and acellular bladder matrix grafts in complex anterior urethral strictures. J Urol. 2008;179(4):1432–1436. doi:10.1016/j.juro.2007.11.101
67. Ram-Liebig G, Barbagli G, Heidenreich A, et al. Results of use of tissue-engineered autologous oral mucosa graft for urethral reconstruction: a multicenter, prospective, observational trial. EBioMedicine. 2017;23(C):185–192. doi:10.1016/j.ebiom.2017.08.014
68. Mantovani F, Trinchieri A, Mangiarotti B, et al. Reconstructive urethroplasty using porcine acellular matrix: preliminary results. Arch Ital Urol Androl. 2002;74(3):127–128.
69. Ansari S, Karram M. Two cases of female urethral reconstruction with acellular porcine urinary bladder matrix. Int Urogynecol J. 2017;28(8):1257–1260. doi:10.1007/s00192-016-3262-7
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
