Back to Journals » Cancer Management and Research » Volume 12
Treatment of Synchronous Liver Metastases from Gastric Cancer: A Single-Center Study
Authors Yu P, Zhang Y , Ye Z, Chen X, Huang L, Du Y, Cheng X
Received 21 May 2020
Accepted for publication 13 August 2020
Published 26 August 2020 Volume 2020:12 Pages 7905—7911
DOI https://doi.org/10.2147/CMAR.S261353
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Yong Teng
Pengfei Yu, Yanqiang Zhang, Zeyao Ye, Xiangliu Chen, Ling Huang, Yian Du, Xiangdong Cheng
Department of Gastric Surgery, Institute of Cancer Research and Basic Medical Sciences of Chinese Academy of Sciences, Cancer Hospital of University of Chinese Academy of Sciences, Zhejiang Cancer Hospital, Hangzhou 310022, People’s Republic of China
Correspondence: Xiangdong Cheng
Department of Gastric Surgery, Institute of Cancer Research and Basic Medical Sciences of Chinese Academy of Sciences, Cancer Hospital of University of Chinese Academy of Sciences, Zhejiang Cancer Hospital, Hangzhou 310022, People’s Republic of China
Tel +86-571-88128041
Email [email protected]
Objective: The therapeutic effects of surgical resection in gastric cancer with liver metastasis remain largely unclear. We sought to examine surgical resection combined with chemotherapy for survival benefit in cases of synchronous liver metastases from gastric cancer (LMGC), and to identify factors affecting patient prognosis.
Methods: Patients diagnosed with synchronous LMGC between January 2010 and December 2015 were enrolled in this study. The effects of gastrectomy and metastasectomy combined with chemotherapy (surgical resection group) and palliative chemotherapy (palliative chemotherapy group) on survival were comparatively assessed.
Results: Of the 132 included cases, 57 (43.2%) and 75 (56.8%) were treated with surgical resection/chemotherapy and palliative chemotherapy, respectively. Overall survival (OS) was markedly prolonged in the surgical resection group compared with the palliative chemotherapy group (33.6 vs 12.4 months, P< 0.001). In patients who underwent surgical resection, R0 resection resulted in prolonged OS in comparison with the non-R0 resection subgroup (45.1 vs 13.5 months, P< 0.001). Surgical resection (hazard ratio [HR]=0.453; 95% confidence interval [CI] 0.276– 0.813; P=0.009) and solitary liver metastasis (HR=0.540; 95% CI 0.315– 0.796; P =0.043) were independent predictors of OS.
Conclusion: Patients with synchronous LMGC might benefit from radical surgical resection combined with appropriate chemotherapy. Additional well-designed prospective studies are required to verify the above findings.
Keywords: gastric neoplasm, liver metastasis, surgical resection, chemotherapy, prognosis
Introduction
Gastric cancer represents the 5th most common cancer and the 3rd leading cause of cancer-related deaths around the world.1 Recurrence and metastasis are the main factors affecting the prognosis of gastric cancer patients, and the liver is the most frequent site of hematogenous metastasis.2 Liver metastases from gastric cancer (LMGC) are diagnosed synchronously in 3–14% of gastric cancer cases and metachronously in up to 37% after curative gastrectomy.3,4 LMGC are mostly multifocal and often complicated with extrahepatic metastasis.5 Previously, palliative chemotherapy and supportive treatment were considered the main therapeutic options for LMGC, but yield a 5-year survival rate of less than 10%.6 Recent studies have reported that radical surgical resection of primary tumors and liver metastases results in significantly prolonged survival time in some LMGC patients after comprehensive treatment including chemotherapy.7,8 However, most of these were retrospective studies with few cases. At present, some unsolved clinical problems remain, including the best indications for surgery, the effects of perioperative chemotherapy, and the factors influencing prognosis. In this study, associations of surgical resection and clinicopathological characteristics with overall survival (OS) were examined to develop more effective treatment strategies for LMGC patients.
Patients and Methods
Patients
Synchronous LMGC was defined as metastases occurring before or during surgery or within 6 months after gastrectomy.9 Patients with synchronous LMGC who underwent gastrectomy and metastasectomy or systemic chemotherapy as the initial treatment at Zhejiang Cancer Hospital (Hangzhou, China) from January 2010 to December 2015 were assessed in this retrospective study.
Inclusion criteria were as follows: age, 18–75 years; Eastern Cooperative Oncology Group (ECOG) performance status (PS), 0–1; liver metastases identified by abdominal enhanced CT, MRI or PET/CT, and limited (no more than five metastases, in one liver lobe or bilobar invasion); adequate organ functions (alanine transaminase [ALT] and aspartate transaminase [AST] levels < 2×normal upper limits [NULs]; serum total bilirubin <1.5×NUL; serum creatinine <1.25×NUL; platelets ≥100×109/L; granulocytes ≥1.5×109/L; hemoglobin amounts ≥90 g/L). Cases with other malignancies or distant extrahepatic metastases were excluded.
Treatment and Evaluation
The patients were submitted to imaging examinations at initial diagnosis to evaluate the extent of disease and resectability. The decision to perform surgical resection was based on consensual opinion after comprehensive assessment of the patient by a multidisciplinary team. Initial gastrectomy and hepatectomy aimed to achieve R0 resection; otherwise, palliative chemotherapy remained as a mainstream treatment. According to the treatment modality, patients were divided into the surgical resection (gastrectomy and metastasectomy combined with chemotherapy) and palliative chemotherapy (palliative chemotherapy only) groups. Then, the clinicopathological features and overall survival of these two groups were compared. Postoperative tumor residual state was classified as R2 (gross residual tumor), R1 (positive margin of resection) and R0 (complete resection with negative margin). Postoperative complication was prospectively defined as any deviation from a predetermined postoperative course within 30 days of surgery, and classified according to the Clavien-Dindo severity classification (CDSC).10 Adverse events were evaluated using the Common Terminology Criteria for Adverse Events, version 4.0 (CTCAE, Ver. 4.0).
Follow-Up
All patients were followed up by outpatient examinations or telephone interviews, once every 3 months for the initial two years, followed by once every 6 months for three to five years, and once yearly thereafter. OS was defined as the time elapsed from the diagnosis of LMGC to death or last follow-up. The cutoff date for OS was January 2019.
Statistical Analysis
Continuous data are mean±standard deviation (SD) or median and range. Student’s t and chi-square tests were performed for comparing continuous and categorical variables, respectively. Kaplan–Meier curves were generated for OS assessment, and between-group comparison was carried out by the Log rank test. Multivariable analysis based on the Cox proportional hazards model was performed to determine factors independently predicting prognosis. SPSS 19.0 (SPSS, USA) was employed for data analysis, with P<0.05 indicating statistical significance.
Results
Patient Features
Between January 2010 and December 2015, a total of 6346 patients with gastric cancer were treated in Zhejiang Cancer Hospital (Hangzhou, China). Among them, 336 patients had synchronous LMGC, and 204 were excluded because of diffuse liver metastases or other distant metastases.
The patients had a median age of 62.5 years (range, 32–75 years). There were 92 males and 40 females, with an average number of liver metastases of 2.6 (range, 1–5) and a mean tumor size of 3.0 cm (range, 0.5–8.9 cm). A total of 57 patients received gastrectomy and metastasectomy combined with chemotherapy (surgical resection group), and 75 patients underwent chemotherapy alone (palliative chemotherapy group).
The baseline features of the 132 patients with synchronous LMGC are listed in Table 1. Primary tumor location, T and N classification, number and size of liver metastases, tumor differentiation, and the levels of tumor markers showed no marked differences between the two groups.
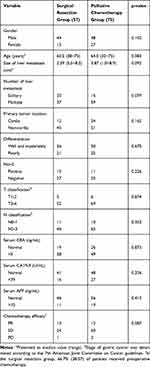 |
Table 1 Clinicopathological Features of the 132 Patients with Synchronous LMGC |
Survival and Prognostic Factors
The median duration of follow-up in the whole group was 37.1 months (range, 1–96 months), with 1-, 3- and 5-year overall survival rates of 57.3%, 19.4% and 15.5%, respectively. Median OS was markedly prolonged in the surgical resection group compared with the palliative chemotherapy group (33.6 months [95% CI 26.6–40.6 months] versus 12.4 months [95% CI 10.0–14.8 months], P<0.001) (Figure 1). In the surgical resection group, 66.7% (38/57) of the patients received preoperative chemotherapy, and their median survival time was slightly but not significantly improved compared with that of the patients without preoperative chemotherapy (34.4 months vs 32.1 months, P>0.05). A total of 68.4% (39/57) of the patients had R0 resection. Median OS times were 45.1 (95% CI 37.0–53.1) and 13.5 (95% CI 11.0–16.0) months in the R0 and non-R0 resection groups, respectively, indicating a significant difference between these two groups (P<0.001, Figure 2).
 |
Figure 1 Kaplan–Meier analysis of overall survival based on treatment for gastric cancer patients with synchronous liver metastases. |
 |
Figure 2 Kaplan–Meier analysis of overall survival based on curative resection of synchronous LMGC. |
Univariate analysis showed that surgical resection, median size of liver metastases, solitary liver metastasis and serum AFP levels were associated with patient prognosis, while gender, age, primary tumor location, differentiation, Her-2 expression, and serum levels of CEA and CA199 had no marked impacts on survival. Multivariate analysis revealed that surgical resection (HR=0.453, 95% CI 0.276–0.813; P=0.009) and solitary liver metastasis (HR=0.540; 95% CI 0.315–0.796; P=0.043) were independent predictors of OS (Table 2).
 |
Table 2 Univariate and Multivariate Analyses of Overall Survival |
Chemotherapy and Adverse Events
Oxaliplatin or paclitaxel combined with fluorouracil was the most commonly used chemotherapy regimen. 29.5% (26/88) of the patients with positive Her-2 expression received trastuzumab treatment. The median duration of chemotherapy was slightly but not significantly prolonged in the palliative chemotherapy group (5.8 [ranging from 1 to 12] cycles) compared with the surgical resection group (4.2 [ranging from 1 to 8] cycles) (P>0.05). Treatment-related adverse events and grade-3 or 4 toxicity were found in 65.9% (87/132) and 34.1% (45/132) of the patients, respectively. Leucopenia/neutropenia (22.7%) and thrombocytopenia (6.8%) were the most common hematological toxicities, while elevated levels of serum AST (5.3%) and diarrhea (3.0%) constituted the most common non-hematological adverse effects.
Postoperative Complications
In the surgical resection group, 9 (15.8%) patients developed postoperative complications (Clavien-Dindo grade II~III), including bile leakage (3 cases), pneumonia (3 cases), abdominal abscess (2 cases), and anastomotic leakage (2 cases). All the above complications were successfully alleviated by conservative treatment.
Discussion
LMGC represents a major cause of death in patients with advanced gastric cancer.11 Previous studies have suggested that chemotherapy and supportive care are the main treatment options for LMGC. However, the response rate of chemotherapy alone is low, and the prognosis is not significantly improved.12 Recent studies have shown that complete resection in combination with more effective chemotherapeutic regimens might improve the survival of some patients.13,14 Nevertheless, the optimal treatment strategy for patients with LMGC remains unclear.
A British study assessed 336 cases of advanced gastric carcinoma with liver metastases, and propensity-matched analysis showed that LMGC patients treated by gastrectomy and hepatectomy (GGH group) had significantly improved 1-year (35.9% vs 50.0%, P=0.049) and 5-year (61.5% vs 75.7%, P=0.031) mortality compared to that of the gastrectomy without hepatectomy (GGNH) group.15 In addition, a meta-analysis reported that the 1-, 3- and 5-year survival rates after radical resection of LMGC were 68%, 31% and 27%, respectively, with a median OS of 21 months.16 Our study found that the median OS was markedly improved in the surgical resection group compared with the palliative chemotherapy group (33.6 vs 12.4 months, P<0.001). Further analysis showed that the patients who received R0 resection had significantly better outcomes than those who did not (45.1 vs 13.5 months, P <0.001). Therefore, a subset of LMGC patients might benefit from gastrectomy combined with radical resection of liver metastases, rather than palliative surgical resection.
Evaluation of prognostic factors might help identify patients who could benefit from surgical treatment. Current studies suggest that lymph node metastasis, differentiation, size and the number of liver metastases may be the main factors influencing the prognosis of patients with LMGC.17,18 Liu et al retrospectively analyzed the clinicopathological data of 37 patients with LMGC who underwent surgical resection, and found a 5-year survival rate reaching 50% in patients without lymph node metastasis, which was significantly better than those with lymph node metastasis.19 The degree of liver metastasis determines whether R0 resection could be obtained after surgery, thereby constituting the main prognostic factor in LMGC. Recent studies have shown that patients with LMGC, type H1 and H2, without peritoneal metastasis, have a cumulative 5-year survival rate of 60% after surgery.20 In this study, we also found that OS was markedly better in patients with solitary liver metastasis than those showing multiple liver metastases. Therefore, limited liver metastasis, especially solitary liver metastasis, can achieve a better survival after aggressive surgical resection.
In patients with originally unresectable and marginally resectable LMGC, preoperative chemotherapy could achieve the purpose of downstaging the primary tumor, allowing a higher R0 resection rate.5 In Liu’s study, a total of 15 patients with LMGC received 2–4 cycles of preoperative chemotherapy, and 11 patients were reported to have achieved a partial response (PR). The overall survival rate of patients with preoperative chemotherapy was found to be better than that of patients without preoperative chemotherapy.19 In this study, the survival time of patients with LMGC was not improved by preoperative chemotherapy; however, the R0 resection rate was much higher than that of patients who did not receive preoperative chemotherapy. Therefore, some patients who could not obtain radical resection initially had such opportunity after effective preoperative chemotherapy. In terms of selection for preoperative chemotherapy, fluorouracil combined with platinum or paclitaxel was the most common regimen, with an overall response rate of 34% to 58%.21,22 The TOGA study showed that in advanced gastric cancer with positive expression of Her-2, trastuzumab combined with chemotherapy could significantly improve the response rate and prolong median OS.23 It was reported that the positive rate of Her-2 in LMGC was higher than that of gastric cancer, especially in patients with the intestinal type.24 The results provided a basis for targeted therapy in gastric cancer patients with liver metastasis.
We acknowledge that the present study had several limitations. First, it was a retrospective study performed in a single center. Further, selection bias could not be completely avoided, especially in patients undergoing surgery. However, the above findings might help adequately select candidate cases for surgical treatment among those who demonstrate significant response to chemotherapy or with limited metastases rather than denying them surgery based on conventional treatment strategies.
In this series, some patients with synchronous LMGC were shown to benefit from radical surgical resection performed in combination with appropriate chemotherapy. Surgical resection and solitary liver metastasis were identified as prognostic factors for survival. Further prospective and randomized clinical studies are required to validate the present findings, and establish optimal therapeutic strategies for these patients.
Abbreviations
LMGC, liver metastasis from gastric cancer; OS, overall survival; ECOG, Eastern Cooperative Oncology Group; PS, performance status; ALT, alanine transaminase; AST, aspartate transaminase; NUL, normal upper limit; CDSC, Clavien-Dindo severity classification; CTCAE, Common Terminology Criteria for Adverse Events; PR, partial response.
Data Sharing Statement
The present data cannot be made publicly available due to ethical reasons. The data in the present study may be requested by contacting the authors at [email protected] or Department of Gastric Surgery, Zhejiang Cancer Hospital, Hangzhou 310022, China.
Ethics Approval and Informed Consent
This study was approved by the medical ethics committee of Zhejiang Cancer Hospital (approval number: IRB-2020-112) and conformed to the provisions of the Declaration of Helsinki. Consent to participate was not applicable due to the retrospective nature of the study, and the data were anonymously analyzed.
Acknowledgments
This study was supported by the Natural Science Foundation of Zhejiang Province of China (LY18H160032 to Pengfei Yu) and the Medical Health Science and Technology Project of Zhejiang Provincial Health Commission (2020KY471 to Pengfei Yu).
Author Contributions
All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors have declared that no competing interests exist for this work.
References
1. Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–E386. doi:10.1002/ijc.29210
2. Nishi M, Shimada M, Yoshikawa K, et al. Results of hepatic resection for liver metastasis of gastric cancer. J Med Invest. 2018;65(1.2):27–31. doi:10.2152/jmi.65.27
3. Saiura A, Umekita N, Inoue S, et al. Clinicopathological features and outcome of hepatic resection for liver metastasis from gastric cancer. Hepatogastroenterology. 2002;49(46):1062–1065.
4. Zacherl J, Zacherl M, Scheuba C, et al. Analysis of hepatic resection of metastasis originating from gastric adenocarcinoma. J Gastrointest Surg. 2002;6(5):682–689. doi:10.1016/S1091-255X(01)00075-0
5. Luo Z, Rong Z, Huang C. Surgery strategies for gastric cancer with liver metastasis. Front Oncol. 2019;9:1353. doi:10.3389/fonc.2019.01353
6. Jerraya H, Saidani A, Khalfallah M, et al. Management of liver metastases from gastric carcinoma: where is the evidence? Tunis Med. 2013;91(1):1–5.
7. Shinohara T, Maeda Y, Hamada T, et al. Survival benefit of surgical treatment for liver metastases from gastric cancer. J Gastrointest Surg. 2015;19(6):1043–1051. doi:10.1007/s11605-015-2775-6
8. Picado O, Dygert L, Macedo FI, et al. The role of surgical resection for stage iV gastric cancer with synchronous hepatic metastasis. J Surg Res. 2018;232:422–429. doi:10.1016/j.jss.2018.06.067
9. Thelen A, Jonas S, Benckert C, et al. Liver resection for metastatic gastric cancer. Eur J Surg Oncol. 2008;34(12):1328–1334. doi:10.1016/j.ejso.2008.01.022
10. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–213. doi:10.1097/01.sla.0000133083.54934.ae
11. Lasithiotakis K, Antoniou SA, Antoniou GA, et al. Gastrectomy for stage IV gastric cancer. A systematic review and meta-analysis. Anticancer Res. 2014;34(5):2079–2085.
12. Andreou A, Viganò L, Zimmitti G, et al. Response to preoperative chemotherapy predicts survival in patients undergoing hepatectomy for liver metastases from gastric and esophageal cancer. J Gastrointest Surg. 2014;18(11):1974–1986. doi:10.1007/s11605-014-2623-0
13. Shirasu H, Tsushima T, Kawahira M, et al. Role of hepatectomy in gastric cancer with multiple liver-limited metastases. Gastric Cancer. 2018;21(2):338–344. doi:10.1007/s10120-017-0730-9
14. Wu P, Wang P, Ma B, et al. Palliative gastrectomy plus chemotherapy versus chemotherapy alone for incurable advanced gastric cancer: a meta-analysis. Cancer Manag Res. 2018;10:4759–4771. doi:10.2147/CMAR.S179368
15. Markar SR, Mackenzie H, Mikhail S, et al. Surgical resection of hepatic metastases from gastric cancer: outcomes from national series in England . Gastric Cancer. 2017;20(2):379–386.
16. Markar SR, Mikhail S, Malietzis G, et al. Influence of surgical resection of hepatic metastases from gastric adenocarcinoma on long-term survival: systematic review and pooled analysis. Ann Surg. 2016;263(6):1092–1101. doi:10.1097/SLA.0000000000001542
17. Kiyasu Y. Long-term recurrence-free survival after metachronous surgery of the stomach and liver for gastric adenocarcinoma and multiple, synchronous liver metastases: a case report and review of literature. Int Surg. 2013;98(3):241–246. doi:10.9738/INTSURG-D-12-00015.1
18. Liu J, Li JH, Zhai RJ, et al. Predictive factors improving survival after gastric and hepatic surgical treatment in gastric cancer patients with synchronous liver metastases. Chin Med J (Engl). 2012;125(2):165–171.
19. Liu YJ, Wang GC, Wan XB, et al. Surgical resection for gastric cancer patients with liver metastasis. Zhonghua Zhong Liu Za Zhi. 2017;39(7):532–535. doi:10.3760/cma.j.issn.0253-3766.2017.07.011
20. Ueda K, Iwahashi M, Nakamori M, et al. Analysis of the prognostic factors and evaluation of surgical treatment for synchronous liver metastases from gastric cancer. Langenbecks Arch Surg. 2009;394(4):647–653. doi:10.1007/s00423-008-0311-9
21. Kito Y, Machida N, Kawai S, et al. Phase II study of S-1 plus oxaliplatin 130 mg/m2 in Japanese patients with advanced gastric cancer. Int J Clin Oncol. 2018;23(6):1084–1089. doi:10.1007/s10147-018-1308-1
22. Bian NN, Wang YH, Min GT. S-1 combined with paclitaxel may benefit advanced gastric cancer: evidence from a systematic review and meta-analysis. Int J Surg. 2019;62:34–43. doi:10.1016/j.ijsu.2018.11.010
23. Bang YJ, Van Cutsem E, Feyereislova A, et al. ToGA Trial Investigators. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a Phase 3, open-label, randomised controlled trial. Lancet. 2010;376(9742):687–697. doi:10.1016/S0140-6736(10)61121-X
24. Dang HZ, Yu Y, Jiao SC. Prognosis of HER2 over-expressing gastric cancer patients with liver metastasis. World J Gastroenterol. 2012;18(19):2402–2407. doi:10.3748/wjg.v18.i19.2402
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
