Back to Journals » OncoTargets and Therapy » Volume 15
Treatment of PD-1 Inhibitor-Associated Toxic Epidermal Necrolysis: A Case Report and Brief Review
Authors Zhao Y, Cao Y, Wang X, Qian T
Received 17 December 2021
Accepted for publication 28 March 2022
Published 8 April 2022 Volume 2022:15 Pages 345—351
DOI https://doi.org/10.2147/OTT.S353743
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sanjeev K. Srivastava
Yidong Zhao,1,* Yuzhen Cao,2,* Xiuyu Wang,3 Tianyi Qian4
1Department of Dermatology, Changshu NO.2 People’s Hospital, Jiangsu, 215500, People’s Republic of China; 2Department of Oncology, Changshu NO.2 People’s Hospital, Changshu NO.2 People’s Hospital, Jiangsu, 215500, People’s Republic of China; 3Department of Nursing, Changshu NO.2 People’s Hospital, Jiangsu, 215500, People’s Republic of China; 4Department of Medical Oncology, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, 100021, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Tianyi Qian, Peking Union Medical College, Chinese Academy of Medical Sciences, Beijing, 100005, People’s Republic of China, Email [email protected]
Background: Sintilimab is a fully human monoclonal antibody targeting PD-1, which has been considered well tolerated among patients and widely applied in malignancies.
Case Presentation: We present a case report of a patient with gallbladder carcinoma treated with sintilimab who developed toxic epidermal necrolysis (TEN). A 72-year-old female presented with fever and maculopapular rash after receiving one dose of sintilimab for metastatic gallbladder carcinoma. Widespread maculopapular rashes with progressive skin detachment occurred within one week. Early skin biopsy of the patient showed apoptotic keratinocytes along with interface dermatitis. She was initially treated with escalating methylprednisolone (from 0.8 to 1.6 mg/kg/d) and subsequently in the combination of intravenous immunoglobulin. Her skin lesions significantly improved, and satisfying re-epithelialization was achieved after 43 days of hospitalization.
Conclusion: Because of the high mortality of grade four immune related adverse event (irAE) on skin, we recommend early monitoring and recognition of symptoms. During management, high-dose glucocorticoids with combined intravenous immune globulin and supportive care may be helpful.
Keywords: sintilimab, anti-PD1, programmed death-1 inhibitor, immune checkpoint inhibitor, toxic epidermal necrolysis, TEN, skin toxicity, immune-related adverse events, irAE
Introduction
Sintilimab is a fully human monoclonal IgG4 antibody that directly binds to programmed cell death receptor-1 (PD-1).1 In malignancies, overexpression of PD-1 receptor ligand (PD-L1) anergizes T cells and thus inhibits anti-tumor immunity. PD-1 inhibitors block PD-L1 from binding to T cells and result in enhancement of immunity and tumor control. Immune checkpoint inhibitors (ICIs) are deemed well tolerated and exhibiting durable anti-tumor activity when compared with cytotoxic agents. Nevertheless, administration of ICIs could cause a group of immune-related adverse events (irAE) due to immune upregulation, of which dermatologic toxicity has been reported as the most frequent manifestations.2 Maculopapular and morbilliform eruptions may occur in 15% of patients during therapy and barely cause significant consequences.2 Life-threatening reactions, namely acute Stevens-Johnson syndrome/toxic epidermal necrolysis (SJS/TEN), may occur as well, with an incidence of less than 1%.3 We report a case of toxic epidermal necrolysis (TEN) associated with sintilimab in gallbladder carcinoma, which was successfully treated during hospitalization.
Case Presentation
A 72-year-old female presented with fever (Tmax 38.0°C) and maculopapular rash after receiving one dose of sintilimab as gallbladder carcinoma systemic therapy (T3N2M1, Stage IVB).
The patient received surgical procedures including cholecystectomy, en bloc hepatic resection, and portal lymphadenectomy 55 days prior to this visit. The lesion histologically contained low-differentiated adenocarcinoma together with signet-ring cell carcinoma. Test identified high microsatellite instability (MSI-H) in the tumor. After surgery, an elevated CEA level (65.4ng/mL) and a re-staging scan using PET-CT showed suspicious mesenteric metastasis. The patient started receiving sintilimab in combination with anlotinib on day-13 as tentative systemic therapy. Written informed consent was obtained from the patient.
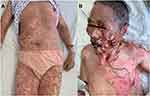
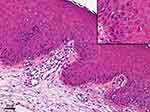
The patient presented with a sporadic mild rash (grade 1, SCORTEN score 2) on body trunk and proximal lower extremities two weeks after receiving one dose of sintilimab. Intravenous infusion of 40 mg of methylprednisolone (0.8mg/kg/d) was applied on the first day. The patient still had a low-grade fever and developed a more extensive rash on day 2, and thus she was given a higher dose of methylprednisolone (1.2 mg/kg/d) in the next two days and admitted to hospital on day 3. Within one day during hospitalization, the rashes connected into large-area maculopapular skin rash with bullae (SCORTEN score 3), and epidermal detachment appeared, in which direct Nikolsky’s sign was present (Figure 1). Skin biopsy revealed karyopyknotic keratinocytes in the epidermis and interface dermatitis with lymphocyte infiltration in the dermo-epidermal junction (Figure 2). Immunoassay showed anti-Dsg1, anti-Dsg3, anti-BP180, anti-BP-230 antibodies were negative in patient’s serum. Dosage of methylprednisolone was increased to 80 mg (1.6 mg/kg/d). Meanwhile, intravenous immunoglobulin was added. The skin rash and bullae continued to progress, and around 70% body surface area of skin and mucosa was affected on day 13 (Figure 1). Intravenous immunoglobulin was increased to 800 mg/kg/d for three days, along with albumin infusion and encapsulation therapy (topical halometasone, erythromycin, and epidermal growth factor spray). The score of toxic epidermal necrolysis (SCORTEN)4 achieved five, and thus the patient was referred to a specialized care unit. We utilized an isolation room and bed with horizontal laminar airflow for infection control. Dressing of skin and mucosa lesions was changed once a day, and medical pain management was applied. It significantly improved her skin lesion within one week (Supplementary Figure 1). Blood and exudate cultures reported negative throughout the course. Fungal infection in the oral cavity appeared and subsequently improved with tapering of glucocorticoid (started on day 20, as SCORTEN decreased to 2) and application of oral nystatin. She was dismissed after 43 days of hospitalization with satisfying re-epithelialization and no infection signs (Figure 3). The patient did not receive further anti-tumor medications. Imaging showed no signs of tumor recurrence at the primary site. A mildly elevated CEA was observed during hospitalization (75.57 ng/mL, 3 weeks after sintilimab administration).
The co-medication of the patient includes calcium antagonists for hypertension treatment (switching from amlodipine to nifedipine on day-4). We evaluated the drug causality using method of ALDEN,5 according to which sintilimab was very probable cause (ALDEN 6’), anlotinib was a possible cause (ALDEN 2’), while nifedipine was an unlikely cause (ALDEN 0’) of the disease. All three drugs were classified as possible causes according to Naranjo’s Scale (sintilimab 3’; anlotinib 1’; nifedipine 1’).6 The detailed algorithms were listed in Supplementary Tables 1 and 2.
Discussion
Gallbladder carcinoma has a low-incidence and poor-prognosis.7,8 Nevertheless, it is the most common biliary tract carcinoma (BTC) worldwide. In 2017, the BilCap study reported the benefit of adjuvant capecitabine in overall survival and relapse-free survival,9 thus capecitabine has been considered as a preferred adjuvant option following potentially curative resection of BTC since then. Advanced BTC displayed limited efficacy upon chemotherapy, of which most studies were negative over the past 20 years, and clinical practice has barely changed.10 As for first-line treatment, cisplatin/gemcitabine was recommended as a reference regimen. However, there is no evidence supporting second-line chemotherapy usage in advanced BTC hitherto.11 The main problem lies in the poor physical status of patients due to disease progression during first-line therapy and complications, such as biliary obstruction. Hence, the application of novel therapeutic targets and the development of practice-changing regimes are in urgent need to improve the prognosis of BTC patients.
Tumors with high mutational load are considered more immunogenic and receive better effect upon immunotherapies.12 Mismatch-repair (MMR) deficiency and microsatellite instability (MSI) are classic indicators that are related to tumor mutational load and thus contribute to the efficacy of checkpoint inhibitors.13 MMR and MSI status have been explored in BTCs, yet the results vary from studies. MMR and MSI are uncommon (3.2%) in BTC patients without hereditary nonpolyposis colorectal cancer.14 According to publications, high-level MSI was reported in 5% of gallbladder carcinoma15 while MMR status (MLH1 and MSH2 negative staining) was observed, respectively, in 51.3% and 59% (N = 39) of gallbladder carcinoma.16 Companion diagnostic approaches using next-generation sequencing, such as tumor mutational burden (TMB), have been newly developed to estimate mutational and predict clinical benefit of anti-PD-1 therapy.17,18 The overall mutational load was reported relatively high in BTCs. The median count of non-silent somatic mutations in gallbladder cancer was 64 (N = 28), which is significantly higher than other kinds of BTCs (39 and 35 in intrahepatic and extrahepatic cholangiocarcinoma, respectively).19 Increased expression of PD-1 and PD-L1 (40% and 15%, respectively) was identified in BTC samples.20 In the KEYNOTE-28 study, 37 of 89 (42%) patients with advanced BTC were identified as PD-L1-positive tumors. Among the pembrolizumab-treated group (N = 24), four patients (17%) achieved partial responses, with another four acquiring stable diseases.21 These results are highly encouraging, and studies are in progress to validate the efficacy of checkpoint inhibitors in BTCs.
Currently, SJS and TEN are considered as a continuous spectrum of disease and are differentially diagnosed according to the extent of skin detachment, measured with the percentage of body surface area (BSA) involved.22 TEN have been reported in nivolumab therapy23–25 while SJS was described more frequently in multiple ICIs, namely nivolumab, ipilimumab, and pembrolizumab26–30 (Table 1). Most cases develop SJS/TEN after the first or second infusion of ICI therapy, including the present case. Two cases of three TEN related to nivolumab were fatal24,25 while the other one patient died four months later,23 and the presented case enriched the experience of treating PD-1 antibody-related extensive TEN.
 |
Table 1 Summary of Immune Checkpoint Inhibitor Induced SJS/TEN Case Reports |
Diagnosis of SJS/TEN is based on clinical features and histopathological findings. Initial manifestations may appear as rapidly progressing macules or papules at body trunk and extremities, along with mucosal involvement and fever. Extensive necrosis and detachment of epidermis follow subsequently. Biopsy of early lesions could manifest as scattered apoptotic keratinocytes in the basal layer of the epidermis. While full-thickness epidermal necrosis and subepidermal bullae may be observed in advanced lesions.31
As for the management of immunotherapy-related grade 4 exfoliative skin toxicity, permanent discontinuation of ICI, and immediate hospitalization are required. Systemic corticosteroid is recommended and initiated at 1.0 to 2.0 mg/kg/d. Dosage tapering after symptom improvement should last 4 to 6 weeks. One could consider a combination of cyclosporine or intravenous immunoglobulin in corticoid non-responders.28,32 Supportive care, including nutrition, pain management, and infection prevention, also plays a pivotal role during treatment. To this aspect, we applied encapsulation of corticoid, antibiotics, and epidermal growth factor on exposed dermis for better re-epithelialization and lower risk of infection. Furthermore, specialized wards, including burn units, can help maintain hydration and control sources of infection, especially in patients having lesions of extensive BSA involvement or patients with SCORTEN score ≥2.33
The specific mechanism underlining ICI-related toxic epidermal necrolysis remains controversial. Generally, SJS/TEN was related to massive apoptosis of keratocytes due to cell-mediated cytotoxic reaction.34 Studies have shown that the cytotoxic T cells involved are drug specific and directed against the native form of the drug.35 However, irAEs caused by immune check point inhibitors are considered being associated with over-activation of the whole immune system rather than a hypersensitive reaction against the drug. It is hypothesized that blockade of immune checkpoint molecules could cause imbalances in immunologic tolerance that lead to severe immune response as TEN, which is mainly mediated by T cells.36 In melanoma, common antigen that co-expressed on both tumor cells and skin has been reported, indicating the role of autologous antigen specific cytotoxic T cells during the development of irAEs.37 We deem that the pathomechanism of the disease might relate both to overall immune system activation and tumor-related autologous antigens, further investigations are required for verification.
Incomplete evaluation of skin lesions is the major limitation of the presented case. We deem that direct and indirect immunofluorescence, serology and pathology tests should be performed when available for diagnosis confirmation and exclusion of other conditions that may mimic SJS/TEN.
Conclusions
This study reported the first case of sintilimab-related toxic epidermal necrolysis that was successfully treated. Further studies are needed to clarify the mechanisms of severe skin toxicity following PD-1 blockade. On the whole, we highlight the necessity of early recognition, timely intervention, and supportive care during management of grade 4 skin toxicity of immune therapy.
Data Sharing Statement
Additional data and materials related to the lab tests, pathologic reports, treatment information, and images are available from the corresponding author upon reasonable request.
Consent
This research was approved by the ethics committee of Changshu NO.2 People’s Hospital. Written informed consent including a patient perspective for publication of clinical details and photographs was obtained from the patient.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Hoy SM. Sintilimab: first global approval. Drugs. 2019;79(3):341–346. doi:10.1007/s40265-019-1066-z
2. Sibaud V. Dermatologic reactions to immune checkpoint inhibitors: skin toxicities and immunotherapy. Am J Clin Dermatol. 2018;19(3):345–361. doi:10.1007/s40257-017-0336-3
3. Wang PF, Chen Y, Song S-Y, et al. Immune-related adverse events associated with anti-PD-1/PD-L1 treatment for malignancies: a meta-analysis. Front Pharmacol. 2017;8:730. doi:10.3389/fphar.2017.00730
4. Bastuji-Garin S,Fouchard N, Roujeau JC, Revuz J, Wolkenstein P, Bastuji-Garin S. SCORTEN: a severity-of-illness score for toxic epidermal necrolysis. J Invest Dermatol. 2000;115(2):149–153. doi:10.1046/j.1523-1747.2000.00061.x
5. Sassolas B, Haddad C, Mockenhaupt M, et al. ALDEN, an algorithm for assessment of drug causality in Stevens-Johnson syndrome and toxic epidermal necrolysis: comparison with case-control analysis. Clin Pharmacol Ther. 2010;88(1):60–68.
6. Naranjo CA,Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30(2):239–245. doi:10.1038/clpt.1981.154
7. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7–30. doi:10.3322/caac.21590
8. Bridgewater J,Lopes A, Wasan H, et al. Prognostic factors for progression-free and overall survival in advanced biliary tract cancer. Ann Oncol. 2016;27(1):134–140. doi:10.1093/annonc/mdv483
9. Primrose JN, Fox R, Palmer DH, et al. Adjuvant capecitabine for biliary tract cancer: the BILCAP randomized study. J Clin Oncol. 2017;35(15_suppl):4006. doi:10.1200/JCO.2017.35.15_suppl.4006
10. Eckel F, Schmid RM. Chemotherapy in advanced biliary tract carcinoma: a pooled analysis of clinical trials. Br J Cancer. 2007;96(6):896–902. doi:10.1038/sj.bjc.6603648
11. Lamarca A, Hubner RA, David Ryder W, et al. Second-line chemotherapy in advanced biliary cancer: a systematic review. Ann Oncol. 2014;25(12):2328–2338. doi:10.1093/annonc/mdu162
12. Alexandrov LB, Nik-Zainal S, Wedge DC, et al. Signatures of mutational processes in human cancer. Nature. 2013;500(7463):415–421. doi:10.1038/nature12477
13. Le DT, Uram JN, Wang H, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372(26):2509–2520. doi:10.1056/NEJMoa1500596
14. Rashid A,Ueki T, Gao YT, et al. K-ras mutation, p53 overexpression. and microsatellite instability in biliary tract cancers: a population-based study in China. Clin Cancer Res. 2002;8(10):3156–3163.
15. Silva VWK, Askan G, Daniel TD, et al. Biliary carcinomas: pathology and the role of DNA mismatch repair deficiency. Chin Clin Oncol. 2016;5(5):62. doi:10.21037/cco.2016.10.04
16. Kohya N, Miyazaki K, Matsukura S, et al. Deficient expression of O(6)-methylguanine-DNA methyltransferase combined with mismatch-repair proteins hMLH1 and hMSH2 is related to poor prognosis in human biliary tract carcinoma. Ann Surg Oncol. 2002;9(4):371–379. doi:10.1007/BF02573872
17. Johnson DB, Frampton GM, Rioth MJ, et al. Targeted next generation sequencing identifies markers of response to PD-1 blockade. Cancer Immunol Res. 2016;4(11):959–967. doi:10.1158/2326-6066.CIR-16-0143
18. Chalmers ZR, Connelly CF, Fabrizio D, et al. Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genome Med. 2017;9(1):34. doi:10.1186/s13073-017-0424-2
19. Nakamura H, Arai Y, Totoki Y, et al. Genomic spectra of biliary tract cancer. Nat Genet. 2015;47(9):1003–1010. doi:10.1038/ng.3375
20. Holcombe RF, Xiu J, Pishvaian MJ, et al. Tumor profiling of biliary tract carcinomas to reveal distinct molecular alterations and potential therapeutic targets. J Clin Oncol. 2015;33(3_suppl):285. doi:10.1200/jco.2015.33.3_suppl.285
21. Bang YJ, Doi T, Braud FD, et al. 525 safety and efficacy of pembrolizumab (MK-3475) in patients (pts) with advanced biliary tract cancer: interim results of KEYNOTE-028. Eur J Cancer. 2015;51:S112. doi:10.1016/S0959-8049(16)30326-4
22. Bastuji-Garin S, Rzany B, Stern RS, Shear NH, Naldi L, Roujeau JC. Clinical classification of cases of toxic epidermal necrolysis, Stevens-Johnson syndrome, and erythema multiforme. Arch Dermatol. 1993;129(1):92–96. doi:10.1001/archderm.1993.01680220104023
23. Nayar N, Briscoe K, Fernandez Penas P. Toxic epidermal necrolysis-like reaction with severe satellite cell necrosis associated with nivolumab in a patient with ipilimumab refractory metastatic melanoma. J Immunother. 2016;39(3):149–152. doi:10.1097/CJI.0000000000000112
24. Vivar KL, Deschaine M, Messina J, et al. Epidermal programmed cell death-ligand 1 expression in TEN associated with nivolumab therapy. J Cutan Pathol. 2017;44(4):381–384. doi:10.1111/cup.12876
25. Griffin LL, Cove-Smith L, Alachkar H, et al. Toxic epidermal necrolysis (TEN) associated with the use of nivolumab (PD-1 inhibitor) for lymphoma. JAAD Case Rep. 2018;4(3):229–231. doi:10.1016/j.jdcr.2017.09.028
26. Ito J, Fujimoto D, Nakamura A, et al. Aprepitant for refractory nivolumab-induced pruritus. Lung Cancer. 2017;109:58–61. doi:10.1016/j.lungcan.2017.04.020
27. Hwang A, Iskandar A, Dasanu CA. Stevens-Johnson syndrome manifesting late in the course of pembrolizumab therapy. J Oncol Pharm Pract. 2019;25(6):1520–1522. doi:10.1177/1078155218791314
28. Saw S, Lee HY, Ng QS. Pembrolizumab-induced Stevens-Johnson syndrome in non-melanoma patients. Eur J Cancer. 2017;81:237–239. doi:10.1016/j.ejca.2017.03.026
29. Haratake N, Tagawa T, Hirai F, et al. Stevens-Johnson syndrome induced by pembrolizumab in a lung cancer patient. J Thorac Oncol. 2018;13(11):1798–1799. doi:10.1016/j.jtho.2018.05.031
30. Pollack MH, Betof A, Dearden H, et al. Safety of resuming anti-PD-1 in patients with immune-related adverse events (irAEs) during combined anti-CTLA-4 and anti-PD1 in metastatic melanoma. Ann Oncol. 2018;29(1):250–255. doi:10.1093/annonc/mdx642
31. Rzany B, Hering O, Mockenhaupt M, et al. Histopathological and epidemiological characteristics of patients with erythema exudativum multiforme major, Stevens-Johnson syndrome and toxic epidermal necrolysis. Br J Dermatol. 1996;135(1):6–11.
32. Brahmer JR, Lacchetti C, Thompson JA. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology clinical practice guideline summary. J Oncol Pract. 2018;14(4):247–249. doi:10.1200/JOP.18.00005
33. McCullough M, Burg M, Lin E, et al. Steven Johnson syndrome and toxic epidermal necrolysis in a burn unit: a 15-year experience. Burns. 2017;43(1):200–205. doi:10.1016/j.burns.2016.07.026
34. Correia O, Delgado L, Ramos JP, Resende C, Torrinha JA. Cutaneous T-cell recruitment in toxic epidermal necrolysis. further evidence of CD8+ lymphocyte involvement. Arch Dermatol. 1993;129(4):466–468. doi:10.1001/archderm.1993.01680250078010
35. Nassif A, Bensussan A, Boumsell L, et al. Toxic epidermal necrolysis: effector cells are drug-specific cytotoxic T cells. J Allergy Clin Immunol. 2004;114(5):1209–1215. doi:10.1016/j.jaci.2004.07.047
36. Weber JS, Yang JC, Atkins MB, et al. Toxicities of immunotherapy for the practitioner. J Clin Oncol. 2015;33(18):2092–2099. doi:10.1200/JCO.2014.60.0379
37. Krenacs T, Kiszner G, Stelkovics E, et al. Collagen XVII is expressed in malignant but not in benign melanocytic tumors and it can mediate antibody induced melanoma apoptosis. Histochem Cell Biol. 2012;138(4):653–667. doi:10.1007/s00418-012-0981-9
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.