Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 16
Treatment of Painful Diabetic Neuropathy Using Frequency Rhythmic Electro Magnetic Neural Stimulation (FREMS); Effectiveness in Daily Practice
Authors Imholz B , Heijster J, Tahrani A , Kooy A
Received 16 December 2022
Accepted for publication 2 May 2023
Published 11 May 2023 Volume 2023:16 Pages 1383—1391
DOI https://doi.org/10.2147/DMSO.S401727
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Konstantinos Tziomalos
Ben Imholz,1 Jack Heijster,1 Abd Tahrani,2 Adriaan Kooy3
1Department of Internal Medicine, ETZ Ziekenhuis, Tilburg, the Netherlands; 2Department of Endocrinology and Diabetes, University Hospitals Birmingham NHS Foundation Trust, Birmingham, UK; 3Department of Internal Medicine, Bethesda Hospital, Treant Care Group, Hoogeveen, the Netherlands
Correspondence: Ben Imholz, Department of Internal Medicine, ETZ-Location Waalwijk, Kasteellaan 2 5141BM Waalwijk, Tilburg, the Netherlands, Email [email protected]
Background: Painful diabetic peripheral neuropathy (PDPN) is common and difficult to treat with limited treatment options. We assessed the efficacy of frequency rhythmic electromagnetic neural stimulation (FREMS) in patients with PDPN.
Methods: An uncontrolled prospective survey of patients with PDPN and pain despite at least two lines of pharmacotherapy. The primary outcome, 50% reduction in pain scores at 1 and/or 3 months post FREMS. FREMS was applied to both legs below the knees using 4 sets of electrodes per leg; the treatment consisted of 10 sessions of 35 min applications given over 14 days. FREMS was repeated every 4 months and patients were followed up for 12 months. Pain was assessed using the neuropathic pain symptom inventory (NPSI) and quality of life (QOL) by the EQ-5D.
Results: Out of 336 subjects, 248 patients met the inclusion criteria (56% men), average age and diabetes duration were 65 and 12.6 years respectively. FREMS was associated with a median decrease NPSI of 31% at M1 (range − 100;+93%), and a median decrease of − 37.5% at M3 (range − 100;+250%). The 50% reduction in pain was reached in 80/248 (32.3%) and 87/248 (35.1%) after M1 and M3 respectively. The change in NPSI was accompanied by a decrease in self reported use of opiates of > 50%.
Conclusion: FREMS treatment was associated with a significant reduction in pain severity over a three months period in patients who did not have adequate response to pharmacotherapy. Randomised (sham)-controlled trials examining the role of FREMS as a treatment for PDPN in non-responders to pharmacotherapy are needed.
Keywords: diabetic neuropathy, electrical stimulation, FREMS, NPSI pain score, quality of life, one-year follow-up
Plain Language Summary
Electrical stimulation by means of the frequency rhythmic electromagnetic neural stimulation (FREMS) characteristics was not earlier reported in a large population of patients, inadequately treated with classical treatment options for painful diabetic neuropathy (DN). The survey shows real-life data in 248 patients. The data show a significant effect after 10 days of treatment lasting for 4 months. In subjects defined as responders, the effect persisted with two additional 10 day treatment sessions during a one-year follow-up. In these subjects a significant >50% decrease in medication use such as morphine was documented. Our survey indicates FREMS as a possible treatment option in difficult to treat painful DN.
Introduction
Painful diabetic peripheral neuropathy (PDPN) is common and affects 20–30% of patients with diabetes mellitus (DM) in Europe with higher prevalence in Africa and the Middle East.1 Different international, European and North American guidelines have generally recommended tricyclic agents (TCA), serotonin–norepinephrine reuptake inhibitors (SNRIs), and γ-aminobutyric acid (GABA) analogues as first and second line treatment, the order of which differs between the different guidelines as does the recommendations to use morphine or tramadol.2
Despite the above-mentioned treatments, a significant proportion of patients with PDPN do not respond to treatments (up to 75%) and need more sophisticated regimens to relieve pain.1–3 In addition, the economic impact of PDPN is significant with an estimated health care cost of 2000–4000 euros per year and loss of productivity cost of 6000–17,000 euros per year depending on pain severity.4 Hence, new modalities for treatment are needed.
Frequency rhythmic electromagnetic neural stimulation (FREMS) is a characteristic type of nerve stimulation (FremsLife, Genua, Italy). The signal is free from nerve adaptation since it uses sequences of modulated electrical stimuli that vary automatically in terms of pulse frequency, duration and voltage amplitude. The FREMS method was designed on the basis of the hypothesis that the summation of sub-threshold electrical stimuli, conveyed through the skin proximal to a motor nerve in a non-invasive system, would induce composite motor action potentials in excitable tissues. A single impulse of low intensity and short duration, such as that used by conventional electrotherapies, is unable to overcome the dielectric skin barrier to excite the underlying nervous or muscular tissue. However, FREMS achieves this effect through specific sequences of weak impulses, characterised by a rapid increase and decrease in pulse frequency and duration, which result in the gradual recruitment of membrane potentials in the stimulated tissues.5 In 2 randomised sham-controlled trials (RCTs) the technique was compared to placebo. In 31 subjects Bosi described a significant decrease in night-time and day-time visual analogue scale (VAS) scores compared to placebo with an improvement of sensory nerve measures such as tactile perception and foot vibration threshold.6 Similar results were obtained in a larger multicentre study (N = 110). However, these RCTs did not explore the best place of FREMS in the treatment paradigm of PDPN (for example, first line vs third or fourth line). In our institute FREMS was used and appeared promising but true insight of its effect was not investigated. Hence, we conducted a survey to assess the impact of FREMS in patients with PDPN who did not respond to other pharmacotherapy agents. The primary aim was to monitor the impact on pain scores, a secondary aim was the impact on quality of life.
Methods
Design
The design is summarised in Figure 1. We conducted an uncontrolled longitudinal survey over 12 months with the primary endpoint measured at 1 and 3 months. At 1 and 3 months, those who responded to the FREMS treatment were followed up for 2 additional treatments (8 months).
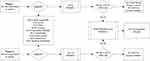 |
Figure 1 Flowchart of patient selection in 2 phases of investigation. |
Medical Ethical Committee
The design of the survey was submitted to “Medische Etische Toetsing Onderzoek Patiënten en Proefpersonen” (METOPP M410/2011) and was approved. All subjects gave written informed consent prior to any survey procedures in accordance with the Declaration of Helsinki.
Population
The survey was conducted in two phases. In the first phase the effect of a single FREMS treatment was evaluated. The results induced the second phase where the efficacy during a one-year follow-up was evaluated. Patients were recruited in the two phases from our outpatient department and via an advert in local newspapers and online (Figure 1). Patients were included in the study if they had a diagnosis of PDPN (based on physician diagnosis) and still had significant pain despite treatment by 2 different pharmacological treatments including at least pregabalin or duloxetine. Patients were excluded if they had other causes of pain such as peripheral vascular disease, or have contra-indications to FREMS as per manufacturer instructions (epilepsy, pregnancy, malignancy, implantable devices such as pacemakers, neurostimulators and defibrillators).
The Treatment Protocol
Treatment with electro stimulation by FREMS was applied with the Aptiva device (FREMSLife, Genova, Italy). The device (right panel) and example of application (left panel) is shown in Figure 2. The technique uses four independent channels with each two pairs of electrodes (left and right leg). Before each session the maximal intensity voltage of neuro stimulation for each pair of electrodes was identified by increasing the intensity stepwise by 1 V/step to maximal tolerated not-painful sustainable voltage by the patient. This treatment is applied for 10 days/sessions over 2 weeks, 5 days/week with a break over the weekend, each session lasting 35 minutes.
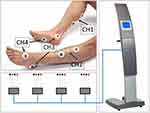 |
Figure 2 The Aptiva device used in the survey (right panel), connected with 4 pairs of electrodes to the legs (left panel). |
Study Endpoints and Assessments
The study's primary end-point was an at least 50% decrease in the neuropathic pain symptom inventory (NPSI7) at month 1 (M1) and/or month 3 (M3). Secondary end points were changes in NPSI at M6, M8, M10 and M12 and changes in EQ-5D at M1, M3, M6, M8, M10 and M12.
A clinically relevant change in pain was defined as an at least one-third reduction of pain score.
The NPSI is validated and well accepted, and contains 10 questions that score different characteristics of neuropathic pain in the last 24 hours from 0–10 and 2 questions that score pain duration and number of pain attacks.7
The quality of life (QOL) was assessed using the EQ-5D.8 The EQ-5D score has two components. In 5 questions disabilities in walking, dressing up and daily activities are scored as none (score = 0), intermediate (score = 1) and total (score = 2). In addition, extent of pain and gloom are scored similarly. These questions give a total disability score (TDS) that ranges from 0 to 10. The second part of the EQ-5D score is a visual linear QOL-score where a line is drawn on a linear scale from 0–100 indicating the perceived QOL status of the subject at the time of answering the questionnaire.
To assess the effect of FREMS on medication used we collected self-reported medication use during the survey.
Statistical Analysis
Categorical data was summarised as frequencies. Continuous data was summarised as mean standard deviation (SD) or mean interquartile range (IQR) depending on data distribution.
Changes in the NPSI and EQ-5D from baseline to 1 and 3 months was analysed using the paired t test. Changes in the NPSI total score over the 3 and 12 months was compared using repeated measures ANOVA. All patients who received at least one FREMS treatment were included in the analysis. A p < 0.05 was considered significant.
Results
A total of 336 patients responded to the invitation. The following patients were excluded: 33 whose neuropathic pain was not due to diabetes; 9 who were treatment-naive, 14 had peripheral vascular disease, 16 whose diabetic neuropathy was not painful, 4 due to FREMS related contra-indications (pacemaker and cancer), and 2 because they were participating in other studies. Hence, 258 subjects were eligible for the study. A further 10 patients were excluded as they cancelled participation before the first treatment session.
A summary of the study flow chart is in Figure 1.
Basic characteristics of the 248 subjects participating are summarised in Table 1.
 |
Table 1 Clinical Characteristics of Subjects |
Changes in Pain Scores
Before FREMS treatment the average NPSI in our subjects was 5.0±1.8. After 1 and 3 months the average changed to 3.4±2,0 and 3.2±2,0 respectively (p < 0.001 vs baseline for both comparisons) (Figure 3). The ANOVA repeated measures showed significant changes in NPSI over the 12 months period (p < 0.001).
 |
Figure 3 Percentage changes in NPSI from baseline in all subjects (#) during period I, ordered from increase to decrease at 1 month (black line) and at 3 months (red line) after start FREMS-1. |
The results are shown in Figure 3 as a “waterfallplot”. The percentage changes of NPSI from baseline at M1 and M3 are given; it shows the individual %-change at M1 (solid line) and M3 (dashed line) ordered from increases to decreases.
50% decreases in NPSI scores were seen in 80/248 subjects at M1 (32.2%) and in 87/248 at M3 (35.1%). Clinically relevant changes, defined as at least a 33.3% decrease in pain, were seen in 111 (44.8%) subjects at M1, in 123 (49.6%) subjects at M3.
Changes in QOL and TDS
The effect after the first treatment is seen in Figure 4. The ANOVA repeated measures showed significant improvements in QOL over the 3 months. Before FREMS the average QOL was 52.6±15.9%. After 1 and 3 months the averages improved to 62.8±14.6% and 63.6±16.7%, respectively (P < 0.001 paired T-test).
The average TDS at baseline was 4.0±1.5. These values were lower at M1 (3.2±1.6) and M3 (3.2±1.7). The changes from baseline to M1 and M3 were significant (P < 0.001 paired T-test).
One Year Follow-Up (NPSI)
As shown in Figure 1, 24 subjects from Phase 1 and 88 subjects from Phase 2 were eligible for the one year treatment phase (ie had >50% reduction on NPSI scores by 1 and/or 3 months). Nine-teen patients were excluded from the analysis (1 patient died and 18 refused additional treatment). Thus a total of 93 patients were included in the analysis of this part of the survey. A total of 79 subjects completed the full one-year follow-up. Reasons for discontinuation were diverse; inability to travel to the hospital (n = 6), complicating comorbidity (n = 4), loss of response (n = 1) and unknown (n = 4).
In all data the ANOVA did not reveal significant differences between NPSI from 3 till 12 months, showing that the initial response to treatment was maintained with repeated sessions up to 12 months (Figure 3). Similar results were found in regards to QOL and TDS.
Self-Reported Use of Medication
Figure 5 shows the self-reported medication use of the subjects implemented in the one-year follow-up. It shows an evident and continuous decrease of medication use during the FREMS treatment period.
Discussion
These results show that a single treatment cycle with FREMS resulted in significant reductions in pain and disability and significant improvements in quality of life in patients with PDPN who were non-responders (or did not tolerate) pharmacotherapy. Concomitant to this improvement in pain, there was a significant reduction in the use of pharmacological agents (including morphine). The treatment did not cause any reported side-effects during the survey period.
The efficacy of FREMS was shown by Bosi et al in two separate RCTs.4,9 However, our data show the usefulness of FREMS in real world practice. More importantly, the RCTs by Bosi et al included any patients with PDPN while we focussed on those who did not respond to previous pharmacotherapy and hence positioning FREMS as a possible third line treatment, which needs to be tested in future RCTs. Our findings are consistent with findings from previous RCTs showing the favourable effects of FREMS on PDPN.4,9
There are several possible plausible mechanisms for the effects FREMS has on PDPN including its effect on neurovascular function. The “continuously changing” electro stimulation has an established effect on sympathetic — vasodilator — functions already described in 2001,10 and was documented for FREMS in 2010.11 It can be expected that these effects improve micro-vascular function and as such effect the shunting of feeding arteries in diabetic neuropathy. The loss of shunting can improve oxygenation of the nerves and influence nociception. In addition an effect of the FREMS signal was documented on vascular endothelial growth factor which by itself may influence perfusion.12
The efficacy of commonly used treatment regimens for PDPN was described in a review recently;2 in RCTs that were FDA approved duloxetine is successful (NNT 5), as is pregabalin (NNT 10).13 However in unselected patients in clinical practice the results are less impressive,14 and side-effects leading to discontinuation is frequent. We showed a good result in our clinical practice FREMS data, which will need recurrent treatments at 3–4 month intervals for chronic pain relief.6
This study has strengths and limitations. The main limitation of this study is the lack of control arm and hence we cannot give good estimates of the placebo effect, which is common in the studies of PDPN. The magnitude of the response, especially the duration of the effect after only 10 days of treatment may suggest an effect not easily attributed to placebo.
However, at least, our survey provides data that can justify an RCT assessing the role of FREMS as a third line treatment option in the management of PDPN and also provide the evidence for FREMS efficacy in a real-world setting. The strengths of our study include a relatively large number of participants compared to other studies in the field;15 a well characterised cohort in that they have failed previous pharmacotherapy and do not have other causes of pain such as malignancy or peripheral vascular disease and we measured the impact on QOL, TDS and medications use.
With FREMS the battle against painful diabetic neuropathy is influenced towards a better outcome, the approach of evaluating macro- and micro-vascular as well as neurological status in a “neuro-vascular outpatient clinic” will be crucial in the ongoing battle of preventing foot damage in the large population of diabetic patients.
In conclusion, a single cycle of FREMS treatment produced significant reductions in pain, disability and medication use and significant improvements in QOL in patients with PDPN who had residual pain or did not respond to previous pharmacotherapy. This proposed use of FREMS needs to be tested in future RCTs to determine efficacy beyond a possible placebo effect and cost effectiveness.
Acknowledgment
The poster was — in part — published by the European Association for the Study of Diabetes: (https://www.easd.org/media-centre/home.html#!resources/treatment-of-painful-diabetic-neuropathy-using-frequency-rhythmic-electro-magnetic-neural-stimulation-effectiveness-in-daily-practice-98e268ee-811d-436b-99c7-fa52b0fa17e3).
Disclosure
Dr Abd Tahrani reports non-financial support from BHR Pharmaceuticals Ltd, during the conduct of the study; grants, personal fees, non-financial support, and other from Novo Nordisk, personal fees from Nestle, grants from Sanofi, personal fees from Gilead, non-financial support from Resmed, outside the submitted work. Dr Abd Tahrani is a Novo Nordisk employee. The authors report no other conflict of interest.
References
1. Azmi S, Petropoulos IN, Ferdousi M, Ponirakis G, Alam U, Malik RA. An update on the diagnosis and treatment of diabetic somatic and autonomic neuropathy. F1000Research. 2019;8:186. doi:10.12688/f1000research.17118.1
2. D’Souza M, Barman R, Joseph A, Abd-Elsayed A. Evidence-based treatment of painful Diabetic Neuropathy: a Systematic Review. Curr Pain Headache Rep. 2022;26(8):583–594. doi:10.1007/s11916-022-01061-7
3. Tesfaye S, Wilhelm S, Lledo A, et al. Duloxetine and pregabalin: high-dose monotherapy or their combination? The COMBO-DN study a multinational, randomized, double-blind, parallel-group study in patients with diabetic peripheral neuropathic pain. Pain. 2013;154:2616–2625. doi:10.1016/j.pain.2013.05.043
4. Alleman CJM, Westerhout KY, Hensen M, et al. Humanistic and economic burden of painful diabetic neuropathy in Europe: a review of the literature. Diabetes Res Clin Pract. 2015;109(2):215–225. doi:10.1016/j.diabres.2015.04.031
5. Bevilacqua M, Barrella M, Toscano R, et al. Disturbances of vasomotion in diabetic (type 2) neuropathy: increase of vascular endothelial growth factor, elicitation of sympathetic efflux and synchronization of vascular flow (vasomotion) during frequency modulated neural stimulation (FREMS).
6. Bosi E, Conti M, Vermigli C, et al. Effectiveness of frequency-modulated electromagnetic neural stimulation in the treatment of painful diabetic neuropathy. Diabetologia. 2005;48:817–823. doi:10.1007/s00125-005-1734-2
7. Bouhassira D, Attal N, Fermanian J, et al. Development and Validation of the Neuropathic Pain Symptom Inventory. Pain. 2004;108:248–257. doi:10.1016/j.pain.2003.12.024
8. Shaw JW, Johnson JA, Coons SJ. US valuation of the EQ-5D health states: development and testing of the D1 valuation model. Med Care. 2005;43(3):203–220. doi:10.1097/00005650-200503000-00003
9. Bosi E, Bax G, Scionti L, et al.; FREMS European Trial Study Group. Frequency-modulated electromagnetic neural stimulation (FREMS) as a treatment for symptomatic diabetic neuropathy: results from a double-blind, randomised, multicentre, long-term, placebo-controlled trial. Diabetologia. 2013;56(3):465–475. doi:10.1007/s00125-012-2795-7
10. Emerson GG, Segal SS. Electrical activation of endothelium evokes vasodilatation and hyperpolarization along hamster feed arteries. Am J Physiol Heart Circ Physiol. 2001;280:H160–H167.
11. Bocchi L, Evangelisti A, Barella M, Scatizzi L, Bevilacua M. Recovery of 0,1 Hz microvascular skin flow in dysautonomic diabetic (type 2) neuropathy by using Frequency Rhythmic Electrical Modulation System (FREMS). Med Eng Phys. 2010;32:407–413. doi:10.1016/j.medengphy.2010.02.004
12. Bevilacqua M, Dominguez LJ, Barella M, Barbagallo M. Induction of vascular endothelial growth factor release by transcutaneous frequency modulated neural stimulation in diabetic polyneuropathy. J Endocrinol Invest. 2007;30:944–947. doi:10.1007/BF03349242
13. Rosenstock J, Tuchman M, LaMoreaux L, Sharma U. Pregabalin for the treatment of painful diabetic peripheral neuropathy: a double-blind, placebo-controlled trial. Pain. 2004;110:628–638. doi:10.1016/j.pain.2004.05.001
14. Butler S, Eek D, Ring L, Gordon A, Karlsten R. The utility/futility of medications for neuropathic pain – an observational study. Scan J Pain. 2019;19(2):327–335. doi:10.1515/sjpain-2018-0317
15. Crasto W, Altaf Q-A, Selvaraj DR, et al. Frequency Rhythmic Electrical Modulation System (FREMS) to alleviate painful diabetic peripheral neuropathy: a pilot, randomised controlled trial (The FREMSTOP study). Diabet Med. 2022;39:e14710. doi:10.1111/dme.14710
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


