Back to Journals » Chronic Wound Care Management and Research » Volume 6
Treatment Of Pain In Wounds With A Topical Long Acting Lidocaine Gel
Authors Treadwell T , Walker D, Nicholson BJ, Taylor M, Alur H
Received 22 July 2019
Accepted for publication 30 October 2019
Published 27 November 2019 Volume 2019:6 Pages 117—121
DOI https://doi.org/10.2147/CWCMR.S224092
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Marco Romanelli
Terry Treadwell,1 Donna Walker,1 BJ Nicholson,1 Maggie Taylor,1 Hemant Alur2
1Institute for Advanced Wound Care, Montgomery, Alabama, USA; 2MilanaPharm, Montgomery, Alabama, USA
Correspondence: Terry Treadwell
Institute for Advanced Wound Care, 2167 Normandie Dr, Montgomery, AL 36111, USA
Tel +1 334 286 3444
Fax +1 334 286 3450
Email [email protected]
Abstract: The treatment of patients with chronic wounds and pain can be frustrating and filled with potential for medication abuse and addiction. In an attempt to improve the management of this chronic wound pain, a gel containing 4% lidocaine in TRI-726 matrix (lidocaine gel) was evaluated in 33 patients with various types chronic painful wounds. In this weeklong study, the new lidocaine gel was applied once on day 0 and patients recorded their perceived pain level for the next 7 days. Mean reported pain scores and pain intensity difference (PID) were statistically significantly lower on days 1–4 compared to day 0 and days 5–7. This new lidocaine gel was effective in reducing the pain in the majority of patients for multiple days after one application. Further studies are warranted to see if long-term use will reduce the amount of pain medication prescribed in this group of patients.
Keywords: topical lidocaine, painful chronic wounds, PID, TRI-726, abuse, addiction
Introduction
In the Unites States, chronic wounds affect the nation’s population and health care costs. It has been estimated that more than six million people suffer from chronic wounds, deriving from decubitus, vascular, inflammatory, and rheumatologic sources.1,2 The number of such people is expected to increase due to the growth in the elderly population and the prevalence of diabetes in such population. Concurrently, the growing population with chronic wounds leads to an increase in medical costs as evidenced by a study showing that chronic wound care cost $9.7 billion in 2004.1 Therefore, an improvement of chronic wound treatment in medical procedures would address a number of social and medical issues.
One of the problems accompanying the presence of a chronic wound is the associated pain that the patient may suffer.3 It has been found that up to 69% of patients with chronic venous ulcers suffer significant pain. Pain may even be severe for patients with an underlying disease process such as diabetic peripheral neuropathy.4–6 Controlling pain in patients with chronic wounds can be a true challenge and for appropriate pain management in these patients, it is necessary to determine the source of the pain, i.e., whether the pain arises from the wound itself or from the underlying disease.3 If the pain is due to the wound itself, treating the wound with moisture retentive wound dressings, controlling infection and the inflammatory environment, assuring adequate circulation, and reducing edema are basic approaches to wound management.2 If the pain is due to the underlying disease such as diabetes, successful pain management requires special care as well as primary care since pain is often worsened by wound treatments, such as dressing changes as well as vulnerable periwound skin.7 In fact, one study showed that it was a major concern for 43% of medical practitioners to control acute pain during wound debridement.8 Another study confirmed that wound treatments themselves such as dressing removal, debridement, and inappropriate dressing selection promote wound-related pain.9 Therefore, it is necessary to use analgesics or anesthetics during wound treatments.
Analgesics categorized into two types, opioids and non-opioids, are frequently used for long-term pain relief in patients with chronic wounds. However, the long-term use of either opioids or non-opioids can lead to tolerance and necessity of dose escalation. The former leads to addiction, dependence, and tolerance while the latter causes a ceiling effect.10,11 To overcome the issue, topical anesthetics are widely used to numb the skin and to relive pain in medical and surgical procedures in anesthesia, ophthalmology, otorhinolaryngology, dentistry, urology, and aesthetic surgery. Among topical anesthetics, lidocaine, tetracaine, benzocaine, and prilocaine in a cream, ointment, or gel are commonly available as prescription and/or over-the-counter (OTC) products.12
Lidocaine (base or hydrochloride salt), either alone or in combination has been used previously for topical anesthesia in painful chronic wounds.6,13–16 It is an amide local anesthetic with a rapid onset and 3–8 hrs of duration of action depending on dose, route, area and purpose of administration.13,15–17 While a plethora of topical lidocaine preparations are available, pain relief in chronic wounds still seems to be short-lived. New and improved topical preparations are thus required. Recently an innovative hydrogel, TRI-726 was introduced for topical administration. The hydrogel is a combination of tri-block copolymer(s) and a natural polysaccharide and focuses on three features 1) in situ gelation, 2) sustained-erosion/release, and 3) adhesion and bioresorption. The vehicle is designed to take advantage of body temperature to undergo sol-to-gel transition and meets the biocompatibility testing requirements of ISO/USP. Characteristics and utility of this vehicle has been demonstrated previously.18,19
This study was done to evaluate the efficacy of 4% lidocaine in this TRI-726 gel (lidocaine gel) in reducing pain in patients with painful chronic wounds. The study was approved and overseen by the Institutional Review Board of Baptist Medical Center South, Montgomery, Alabama, USA, for the involvement of human subjects and was conducted in accordance with the Declaration of Helsinki guidelines.
Materials And Methods
Materials
Lidocaine hydrochloride, citric acid, sodium citrate, mono- and di-basic potassium phosphate, sodium hydroxide (NaOH), and hydrochloric acid (HCl) were purchased from Spectrum Chemicals. The tri block co-polymers/poloxamers (F127, F108 and F68) and xanthan gum were procured from BASF and CP Kelco, respectively. All excipients were used as received without further purification.
Methods
Preparation Of 4% Lidocaine Gel
Hydrogel, TRI-726’s composition, and development are described in detail elsewhere.16,17 Briefly, all ingredients, except the poloxamers, were dissolved in purified water. Poloxamers were then added to this solution by the “cold method” of incorporation.16,17 The pH of the final formulation, if required, was adjusted to 4–6 using either HCl or NaOH. The new lidocaine gel thus prepared under cGMP was filled in a 5 mL, luer-lock polypropylene syringe. The filled syringes were gamma sterilized as the final step.
Patients
Following patient presentation and complaint of painful chronic wound, each patient was evaluated and given an opportunity for voluntary participation in the study. If the patient agreed, study procedures were explained, and a written informed consent was obtained before initiating any treatments. Selection criteria excluded any patients that were on any type of anesthetic medication including lidocaine for their chronic wound. For inclusion in the study, the patient had to present 1) a chronic painful wound (5–50 cm2) and 2) a wound judged not to require debridement.
Study Protocol
Day 0
Patients were evaluated and assessed for eligibility. If all study requirements were met and the patient provided written informed consent, the patient was enrolled in the study and underwent following procedure. Each patient was asked to list his/her pain level on a Numeric Rating Scale (NRS) with 0 being “no pain” and 10 being the “worst pain imaginable.” Once the wound to be treated was clearly identified, it was cleansed with sterile normal saline or sterile water to remove extraneous material, if any. The wound was gently dried with a clean piece of gauze. A thick layer of gel (a maximum of 5 g to cover the wound to the thickness of a dime) was applied to the wound bed and covered with a sterile petrolatum dressing. Next, the wound was wrapped in a standard bandage appropriate for the wound, which remained on until their return on day 7. Patients were sent home with a diary to note their daily pain level. They were also advised to note any adverse reactions.
Day 7
Bandages and dressings were carefully removed. Patient diaries were collected following their evaluation.
Statistical Analysis
All analyses were performed using either the statistical software package (SAS statistical software, SAS Institute Inc, Cary, NC) or the data analysis software (Excel, Microsoft Corporation, Bellevue, WA). Since the pain scores obtained on day 0 were before lidocaine gel application, they were considered as the baseline pain scores. Pain scores from days 0–7 were compared using ANOVA (single factor repeated measures) initially to determine significance, followed by a Tukey HSD post-hoc pairwise test to analyze the differences in pain scores from day 0–7. p-Values of <0.05 were considered significant.
Results
The evaluation included 33 patients of both sexes (14 men (42%) and 19 women (58%)) suffering from pain due to chronic open wounds. The age of the patients ranged from 53 to 89 years. 12 patients had venous leg ulcers (36%), 11 had diabetic foot ulcers (33%), 4 had pressure ulcers (12%), 2 had vasculitic ulcers (6%), 2 had a traumatic wound (6%), 1 had an abdominal wound (3%), and 1 had a second-degree burn (3%) (Table 1).
 |
Table 1 Types Of Wound Presented By The Enrolled Patients |
Initial pain score of the patients prior to the application of lidocaine gel is shown in Table 2. Six (6) patients (18%) recorded a pain score of 10 (worst imaginable pain), 24 patients (72%) indicated 6 or more on the pain scale, and no patient recorded 0 (no pain) pain, which suggests that the participating patients suffered from significant pain from their chronic wounds.
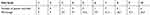 |
Table 2 Distribution Of Enrolled Patients By Pain Scale On Day 0 |
Table 3 shows the pattern of the pain relief over 1 week after the application of the new lidocaine gel. Of all the patients, 2 patients (6%) had no pain relief or were non-responsive (did not feel numbness/relief to gel application throughout the study period) to this new lidocaine gel. Of the 31 responders who reported an improvement, all recorded that they felt pain relief by day 1. Approximately 94% and 77% of the responders continued to feel pain relief by day 2 and 3, respectively. The result shows that the formulated lidocaine gel was effective in providing pain relief after one application in majority of the patients for multiple days. Over half of the responders (58%) reported that the effectiveness of the lidocaine gel lasted for up to 4 days, which is was not expected and cannot be easily explained. However, the efficacy of the lidocaine gel to yield therapeutic benefits was provided.
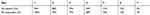 |
Table 3 Pattern Of Pain Relief Over The Study Period Following The Application Of Lidocaine Gel |
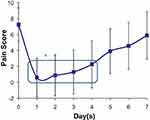
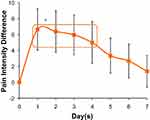
Figure 1 shows the average pain score reported by the patients over 1 week following a single application of the new lidocaine gel on day 0. The average pain score was the lowest on day 1 and slowly increased for the next 6 days. The average pain scores from days 1–4 were statistically significantly lower (ρ<0.01) compared to the baseline (day 0) and days 5–7 pain scores. Figure 2 shows the average pain intensity difference (pain scores normalized to baseline value) over 1 week following a single application of the new lidocaine gel on day 0. The pain intensity difference was maximum on day 1 and slowly decreased over the next 6 days. The average pain intensity difference from days 1–4 were statistically significantly lower (ρ<0.01) compared to the baseline (day 0) and days 5–7 pain intensity difference.
Table 4 shows a comparison of the average pain score prior to and at 1 week after the application of the new lidocaine gel. The average pain score of all patients was 7.2 prior to the application and 6 at 1 week after the application. Among the responders reporting an improvement, the average pain scale was 7 prior to the application and 5.8 at 1 week after the gel application. This table demonstrates that the pain experienced by the enrolled patients was lower even at 1 week than they experienced at the beginning of the study.
 |
Table 4 Comparison Of Average Pain Score Before And 1 Week After Lidocaine Gel Application |
Patients’ comfort level and acceptance of the new lidocaine gel were very high throughout the study. No adverse reactions were reported during the entire study period.
Discussion
Lidocaine, an amide anesthetic has been used as a topical anesthetic to control pain in chronic wound pain for many years.6,13–15 Pain relief with most of the commercially available topical lidocaine preparations lasts a few hours (3–8 hrs) requiring multiple daily applications.13,15–17 The evaluated 4% lidocaine in TRI-726 gel, on the contrary, provided pain relief for multiple days after one application in majority of the patients.
Conclusions
The treatment of patients with chronic wounds and pain can be frustrating and filled with potential for medication abuse and addiction. In an attempt to manage the chronic wound pain, a gel containing 4% lidocaine in TRI-726 matrix was evaluated. Although this study evaluated the efficacy of the new lidocaine gel in a small number of patients, it provided pain relief for multiple days after single application. This result was unexpected and warrants further studies to see if long-term use will reduce the amount of pain medication prescribed in this group of patients, thus curbing the potential for pain medication abuse and addiction.
Disclosure
Dr Alur reports a patent US20180104200A1, pending. Dr Alur is an employee of MilanaPharm, Montgomery, Alabama. The authors report no other conflicts of interest in this work.
References
1. Loesche M, Gardner SE, Kalan L, et al. Temporal stability in chronic wound microbiota is associated with poor healing. J Invest Dermatol. 2017;137(1):237–244. doi:10.1016/j.jid.2016.08.009
2. Powers JG, Higham C, Broussard K, Phillips TJ. Wound healing and treating wounds: chronic wound care and management. J Am Acad Dermatol. 2016;74(4):607–625. doi:10.1016/j.jaad.2015.08.070
3. Price P, Fogh K, Glynn C, Krasner DL, Osterbrink J, Sibbald RG. Managing painful chronic wounds; the wound pain management model. Int Wound J. 2007;4(Suppl 1):4–15. doi:10.1111/j.1742-481X.2007.00311.x
4. Hoffman D, Ryan T, Arnold F, et al. Pain in venous leg ulcers. J Wound Care. 1997;6(5):222–224. doi:10.12968/jowc.1997.6.5.222
5. Phillips T, Stanton B, Provan A, Lew R. A Study of the impact of leg ulcers on quality of life: financial, social, and psychologic implications. J Am Acad Dermatol. 1994;31(1):49–53. doi:10.1016/S0190-9622(94)70134-2
6. Lok C, Paul C, Amblard P, et al. EMLA cream as a topical anesthetic for the repeated mechanical debridement of venous leg ulcers: a double-blind, placebo-controlled study. J Am Acad Dermatol. 1999;40(2 Pt 1):208–213. doi:10.1016/S0190-9622(99)70190-8
7. Woo KY, Coutts PM, Price P, Harding K, Sibbald RG. A randomized crossover investigation of pain at dressing change comparing 2 foam dressings. Adv Skin Wound Care. 2009;22(7):304–310. doi:10.1097/01.ASW.0000305483.60616.26
8. Treadwell T Sharp debridement survey, January 2014.
9. Price PE, Fagervik-Morton H, Mudge EJ, et al. Dressing-related pain, in patients with chronic wounds: an international patient perspective. Int Wound J. 2008;5(2):159–171. doi:10.1111/j.1742-481X.2008.00471.x
10. Serena TE, Yaakov RA, Aslam S, Aslam RS, Preventing, minimizing, and managing pain in patients with chronic wounds: challenges and solution. Chronic Wound Care Manage Res. 2016;3:85–90. doi:10.2147/CWCMR.S85463
11. Reddy M, Kohr R, Queen D, Keast D, Sibbald G. Practical treatment of wound pain and trauma: a patient-centered approach. An overview. Ostomy Wound Mange. 2003;49(Suppl 4):2–15.
12. Kumar M, Chawla R, Goyal M. Topical anesthesia. J Anaesthesiol Clin Pharmacol. 2015;31(4):450–456. doi:10.4103/0970-9185.169049
13. Tipett A. Palliative wound treatment promotes healing. Wounds. 2015;27(1):12–19.
14. Pontani A, Feste M, Lloyd KP, Spalding JB A crossover clinical study of forty-seven patients with painful deep wounds showed use of a hydrogel containing 2% lidocaine hcl and collagen as contact layer was significant in alleviating dressing related pain. Poster presented at: 23rd Clinical Symposium on Advances in Skin & Wound Care: The Conference for Prevention and Healing; October 27–30, 2008; Las Vegas, NV.
15. Golzari SEJ, Soleimanpour H, Mahmoodpoor A, Safari S, Ala A. Lidocaine and pain management in the emergency department: a review article. Anesth Pain Med. 2014;4(1):e15444.
16. Tadicherla S, Berman B. Percutaneous dermal drug delivery for local pain control. Ther Clin Risk Manag. 2006;2(1):99–113.
17. Rowbotham MN, Davies PS, Fields HL. Topical lidocaine gel relieves postherpetic neuralgia. Ann Neurol. 1995;37(2):246–253. doi:10.1002/ana.410370216
18. Mondal P, Alur HH, Johnston TP. Evaluation of TRI-726 as a drug delivery matrix. Drug Dev Ind Pharm. 2011;37(8):995–1001. doi:10.3109/03639045.2011.555913
19. Johnston TP, Mondal P, Pal D, MacGee S, Stromberg AJ, Alur H. Canine periodontal disease control using a clindamycin hydrochloride gel. J Vet Dent. 2011;28(4):224–229. doi:10.1177/089875641102800402
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.