Back to Journals » Infection and Drug Resistance » Volume 11
Treatment of candidemia in a nationwide setting: increased survival with primary echinocandin treatment
Authors Lausch KR, Søgaard M, Rosenvinge FS, Johansen HK, Boysen T, Røder BL, Mortensen KL , Nielsen L, Lemming L, Olesen B, Leitz C, Kristensen L, Dzajic E, Østergaard LJ , Schønheyder HC , Arendrup MC
Received 5 June 2018
Accepted for publication 2 August 2018
Published 23 November 2018 Volume 2018:11 Pages 2449—2459
DOI https://doi.org/10.2147/IDR.S176384
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Joachim Wink
Karen Rokkedal Lausch,1 Mette Søgaard,2,3 Flemming Schønning Rosenvinge,4,5 Helle Krogh Johansen,6 Trine Boysen,7 Bent Løwe Røder,8 Klaus Leth Mortensen,1,9 Lene Nielsen,10 Lars Lemming,9 Bente Olesen,10 Christine Leitz,11 Lise Kristensen,9,12 Esad Dzajic,13 Lars Jørgen Østergaard,1 Henrik Carl Schønheyder,14,15 Maiken Cavling Arendrup6,16,17
1Department of Infectious Disease, Aarhus University Hospital, 8200 Aarhus, Denmark; 2Department of Cardiology, Aalborg University Hospital, 9000 Aalborg, Denmark; 3Aalborg Thrombosis Research Unit, Department of Clinical Medicine, Faculty of Health, Aalborg University, 9000 Aalborg, Denmark; 4Department of Clinical Microbiology, Odense University Hospital, 5000 Odence C, Denmark; 5Department of Clinical Microbiology, Lillebaelt Hospital, 5500 Middelfart, Denmark; 6Department of Clinical Microbiology, Rigshospitalet, 2100 Copenhagen, Denmark; 7Department of Clinical Microbiology, Hvidovre Hospital, 2650 Hvidovre, Denmark; 8Department of Clinical Microbiology, Hospital of Slagelse, Slagelse Sygehus, 4200 Slagelse, Denmark; 9Department of Clinical Microbiology, Aarhus University Hospital, 8200 Aarhus, Denmark; 10Department of Clinical Microbiology, Herlev and Gentofte Hospital, University of Copenhagen, 2730 Herlev, Denmark; 11Department of Clinical Microbiology, Viborg Regionshospital, 8800 Viborg, Denmark; 12Department of Clinical Microbiology, Herning Regionshospital, 7400 Herning, Denmark; 13Department of Clinical Microbiology, Sydvestjysk Sygehus, 6700 Esbjerg, Denmark; 14Department of Clinical Microbiology, Aalborg University Hospital, 9000 Aalborg, Denmark; 15Department of Clinical Medicine, University of Aalborg, 9000 Aalborg, Denmark; 16Unit of Mycology, Statens Serum Institute, 2300 København, Denmark; 17Department of Clinical Medicine, University of Copenhagen, 2200 Copenhagen, Denmark
Background: In accordance with international guidelines, primary antifungal treatment (AFT) of candidemia with echinocandins has been nationally recommended in Denmark since 2009. Our nationwide cohort study describes the management of candidemia treatment focusing on the impact of prophylactic AFT on species distribution, the rate of adherence to the recommended national guidelines for AFT, and the effect of AFT on patient outcomes.
Materials and methods: Incident candidemia cases from a 2-year period, 2010–2011, were included. Information on AFT was retrospectively collected from patient charts. Vital status was obtained from the Danish Civil Registration System. HRs of mortality were reported with 95% CIs using Cox regression.
Results: A total of 841 candidemia patients was identified. Prior to candidemia diagnosis, 19.3% of patients received AFT (162/841). The risk of non-albicans candidemia increased after prior AFT (59.3% vs 45.5% among nontreated). Echinocandins as primary AFT were given for 44.2% (302/683) of patients. Primary treatment with echinocandins resulted in adequate treatment in a higher proportion of patients (97.7% vs 72.1%) and was associated with lower 0- to 14-day mortality compared with azole treatment (adj. HR 0.76, 95% CI: 0.55–1.06). Significantly lower 0- to 14-day mortality was observed for patients with Candida glabrata and Candida krusei with echinocandin treatment compared with azole treatment (adj. HR 0.50, 95% CI: 0.28–0.89), but not for patients with Candida albicans or Candida tropicalis.
Conclusion: The association shown between prior AFT and non-albicans species underlines the importance of treatment history when selecting treatment for candidemia. Compliance with national recommendations was low, but similar to previously reported international rates. Primary treatment of candidemia with echinocandins compared with azoles yielded both a higher proportion of adequately treated patients and improved mortality rates. This real-life setting supports guidelines recommendation, and further focus on compliance with these seems warranted.
Keywords: candidemia, candida, antifungal treatment, echinocandin, azole, Candida albicans, Candida glabrata
Introduction
Management of candidemia is challenging due to suboptimal diagnostics and complex protocols for prophylactic, pre-emptive, empirical, and targeted treatments of different patient categories. The first guidelines from the Infectious Disease Society of America (IDSA) for the management of candidemia were published in 20001 and updated in 2009 as well as in 2016.2,3 The first national treatment recommendations were released in 2004 (Danish Pharmaceutical Product information, www.dli.dk, www.pro.medicin.dk). Recommendations were revised annually, and in 2009, echinocandins became the primary choice of antifungal treatment (AFT) with amphotericin B listed as alternative; for children and hemodynamically stable patients, azoles could still be used. Mandatory National Danish Guidelines for hospital use of antifungals came in 2012 from The Danish Council for the Use of Expensive Hospital Medicine.4 Since then, echinocandins have been the first choice for initial treatment with de-escalation according to susceptibility patterns. Thus, the Danish guidelines followed the international recommendations by replacing azoles with echinocandins as first-line recommendation for the treatment of candidemia.2,5–7 Denmark has a high and increasing proportion of Candida glabrata.8,9 Thus, the shift to echinocandins was motivated by 1) the increasing incidence of the more resistant Candida species such as C. glabrata and Candida krusei and 2) the possible superior efficacy of echinocandins against C. albicans.10
Guidelines are based on randomized trials,10–14 but results from “real-life” settings with assorted patient populations have been more diverse and guidelines more challenging to verify.15–19 Moreover, adherence to guidelines and the clinical effect of AFT have not previously been explored in a nationwide setting. Therefore, this cohort study aimed to describe the management of candidemia, including impact of prior AFT on species distribution, adherence to national recommendations, and clinical outcomes.
Materials and methods
Setting, study population, and data collection
This study included all incident unique cases of candidemia among adults ≥16 years old in Denmark between 2010 and 2011. Patients were identified as part of an ongoing national fungemia surveillance program.20
Information regarding department responsible for the candidemia diagnosis and AFT was retrospectively collected from patient charts by local clinical microbiologists. Treatment information included the following: timing related to blood culture collection (BCC) date, dosage, and duration of initial and subsequent antifungal therapies.
The Danish healthcare system provides health care for all citizens free of charge.
Definitions
Polyfungal cases, defined as two or more Candida species present in blood cultures within 48 hours, were included in the analyses. Polyfungal cases with ≥48 hours between species isolation were excluded due to complications in interpretation of adequate treatment and follow-up time (n=15). Prior, initial, and secondary AFT was categorized as azoles (96.2%–99.1% fluconazole), echinocandins (88.6%–95.5% caspofungin, 4.5%–11.4% anidulafungin), and amphotericin B. De-escalation of AFT was defined as a change from echinocandins or amphotericin B to azoles or from amphotericin B to echinocandins. Escalation of treatment was defined as a change in the opposite direction. Adequate AFT was assessed according to antifungal susceptibility testing.
Microbiology
Information on species identification and susceptibility was retrieved from the National Mycology Reference Laboratory at Statens Serum Institut, Copenhagen, Denmark. Procedures, species distribution, and susceptibility patterns for isolates have been described elsewhere.8 Antifungal susceptibility testing was performed according to available European Committee on Antimicrobial Susceptibility Testing (EUCAST) breakpoints and epidemiological cutoff values (ECOFF).21
Mortality
Mortality data were obtained from The Danish Civil Registration System, in which the vital status, including death and emigration, of Danish citizens is registered and updated daily. Different time intervals were used to estimate treatment efficacy. First, for AFT given prior to the BCC date, mortality at day 7 after BCC was used as a marker for effectiveness. This was chosen as the period influence on mortality is shorter for AFT given prior to BCC. Second, for AFT given after BCC date, mortality at day 14 was used as a marker of targeted therapy given the standard recommendation of 14 days’ duration of therapy. In addition, 30-day and 1-year mortality was assessed. Subanalysis was performed for C. glabrata and C. krusei to investigate the effect of treatment with different antifungal compounds for these more common species, which harbor intrinsic resistance.
Ethics approval
Data collection was approved by the Danish Health authorities (Journal no 3-3013-364/1/) and the Danish Data Protection Agency (Journal no 2004-54-1627).
Statistical analyses
Statistical analyses were carried out in Stata®, vs 14 (StataCorp). Quantitative variables were reported as median and IQR; qualitative variables as number (%). Prevalence proportions were reported with 95% CIs. HRs for treatment effects on mortality was assessed using Cox regression with 95% CIs. Directed acyclic graphs were used to assess minimal sufficient adjustment sets for estimating the total effect of treatment on mortality (Figures S2 and S3).
Results
Candidemia episodes
A total of 841 primary cases of candidemia in adults was included (Figure S1). The median age was 67 years (IQR 58–76) and 60.5% were males.
Species distribution, department, and prior AFT
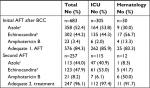
Species distribution varied according to the department where the candidemia diagnosis occurred. Non-albicans species accounted for the majority of cases in medicine (55.0%) and hematology (75.0%) (Figure 1, overall species distribution in Table S1). AFT was given prior to BCC in 19.3% (162/841) of all patients. The proportion of patients receiving AFT prior to BCC varied across departments, lowest in medicine (7.7% [13/169]), and highest in hematology (46.9% [8/32]) (Figure 1).
  | Figure 1 Species distribution and prior AFT by hospital department at the time of candidemia diagnosis. Abbreviation: AFT, antifungal treatment. |
The proportion of C. albicans was lower in patients with prior AFT, most evident for the echinocandins compared with patients without prior AFT (27.3% vs 54.5%; Table 1). The proportion of C. krusei was substantially higher in patients with prior AFT ([12.9% for azoles and 9.1% for echinocandins] vs 2.2% without prior AFT). Similarly, C. glabrata was more prevalent in patients with prior AFT, especially evident for echinocandins (36.4% vs 28.0%). The association between prior AFT and the proportion of C. albicans was also observed at the department level except from the ICU and medicine (Figure 1). Prior AFT was given for >7 days in 39.5% (64/162) of the treated patients and revealed a similar trend on species distribution as described above.
  | Table 1 Species distribution according to prior AFT Notes: aOne hundred twenty-seven cases received fluconazole, three cases received posaconazole, and two cases received voriconazole. bTwenty-one cases received caspofungin and one case received anidulafungin. cOther species, ie, Candida dubliniensis, Candida lusitaniae, and Candida kefyr (see Table S1). Abbreviation: AFT, antifungal treatment. |
AFT after blood culture
Following BCC, 81.2% of patients received AFT. Among patients who survived until the results of blood cultures became available, 92.5% (664/718) received AFT. The recommendation of echinocandins as first-line treatment choice was followed in 44.2% (Table 2). Azoles were the main drug of choice (52.4%, 358/682) except in the Department of Hematology in which 56.7% (17/30) received echinocandins. Overall, the primary AFT was adequate in 84.4% (95% CI: 81.4–87.0). Level of adequate AFT ranged from 72.1% (95% CI: 67.1–76.7) for patients treated with azoles to 97.7% (95% CI: 95.3–99.1) for treatment with echinocandins and 100% (95% CI: 85.2–100) for treatment with amphotericin B (Figure 2).
Change of treatment to a second antifungal agent occurred in 37.6% (257/684) of treated patients after a median duration of 2 days of the first AFT. Escalation of AFT from azoles to echinocandins or amphotericin B was seen more often than change in the opposite direction (55.3%, 142/257). Overall, 96.1% (247/257) of second-line choices were adequate (Figure 2). The recommended de-escalation from echinocandins to azoles according to susceptibility occurred in 103 of 148 patients (69.6%) infected with susceptible species surviving ≥5 days (making the patients eligible for treatment change). Median duration of treatment among survivors (≥14 days) was 15 (IQR 13–20) days. Dosing information was available for 87.9%. For patients receiving primary treatment with fluconazole, 50.8% (133/262) received the recommended loading dose of 800 mg. For patients receiving echinocandins, a loading dose of either 70 mg caspofungin or 200 mg anidulafungin was administered in 81.7% (215/263) of patients
Mortality and treatment
Mortality status was available for 99.5% (837/841). A notable proportion of patients died before blood culture results became available (14.2%, 119/837). These patients were older and more frequently admitted to the ICU; however, fewer of these patients had a central venous catheter (CVC) or received total parenteral nutrition compared with the remainder patients.
A sum of 18.5% did not receive AFT after BCC and 20.6% (32/155) of these survived ≥14 days after BCC. Patients surviving ≥14 days without treatment did not differ from the total cohort of candidemia.
The overall 7- and 14-day mortality among primary cases of candidemia was 23.7% (198/837) and 33.7% (281/835), increasing to 43.3% (362/8337) at day 30 and 62.1% (520/837) at 1 year follow-up. Treatment after BCC was associated with a significantly lower 14-day mortality compared with nontreated patients (adj. HR 0.12, 95% CI: 0.10–0.16, Table 3). Initial treatment with echinocandins was associated with lower 14-day mortality compared with azole treatment (adj. HR 0.76, 95% CI: 0.55–1.06), and the same trend was seen at both 30 days and 1 year follow-up (Table 3). The association was more pronounced for C. glabrata and C. krusei cases at all time points (14-day mortality adj. HR 0.50, 95% CI: 0.28–0.89), whereas no difference was observed for C. albicans and C. tropicalis cases (14-day mortality adj. HR 1.00, 95% CI: 0.65–1.55). Adequate initial treatment was associated with lower 14-day mortality (adj. HR 0.80, 95% CI: 0.53–1.19), whereas for C. glabrata and C. krusei adequate treatment was associated with lower mortality at all time points (14-day mortality adj. HR 0.62, 95% CI: 0.36–1.08).
Discussion
This cohort study evaluated AFT management practices and outcomes in a nationwide clinical setting. Adherence to AFT guidelines following BCC was low as less than half of the patients received echinocandins as initial treatment. Our findings indicated that initial treatment with echinocandins resulted in a greater number of patients achieving sufficient levels of treatment and a lower mortality rate compared with patients treated with azoles. This decrease in mortality was significant among patients with C. glabrata and C. krusei.
A total of 21% of the candidemia patients received prior AFT, which is similar to previously published studies (12%–21%).22–26 Previous studies also demonstrated that prior AFT was associated with more non-albicans species.27,28 Data from the hematology department in our data supported this, where 46.9% received prior AFT and 25.0% of the candidemia isolates were C. albicans. More frequent caspofungin exposure was also associated with Candida strains with reduced caspofungin susceptibility in a matched case–control hematological patients.29 Likewise, prophylactic AFT altered subsequent colonization species toward non-albicans species in a randomized controlled trial.30 A recent Danish study examined colonization species after AFT for candidemia and showed a change toward a higher proportion of strains with intrinsic resistance to azoles following ≥7 days of fluconazole. Furthermore, an increase in acquired resistance to C. glabrata after both azole and echinocandin treatment was observed.31 The current study supports the evidence that prior AFT is associated with a greater proportion of non-albicans at the patient level and at the department level comparing species distribution at the Department of Hematology where a high proportion of patients receive prior AFT with species distribution in departments with lower use of AFT prior to candidemia diagnosis. This association could not be confirmed comparing ICU, medicine, and surgery possible due to small sample size, but potentially also reflecting that other factors influence the species distribution including age, duration of prior AFT, and potential AFT prior to the current hospitalization. Nevertheless, these findings underline the importance of information regarding prior AFT when selecting AFT for a subsequent candidemia.
The low adherence to the recommended guidelines of initial treatment with echinocandins (44.3%) is comparable with other studies that report using echinocandins as primary treatment in 17%–57% of patients with candidemia.16,18,22,23,28 We are unaware of prior nationwide studies on the compliance with treatment recommendations. A study from 87 ICUs in France identified the compliance rate with IDSA or ESCMID guidelines as “rather poor,” given that 62.5% of ICU patients with proven invasive candidiasis (mainly candidemia) received echinocandins as primary treatment.19 We showed that treatment with echinocandins increased the proportion of adequately treated patients and that the overall proportion of candidemia patients receiving adequate treatment was higher than previously described (84.3% vs 57%–68%).18,32,33 Following the completion of our study, mandatory National Guidelines for AFT were implemented in Denmark in 2012 and we cannot exclude that treatment practice has improved over time. However, the mandatory guidelines for AFT resemble the treatment recommendations from 2009 to 12 and except from one recent initiative to survey AFT with an antifungal stewardship programs in the capital region of Denmark, no specific focus have been targeted to AFT guidelines.
Evaluation of treatment effect on outcome in candidemia patients is complicated and especially comparing patients receiving AFT with nontreated patients carries a risk of substantial bias. Surviving long enough to receive treatment, as well as being selected for treatment, highlights a subgroup of patients with a better presumptive survival expectancy. While this issue is frequently unaccounted for in candidemia evaluations, it is routinely accounted for in oncology research investigating survival of responders to nonresponders.34 We included the analysis of treated cases vs nontreated cases at day 14 to illustrate the extremely (unrealistic) advantageous treatment effect rates (HR 0.13, 95% CI: 0.10–0.16) compared with the nontreated. However, we find the comparison of treatment vs nontreatment unusable for further considerations.
We noted a lower 14-day mortality among patients receiving echinocandins compared with patients receiving azoles, which was further substantiated by a lower mortality rate among patients with C. glabrata or C. krusei. Such superior efficacy against intrinsically more azole-resistant species is expected from a mycology point of view and supports the recommendation of echinocandins as initial treatment choice in a setting with high incidence of C. glabrata, such as in Denmark, the USA, Scotland, Belgium, France, Finland, Sweden, and Australia.23,35–39 Lower mortality among C. glabrata cases treated with echinocandins was also found in a previous Danish study.40 In contrast, no correlation was found on primary AFT and mortality in a single-center Italian study in which only 6.9% were diagnosed with C. glabrata16 nor did the prior French ICU study show any difference in mortality on primary AFT with 16.6% C. glabrata.19 Furthermore, the 2010–2011 CANDIPOP project from 29 hospitals in Spain found no overall difference41 or among the 13.4% of cases with C. glabrata.15 Another important factor is a possible greater number of comorbidities among C. glabrata cases vs other species. This would reduce the attributable mortality of candidemia and consequently dilute the efficacy of echinocandins; therefore, our findings are adjusted for key comorbidities. A patient-level quantitative review of seven randomized trials for the treatment of invasive candidiasis showed improved survival after use of echinocandins.13 No new randomized controlled trials of AFT have been performed since the introduction of echinocandins as primary treatment for candidemia in the 2012 guidelines. The gap between results from RCT and verification in epidemiologic studies highlights the importance of “real-life” evaluations to inform the next iterations of National Guidelines.
There are limitations to our data including possible differences in data collection as multiple doctors contributed. Survey forms were used to minimalize this issue. Another limitation is the insufficient data on CVC removal and kidney function. Due to frequent changes in treatment selection and transfer of patients, between-hospital data on dosing were missing in 87.9% of the patients. On the contrary, the Danish personal security number system insured the inclusion of incident candidemia patients and eliminated the potential for duplicate cases. Finally, due to the lack of detailed clinical data regarding severity of the underlying disease, the potential bias was not included in our analysis. As severely ill patients are likely more often treated with echinocandins rather than fluconazole, a likely consequence is that we may have underestimated the superiority of the echinocandins.
In conclusion, this is the first study to evaluate AFT in a national setting. Our findings support and extend the knowledge on the impact of prior AFT on species distribution. Echinocandins were favorable both with regard to the proportion of adequately treated patients and prognosis. Thus, our findings support national guideline recommendations of echinocandins as primary treatment for candidemia in Denmark. Further nationwide studies assessing enforcement of recommended guideline adherence and effect of AFT are needed for comparable and updated data.
Acknowledgments
MCA reports grants from Amplyx, Basilea, Cidara, F2G, and Gilead and personal fees from Astellas, Basilea, Gilead, MSD, Pfizer, and T2Biosystems outside the submitted work; KLM reports grants from Pfizer, personal fees from Astra Zeneca and Horizon Pharmaceuticals outside the submitted work; LL reports travel grants from MSD and Pfizer outside the submitted work; HCS reports travel grants from MSD outside the submitted work; KRL reports research grant and speakers honoraria from Gilead outside the submitted work.
Disclosure
The authors report no conflicts of interest in this work.
References
Rex JH, Walsh TJ, Sobel JD, et al. Practice guidelines for the treatment of candidiasis. Clin Infect Dis. 2000;30(4):662–678. | ||
Pappas PG, Kauffman CA, Andes DR, et al. Executive summary: clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;62(4):409–417. | ||
Pappas PG, Kauffman CA, Andes D, et al. Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis. 2009;48(5):503–535. | ||
Behandlingsvejledning inklusiv laegemiddelrekommandation for systemisk antimykotisk behandling; 2016. Available from: http://www.regioner.dk/media/3739/beh-og-rek-antimykotika-vers-2-0-okt-2016.pdf. Accessed June 27, 2017. | ||
Cornely OA, Bassetti M, Calandra T, et al. ESCMID* guideline for the diagnosis and management of Candida diseases 2012: non-neutropenic adult patients. Clin Microbiol Infect. 2012;18(Suppl 7):19–37. | ||
Bow EJ, Evans G, Fuller J, et al. Canadian clinical practice guidelines for invasive candidiasis in adults. Can J Infect Dis Med Microbiol. 2010;21(4):e122–e150. | ||
Ruhnke M, Rickerts V, Cornely OA, et al. Diagnosis and therapy of Candida infections: joint recommendations of the German Speaking Mycological Society and the Paul-Ehrlich-Society for Chemotherapy. Mycoses. 2011;54(4):279–310. | ||
Arendrup MC, Dzajic E, Jensen RH, et al. Epidemiological changes with potential implication for antifungal prescription recommendations for fungaemia: data from a nationwide fungaemia surveillance programme. Clin Microbiol Infect. 2013;19(8):e343–e353. | ||
Astvad KMT, Johansen HK, Røder BL, et al. Update from a 12-year nationwide fungemia surveillance: increasing intrinsic and acquired resistance causes concern. J Clin Microbiol. 2017;56(4):pii:e01564-17. | ||
Reboli AC, Shorr AF, Rotstein C, et al. Anidulafungin compared with fluconazole for treatment of candidemia and other forms of invasive candidiasis caused by Candida albicans: a multivariate analysis of factors associated with improved outcome. BMC Infect Dis. 2011;11:261. | ||
Reboli AC, Rotstein C, Pappas PG, et al. Anidulafungin versus fluconazole for invasive candidiasis. N Engl J Med. 2007;356(24):2472–2482. | ||
Rex JH, Bennett JE, Sugar AM, et al. A randomized trial comparing fluconazole with amphotericin B for the treatment of candidemia in patients without neutropenia. Candidemia Study Group and the National Institute. N Engl J Med. 1994;331(20):1325–1330. | ||
Andes DR, Safdar N, Baddley JW, et al. Impact of treatment strategy on outcomes in patients with candidemia and other forms of invasive candidiasis: a patient-level quantitative review of randomized trials. Clin Infect Dis. 2012;54(8):1110–1122. | ||
Kullberg BJ, Sobel JD, Ruhnke M, et al. Voriconazole versus a regimen of amphotericin B followed by fluconazole for candidaemia in non-neutropenic patients: a randomised non-inferiority trial. Lancet. 2005;366(9495):1435–1442. | ||
Puig-Asensio M, Fernández-Ruiz M, Aguado JM, et al. Propensity score analysis of the role of initial antifungal therapy in the outcome of Candida glabrata bloodstream infections. Antimicrob Agents Chemother. 2016;60(6):3291–3300. | ||
Murri R, Scoppettuolo G, Ventura G, et al. Initial antifungal strategy does not correlate with mortality in patients with candidemia. Eur J Clin Microbiol Infect Dis. 2016;35(2):187–193. | ||
de Rosa FG, Corcione S, Filippini C, et al. The Effect on mortality of fluconazole or echinocandins treatment in candidemia in internal medicine wards [corrected]. PLoS ONE. 2015;10(5):e0125149. | ||
Luzzati R, Merelli M, Ansaldi F, et al. Nosocomial candidemia in patients admitted to medicine wards compared to other wards: a multicentre study. Infection. 2016;44(6):747–755. | ||
Leroy O, Bailly S, Gangneux JP, et al. Systemic antifungal therapy for proven or suspected invasive candidiasis: the AmarCAND 2 study. Ann Intensive Care. 2016;6(1):2. | ||
Arendrup MC, Bruun B, Christensen JJ, et al. National surveillance of fungemia in Denmark (2004 to 2009. J Clin Microbiol. 2011;49(1):325–334. | ||
EUCAST. EUCAST breakpoints and ECOFF. Available from: http://www.eucast.org/clinical_breakpoints/. Accessed May 09, 2018. | ||
Pfaller M, Neofytos D, Diekema D, et al. Epidemiology and outcomes of candidemia in 3648 patients: data from the Prospective Antifungal Therapy (PATH Alliance®) registry, 2004-2008. Diagn Microbiol Infect Dis. 2012;74(4):323–331. | ||
Cleveland AA, Farley MM, Harrison LH, et al. Changes in incidence and antifungal drug resistance in candidemia: results from population-based laboratory surveillance in Atlanta and Baltimore, 2008–2011. Clin Infect Dis. 2012;55(10):1352–1361. | ||
Puig-Asensio M, Ruiz-Camps I, Fernández-Ruiz M, et al. Epidemiology and outcome of candidaemia in patients with oncological and haematological malignancies: results from a population-based surveillance in Spain. Clin Microbiol Infect. 2015;21(5):491.e1–491.e10. | ||
Almirante B, Rodríguez D, Park BJ, et al. Epidemiology and predictors of mortality in cases of Candida bloodstream infection: results from population-based surveillance, Barcelona, Spain, from 2002 to 2003. J Clin Microbiol. 2005;43(4):1829–1835. | ||
Hirano R, Sakamoto Y, Kitazawa J, Yamamoto S, Kayaba H. Epidemiology, practice patterns, and prognostic factors for candidemia; and characteristics of fourteen patients with breakthrough Candida bloodstream infections: a single tertiary hospital experience in Japan. Infect Drug Resist. 2018;11:821–833. | ||
Lortholary O, Desnos-Ollivier M, Sitbon K, et al. Recent exposure to caspofungin or fluconazole influences the epidemiology of candidemia: a prospective multicenter study involving 2,441 patients. Antimicrob Agents Chemother. 2011;55(2):532–538. | ||
Puig-Asensio M, Pemán J, Zaragoza R, et al. Impact of therapeutic strategies on the prognosis of candidemia in the ICU. Crit Care Med. 2014;42(6):1423–1432. | ||
Blanchard E, Lortholary O, Boukris-Sitbon K, et al. Prior caspofungin exposure in patients with hematological malignancies is a risk factor for subsequent fungemia due to decreased susceptibility in Candida spp.: a case-control study in Paris, France. Antimicrob Agents Chemother. 2011;55(11):5358–5361. | ||
Mann PA, Mcnicholas PM, Chau AS, et al. Impact of antifungal prophylaxis on colonization and azole susceptibility of Candida species. Antimicrob Agents Chemother. 2009;53(12):5026–5034. | ||
Jensen RH, Johansen HK, Søes LM, et al. Posttreatment antifungal resistance among colonizing Candida isolates in candidemia patients: results from a systematic multicenter study. Antimicrob Agents Chemother. 2016;60(3):1500–1508. | ||
Puig-Asensio M, Padilla B, Garnacho-Montero J, et al. Epidemiology and predictive factors for early and late mortality in Candida bloodstream infections: a population-based surveillance in Spain. Clin Microbiol Infect. 2014;20(4):O245–O254. | ||
Barchiesi F, Orsetti E, Gesuita R, Skrami E, Manso E; Candidemia Study Group. Epidemiology, clinical characteristics, and outcome of candidemia in a tertiary referral center in Italy from 2010 to 2014. Infection. 2016;44(2):205–213. | ||
Anderson JR, Cain KC, Gelber RD. Analysis of survival by tumor response. J Clin Oncol. 1983;1(11):710–719. | ||
Pappas PG, Lionakis MS, Arendrup MC, Ostrosky-Zeichner L, Kullberg BJ. Invasive candidiasis. Nat Rev Dis Primers. 2018;4:18026. | ||
Trouvé C, Blot S, Hayette MP, et al. Epidemiology and reporting of candidaemia in Belgium: a multi-centre study. Eur J Clin Microbiol Infect Dis. 2017;36(4):649–655. | ||
Rajendran R, Sherry L, Deshpande A, et al. A prospective surveillance study of candidaemia: epidemiology, risk factors, antifungal treatment and outcome in hospitalized patients. Front Microbiol. 2016; 7:915. | ||
Hesstvedt L, Arendrup MC, Poikonen E, et al. Differences in epidemiology of candidaemia in the Nordic countries - what is to blame? Mycoses. 2017;60(1):11–19. | ||
Ericsson J, Chryssanthou E, Klingspor L, et al. Candidaemia in Sweden: a nationwide prospective observational survey. Clin Microbiol Infect. 2013;19(4):E218–E221. | ||
Arendrup MC, Sulim S, Holm A, et al. Diagnostic issues, clinical characteristics, and outcomes for patients with fungemia. J Clin Microbiol. 2011;49(9):3300–3308. | ||
López-Cortés LE, Almirante B, Cuenca-Estrella M, et al. Empirical and targeted therapy of candidemia with fluconazole versus echinocandins: a propensity score-derived analysis of a population-based, multicentre prospective cohort. Clin Microbiol Infect. 2016;22(8):733.e1–73733. |
Supplementary materials
  | Table S1 Species distribution |
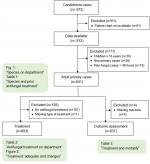  | Figure S1 Flow chart of the study population. |
  | Figure S2 Directed Acyclic Graphs (DAG s): Prior antifungal treatment and 7-day mortality.
|
  | Figure S3 Directed Acyclic Graphs (DAGs) showing antifungal treatment after blood culture collection and 14-day mortality.
|
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.