Back to Journals » Journal of Pain Research » Volume 15
Treatment of Calcified Lumbar Disc Herniation by Intervertebral Foramen Remolding: A Retrospective Study
Authors Yuan AL , Shen X, Chen B
Received 13 January 2022
Accepted for publication 13 June 2022
Published 16 June 2022 Volume 2022:15 Pages 1719—1728
DOI https://doi.org/10.2147/JPR.S357033
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Krishnan Chakravarthy
Ao-lin Yuan,1 Xin Shen,2 Bin Chen1
1Minimally Invasive Spine Surgery, Chengde Medical University Affiliated Hospital, Chengde, Hebei Province, People’s Republic of China; 2Breast and Thyroid Surgical Oncology, Hebei Medical University Second Affiliated Hospital, Shijiazhuang, Hebei Province, People’s Republic of China
Correspondence: Bin Chen, Minimally Invasive Spine Surgery, Chengde Medical University Affiliated Hospital, Chengde, Hebei Province, People’s Republic of China, Email [email protected]
Purpose: This study aimed to investigate the use of the percutaneous intervertebral foramen lens technology for secondary molding of the intervertebral foramen in the treatment of calcified lumbar discs.
Methods: The study included 104 patients who were divided into two groups. Group A comprised 50 patients with calcified lumbar disc herniation and group B comprised 54 patients with non-calcified lumbar disc herniation diagnosed by computed tomography and magnetic resonance imaging. Patients underwent a percutaneous endoscopic lumbar discectomy at our hospital from January 1, 2017, to December 31, 2019. Demographic characteristics before the surgery and perioperative outcomes were retrospectively reviewed. The treatment outcome was analyzed using the numerical rating scale (NRS) score, Oswestry Disability Index (ODI) score, and modified Macnab criteria.
Results: Patients in groups A and B showed significant improvement in both the NRS and ODI scores after the surgery and maintained relatively low ODI and NRS scores during subsequent follow-ups. According to the evaluation under the modified MacNab standard, the good–excellent rate of clinical efficacy was 94% in group A and 92.6% in group B at the 3 month follow-up. In group A, one patient developed neck pain during the surgery, which was diagnosed as spinal hypertension syndrome, and the surgery was suspended until the patient’s condition improved. No similar complications occurred in group B. In both the groups, no patient reported any dural leak, infection, or other related complications.
Conclusion: The use of transforaminal remolding technology can significantly improve the symptoms and dysfunction of patients with calcified and non calcified lumbar disc herniation. There are few intraoperative and postoperative complications and have little impact on vertebral stability. It can provide a reference for the treatment of special types of lumbar disc herniation.
Keywords: lumbar disc herniation, calcification, percutaneous transforaminal endoscopic surgery, nerve root compression, visual analog scale, Oswestry, disability index, Macnab criteria
Introduction
Calcified lumbar disc herniation is characterized by vertebral canal bone occupancy, which is a special type of lumbar disc herniation with the calcified herniated site.1,2 It has been reported an incidence ranging from 4.7% to 15.9%. The incidence of the disease is gradually increasing and has a younger trend.3–5 Unlike calcification occurring in children, the course of the disease in adults is usually long, and rarely resolves spontaneously.6 Studies on calcified disc herniation mainly focus on the thoracic vertebrae, while calcified disc herniation on the lumbar vertebrae is rare and difficult to treat.7–9 Traditional open fusion surgery is widely used in the clinic.10 Although it shows good efficacy, it is accompanied by many complications, such as intractable pain in the lower back and degeneration in adjacent stages.2,9,10
Calcified lumbar disc herniation is easily confused with Posterior Ring Apophysis Separation and lumbar disc herniation with calcification. Calcified lumbar disc herniation and Posterior Ring Apophysis Separation can be distinguished by CT.11,12 CT findings of Posterior Ring Apophysis Separation: there were obvious bone defects at the posterior margin of the vertebral body; the bone protruding into the spinal canal is usually located at the edge of the protruding disc and is roughly complementary to the bone defect at the posterior edge of the vertebral body. CT findings of calcified lumbar disc herniation: calcified foci are usually punctured or lamellar in the interior of the herniated disc, and there is no bone defect at the posterior edge of the vertebral body. Most of the patients with lumbar disc herniation with calcification are female children.13,14 The calcification is concentrated in the thoracic vertebra and generally resolves around the age of 20 years13–16 and usually accompanied by vertebral-plate irregularities and possible intervertebral body fusion.17,18
Percutaneous foraminal endoscopic treatment of calcified lumbar disc herniation is not as satisfactory as that of conventional lumbar disc herniation.8 This may be due to the limited scope of endoscopic operation, intraoperative interference with normal bone structures, and tension of nerve roots. How to decompress the compressed nerve root accurately on the premise of reducing nerve disturbance in the spinal canal and maintaining normal movement of the spine, while alleviating the symptoms has become a common problem faced by spinal surgeons. This study retrospectively analyzed the efficacy of the transforaminal approach in the treatment of calcified and noncalcified lumbar disc herniation and discussed the removal method of calcified lesions to provide a reference for clinical treatment.
Materials and Methods
This retrospective study recruited 104 consecutive patients from January 1, 2017, to December 31, 2019, according to the following inclusion criteria: 1) single-stage percutaneous endoscopic lumbar discectomy; 2) imaging data confirmed single-segment lumbar disc herniation and ruled out other spinal diseases such as lumbar spondylolisthesis.; and 3) significant lumbago and leg pain but no significant improvement after 6 months of conservative treatment. The 104 patients were divided into groups A and B. The specific grouping details are presented in the block diagram in Figure 1.
 |
Figure 1 Flow chart of the study. |
Table 1 shows the baseline data of patients in groups A and B: 50 patients in group A and 54 patients in group B. There were no significant differences in age, sex, surgical level, and body mass index between the two groups.
 |
Table 1 Preoperative Demographic Characteristics |
Outcome Assessment
Documented demographic characteristics and perioperative outcomes were evaluated. Leg pain before and after the surgery was assessed using the numerical rating scale (NRS) with a score ranging from 0 to 10. Functional disability before and after the surgery was measured by the Simplified Chinese Version of the Oswestry Disability Index (ODI). Surgical outcomes were also evaluated according to the modified Macnab criteria, as described previously.
Follow-Up
Patients were followed up at 1 week, 3 months, 6 months, and 1 year after surgery. The NRS and ODI scores were assessed at the four follow-ups, whereas outcomes were evaluated according to the Macnab criteria only at the 3-month follow-up.
Statistical Analysis
Data were statistically analyzed using the SPSS 25 program (USA, IBM corporation). Data normality was tested using the Shapiro–Wilk test. Parametric data are presented as mean ± standard deviation, whereas nonparametric data are presented as median (interquartile range). Nonparametric data and ordinal categorical variables were tested using the Mann–Whitney U-test. A p value of <0.05 was considered statistically significant.
Surgical Procedure
The surgery was performed using an endoscopic surgery system. The patient’s position is shown in Figure 2. Fluoroscopy was used for body surface projection. A vertical line through the extension of the upper endplate line of the lower vertebral body was made, and the body surface projection of the base of the articular process on the responsibility space and the body surface projection of the center of the responsibility space were established as the extension line. Intersection points of L4-5, L3-4 and L2-3 were approximately 12–14 cm, 10–12 cm and 8–10 cm from the posterior midline, respectively, and the intersection point of the two lines was the established as the puncture point. After routine disinfection, local anesthesia was administered with 20 mL 1% lidocaine, 18 G puncture needles were placed obliquely along the superior articular process of the superior responsibility space, and 0.5% lidocaine was injected for local anesthesia of the foraminal area. 8 mm skin at the insertion point was cut approximately and a one-level cannula was placed along the positioning needle. Positioning was confirmed by fluoroscopy. The base of the superior articular process was polished with bone drills of 6, 7, and 8 mm in turn to conduct the first foraminal molding, establish the working channel, place the endoscopic system, and deal with the soft tissues in the foraminal area. The ventral bone at the base of the superior articular process was excised with a bone knife under a endoscope, and the foramina were reshaped so that a relatively sufficient perspective and operating space could be obtained (also under the microscope). For large calcification foci, the soft tissues around the calcified intervertebral disc were first treated to expose the base of the calcification foci connected to the vertebral body. The bone knife and cannula were rotated under the microscope to separate the calcification foci and remove them in small pieces. Small free calcifications could be removed directly. The blood vessels on the surface of the nerve root were recovered under endoscopic, and the nerve root showed regularly (Figure 3). The straight leg raising test was negative intraoperatively, and the nerve root could slip freely. The ruptured annulus was carefully repaired by radiofrequency ablation, and the endoscopic system was withdrawn. A drainage tube was placed, the incision was sutured, and the surgery was completed.
Results
Demographic and Pathophysiological Data
Demographic and pathophysiological data are presented in Table 1, and patients’ perioperative outcomes are shown in Table 2. The average operation duration of group A was 64.9 ± 17.2 minutes and of group B was 57.2 ± 15.8 minutes. The operation duration of group A was slightly longer than that of group B, and the difference was statistically significant (p<0.05).There were no significant differences in Age, Sex, Segments, BMI, Blood loss, Hospital stay, Drainage between the two groups (p>0.05).
 |
Table 2 Perioperative Outcomes |
Clinical Results
We followed up the patients for at least 1 year. The pain severity of both the groups was assessed using NRS scores, and the follow-up data are presented in Table 3. The mean preoperative NRS score of group A was 7.3±1.3, and it decreased to 2.3±0.8 1 week after the surgery and to 1.0±0.6 at the 12-month follow-up. The mean NRS score of group B decreased from 7.2±0.9 to 2.1±1.5 in the 1 week post-operation period and to 0.9±0.8 at 12 months after the surgery. There were no significant differences in NRS scores between the two groups before the surgery and at 1 week, 3 months, 6 months, and 12 months after the surgery (p>0.05).
 |
Table 3 The Preoperative and Postoperative NRS Data |
The functional disability of the two groups before and after the surgery was measured using the Simplified Chinese Version of the ODI, and their changes are presented as a bar chart in Figure 4.
In group A, the good-to-excellent rate, as evaluated by the patients, was 94%. Two patients showed fair results, and one patient reported poor outcome (Figure 5A). In group B, the good-to-excellent rate, as evaluated by the patients, was 92.6%. Four patients showed fair results, and no patients reported poor outcome (Figure 5B).
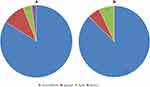 |
Figure 5 Modified Macnab criteria. (A) Modified Macnab criteria of group A. (B) Modified Macnab criteria of group B. |
Complications
During the surgery, one patient in group A developed neck pain; therefore, the surgery was terminated immediately and the height of normal saline used for irrigation was lowered. Thereafter, the patient’s symptoms were significantly relieved, the surgery continued smoothly, and no postoperative complications occurred. The other patients did not develop any intraoperative and postoperative complications, such as infections and dural leaks. Patients in group B had a smooth operation, with no operative complications.
Discussion
At present, the most extensively applied approaches for endoscopic treatment of calcified lumbar disc herniation are the transforaminal and -laminar approaches.19 One of the most important points of the operation is establishing the working channel, to fully expose the calcification under the microscope. Ruetten et al20,21 reported the surgical method of the translaminar approach generally in the prone position, and the intraoperative anatomy was similar to that of traditional open surgery, which was in line with most operators’ habits and had a better effective outcomes on axillary and central lumbar disc herniation. However, with this approach, it is difficult to treat the protrusion located at the shoulder of the nerve root, sufficient resection of the medial facet is required to obtain sufficient operating space.22 Patients with calcified lumbar intervertebral disc herniation often have a longer course of disease, severe vertebral degeneration, and narrow lamina space. To successfully place the endoscope, on the basis of sufficient medial facetectomy, more bone may need to be removed. This degree of osteotomy may cause injury not only to the exit root but also the walking root. The medial superior articular process and medial pedicle crypt are thick and hard, and it is difficult to establish the channel through osteotomy. Moreover, pulling the nerve root to expose calcification can easily cause nerve injury. Therefore, we performed a transforaminal approach to remove the bone at the ventral base of the superior articular process with a canard-bill cannula and a endoscopic osteotomy with continuous visibility. This approach mainly has the following advantages: facet joints were not damaged during operation, and make little effect on vertebral stability. The approach from the physiological and anatomical space will not affect the structure behind the spine and the ligamentum flavum, and can reduce postoperative scar adhesion in the spinal canal.23 Even if the operation fails, it is relatively easy to turn to traditional open surgery. The base of the superior articular process is far from the exit nerve root, so achieving nerve injury by osteotomy is impossible, and the operation time is shortened.24 The duck-bill cannula approximately envelops the superior articular process and is a barrier between the superior articular process and the nerve root during osteotomy to reduce nerve disturbance. The endoscopic power system and the side laser system may lead to transient deterioration of neurological function, and the cost is high.7,25 However, under the microscope, osteotomy time is short and causes less damage to nerve roots. The manipulation of bone under visual conditions will improve the safety of the operation. There are still some problems in this procedure. As the intervertebral foramina of the lower lumbar spine were gradually reduced, the blocking effect of the superior articular process became increasingly serious. Especially in the L5-S1 segment, due to the obstruction of the iliac ridge and the large transverse process of L5, it becomes more difficult to establish the channel.26 Most surgeons used the interlaminar approach to solve this problem.27,28 We adopted a lateral decubitus position and raised the patient’s lumbar pad so that the intervertebral space could be fully opened to minimize the impact of the position on the operation. The lateral opening was reduced to avoid obstruction of the iliac ridge to the cannula as much as possible, but it was still difficult to address the relatively extensive calcification. Therefore, on the basis of not destroying the articular surface as much as possible, we moderately expanded the resection range of the ventral articular process on the upper S1. Studies have shown that resection of the anteromedial 1/3 of the superior articular process, the anterior part of the lower facet joint, and the part between them can increase the foraminal area by 45% without affecting the spine stability.23,29 The range of facet excision was controlled within 1/3 in most patients, and during follow-up, there was no obvious vertebral instability. Interestingly, we excised more than 1/3 of the upper articular process of S1 in some patients and excised part of the joint capsule, but no obvious vertebral instability occurred in the postoperative follow-up, So we believe may be due to the low activity of the L5-S1 segment, as well as the postoperative rehabilitation exercise guidance for each patient.
According to reports by Dabo et al,8 when central or paracentral calcified lumbar disc herniation is treated with an interlaminar approach, early postoperative paraesthesia is more likely to occur than conventional soft lumbar disc herniation, and the postoperative traditional medicine utilization rate is higher. Chen et al30 found that postoperative lower limb sensory dysfunction mainly occurred in the translaminar approach. When the nerve root is pulled across the midline, the risk of nerve root injury is increased greatly.31,32 We think this may be related to calcification type lumbar disc, oppression dural sac, highlight the material hard and dural sac to produce a kind of “half package”, when revealed calcification for larger degree of nerve root and dural sac pull, when calcifications is located in the central, in order to expose calcifications, will increase the degree of the pull. Therefore, it is very important to obtain the right angle and sufficient operating space before removing calcifications. Chen et al30 reported that the peak method can reduce the traction of nerve roots and reduce postoperative neurological deterioration and other complications in calcification removal. However, intraoperatively, we found that the base of calcification was generally located at the posterior edge of the vertebral body, and some calcifications could even span the entire vertebral space. To expose the peak of calcification at this time, not only is the amount of osteotomy required during foraminal secondary forming increased, but the pulling of nerve roots is also inevitable Therefore, after the formation of the secondary intervertebral foramen, the base of the calcified foci was exposed, and the calcified foci were excised by means of cannula rotation combined with a endoscopic bone knife. The part of the base of the calcified foci that was connected with the vertebral body was excised to make the calcified foci free, and the calcified foci were removed by partitioning under the microscope (Figures 6 and 7). For large calcification foci that are difficult to segment, the cannula can be removed with nucleus pulposus forceps, and the channel can be placed again after removal. This approach reduces the risk of more osteotomies affecting the stability of the vertebral body and has relatively little impact on nerve roots. Fibroannular damage may be associated with an increased risk of reherniation and may accelerate disc degeneration.33 Removal of the area where the calcified focus connects to the posterior edge of the vertebral body may damage the normal annulus, so careful annulus plasty is required to avoid postoperative re-protrusion due to annulus damage.
In our study, groups A and B showed no relevant complications such as recurrent herniation, weakness or numbness after operation, and patients in both groups achieved good outcomes, with significant improvements in NRS and ODI scores compared with those before operation during a follow-up period of at least one year. Group B had lower NRS and ODI scores than group A at postoperative contemporaneous follow-up, but the difference between the two was not statistically significant. But one patient in group A presented with intraoperative neck pain, which we believe may be related to the surgical length of the decompression of calcified versus noncalcified type lumbar disc herniation, and a larger secondary molding of the intervertebral foramen area without compromising the stability of the vertebral body may be necessary to find a suitable angle to remove the calcifications.Careful exploration for adhesions between nerve roots and parts of the calcified lesion is also necessary in patients with a calcified lumbar disc herniation, with more care during decompression to avoid damage to the nerve root, which will both allow longer surgery. Our results also showed that group A required significantly more operative time than group B. In addition to this, bleeding from the bone surface may result in an unclear field of view, which tends to resolve intraoperatively by increasing irrigation pressure, which may also be responsible for this intraoperative complication.
However, our study also had some limitations. First, the sample size was small, and the relationship between the size, location and shape of the calcification foci and prognosis was not further discussed. Second, the short follow-up time made it impossible to further analyze its long-term efficacy. In the future, we will enlarge the sample size and undergo long follow-up time to classify calcified lumbar disc herniation according to the characteristics of calcified foci, and explore the relationship between different factors and prognosis.
Data Sharing Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
This research was approved by the ethics committee of Chengde Medical University Affiliated Hospital. All participants agreed with the data and publication of the manuscript. All methods were performed in accordance. All methods were performed in accordance with the relevant guidelines and regulations. Informed consent was obtained from all subjects and/or their legal guardian(s).All authors make a commitment to patient data confidentiality and compliance with the declaration of Helsinki.
Patient Consent for Publication
Written informed consent was obtained from all participants and, informed consent was obtained from all subjects and/or their legal guardian(s).
Funding
No funding was received.
Disclosure
The authors declare that they have no conflicts of interest in relation to this work.
References
1. Kim HS, Adsul N, Ju YS, et al. Full endoscopic lumbar discectomy using the calcification floating technique for symptomatic partially calcified lumbar herniated nucleus pulposus. World Neurosurg. 2018;119:500–505. doi:10.1016/j.wneu.2018.06.133
2. Wang H, Zhou T, Gu Y, Yan Z. Evaluation of efficacy and safety of percutaneous transforaminal endoscopic surgery (PTES) for surgical treatment of calcified lumbar disc herniation: a retrospective cohort study of 101 patients. BMC Musculoskelet Disord. 2021;22(1):65. doi:10.1186/s12891-020-03938-3
3. Cong L, Zhu Y, Tu G. A meta-analysis of endoscopic discectomy versus open discectomy for symptomatic lumbar disk herniation. Eur Spine J. 2016;25(1):134–143. doi:10.1007/s00586-015-3776-6
4. Cheng XG, Brys P, Nijs J, et al. Radiological prevalence of lumbar intervertebral disc calcification in the elderly: an autopsy study. Skeletal Radiol. 1996;25(3):231–235. doi:10.1007/s002560050070
5. Karamouzian S, Eskandary H, Faramarzee M, et al. Frequency of lumbar intervertebral disc calcification and angiogenesis, and their correlation with clinical, surgical, and magnetic resonance imaging findings. Spine. 2010;35(8):881–886. doi:10.1097/BRS.0b013e3181b9c986
6. Du JJ, Chen YF, Peng Y, Li XJ, Ma W. Calcification of the intervertebral disc and ossification of posterior longitudinal ligament in children. BMC Musculoskelet Disord. 2018;19(1):316. doi:10.1186/s12891-018-2227-z
7. Ahn Y, Oh HK, Kim H, Lee SH, Lee HN. Percutaneous endoscopic lumbar foraminotomy: an advanced surgical technique and clinical outcomes. Neurosurgery. 2014;75(2):124–133. doi:10.1227/NEU.0000000000000361
8. Dabo X, Ziqiang C, Yinchuan Z, et al. The clinical results of Percutaneous Endoscopic Interlaminar Discectomy (PEID) in the treatment of calcified lumbar disc herniation: a case-control study. Pain Physician. 2016;19(2):69–76. doi:10.36076/ppj/2016.19.69
9. Yu L, Wen JK, Wang S, Wang WH, Yu JM, Ye XJ. Removal of calcified lumbar disc herniation with endoscopic-matched ultrasonic osteotome - our preliminary experience. Br J Neurosurg. 2020;34(1):80–85. doi:10.1080/02688697.2019.1687850
10. Nellensteijn J, Ostelo R, Bartels R, Peul W, van Royen B, van Tulder M. Transforaminal endoscopic surgery for symptomatic lumbar disc herniations: a systematic review of the literature. Eur Spine J. 2010;19(2):181–204. doi:10.1007/s00586-009-1155-x
11. Wu XY, Ma W. Posterior lumbar ring apophysis fracture. Orthop Surg. 2011;3(1):72–77. doi:10.1111/j.1757-7861.2010.00122.x
12. Singhal A, Mitra A, Cochrane D, Steinbok P. Ring apophysis fracture in pediatric lumbar disc herniation: a common entity. Pediatr Neurosurg. 2013;49(1):16–20. doi:10.1159/000355127
13. Sonnabend DH, Taylor TK, Chapman GK. Intervertebral disc calcification syndromes in children. J Bone Joint Surg Br. 1982;64(1):25–31. doi:10.1302/0301-620X.64B1.7068715
14. Dias MS, Pang D. Juvenile intervertebral disc calcification: recognition, management, and pathogenesis. Neurosurgery. 1991;28(1):130–135. doi:10.1227/00006123-199101000-00018
15. Melnick JC, Silverman FN. Intervertebral disk calcification in childhood. Radiology. 1963;80:399–408. doi:10.1148/80.3.399
16. Dai LY, Ye H, Qian QR. The natural history of cervical disc calcification in children. J Bone Joint Surg Am. 2004;86(7):1467–1472. doi:10.2106/00004623-200407000-00015
17. Chanchairujira K, Chung CB, Kim JY, et al. Intervertebral disk calcification of the spine in an elderly population: radiographic prevalence, location, and distribution and correlation with spinal degeneration. Radiology. 2004;230(2):499–503. doi:10.1148/radiol.2302011842
18. Oda J, Tanaka H, Tsuzuki N. Intervertebral disc changes with aging of human cervical vertebra. From the neonate to the eighties. Spine. 1988;13(11):1205–1211. doi:10.1097/00007632-198811000-00001
19. Wu PH, Kim HS, Jang IT, Narrative A. Review of development of full-endoscopic lumbar spine surgery. Neurospine. 2020;17(Suppl 1):S20–S33. doi:10.14245/ns.2040116.058
20. Ruetten S, Komp M, Merk H, Godolias G. Surgical treatment for lumbar lateral recess stenosis with the full-endoscopic interlaminar approach versus conventional microsurgical technique: a prospective, randomized, controlled study. J Neurosurg Spine. 2009;10(5):476–485. doi:10.3171/2008.7.17634
21. Ruetten S, Komp M, Merk H, Godolias G. Use of newly developed instruments and endoscopes: full-endoscopic resection of lumbar disc herniations via the interlaminar and lateral transforaminal approach. J Neurosurg Spine. 2007;6(6):521–530. doi:10.3171/spi.2007.6.6.2
22. Choi KC, Kim JS, Ryu KS, Kang BU, Ahn Y, Lee SH. Percutaneous endoscopic lumbar discectomy for L5-S1 disc herniation: transforaminal versus interlaminar approach. Pain Physician. 2013;16(6):547–556.
23. Li ZZ, Hou SX, Shang WL, Song KR, Zhao HL. Modified percutaneous lumbar foraminoplasty and percutaneous endoscopic lumbar discectomy: instrument design, technique notes, and 5 years follow-up. Pain Physician. 2017;20(1):E85–E98. doi:10.36076/ppj.2017.1.E85
24. Yang JS, Chu L, Chen CM, et al. Foraminoplasty at the tip or base of the superior articular process for lateral recess stenosis in percutaneous endoscopic lumbar discectomy: a multicenter, retrospective, controlled study with 2-year follow-up. Biomed Res Int. 2018;2018:7692794. doi:10.1155/2018/7692794
25. Knight MT, Jago I, Norris C, Midwinter L, Boynes C. Transforaminal endoscopic lumbar decompression & foraminoplasty: a 10 year prospective survivability outcome study of the treatment of foraminal stenosis and failed back surgery. Int J Spine Surg. 2014;8:21. doi:10.14444/1021
26. Yan S, Zhang Y, Wang K, et al. Three-dimensional morphological characteristics of lower lumbar intervertebral foramen with age. Biomed Res Int. 2018;2018:8157061. doi:10.1155/2018/8157061
27. Wang D, Xing J, Shao B, et al. A surgical decompression procedure for effective treatment of calcified lumbar disc herniation. J Int Med Res. 2020;48(7):300060520938966. doi:10.1177/0300060520938966
28. Song H, Hu W, Liu Z, Hao Y, Zhang X. Percutaneous endoscopic interlaminar discectomy of L5-S1 disc herniation: a comparison between intermittent endoscopy technique and full endoscopy technique. J Orthop Surg Res. 2017;12(1):162. doi:10.1186/s13018-017-0662-4
29. Osman SG, Nibu K, Panjabi MM, Marsolais EB, Chaudhary R. Transforaminal and posterior decompressions of the lumbar spine. A comparative study of stability and intervertebral foramen area. Spine. 1997;22(15):1690–1695. doi:10.1097/00007632-199708010-00002
30. Chen Y, Wang JX, Sun B, et al. Percutaneous endoscopic lumbar discectomy in treating calcified lumbar intervertebral disc herniation. World Neurosurg. 2019;122:e1449–e1456. doi:10.1016/j.wneu.2018.11.083
31. Okuda S, Miyauchi A, Oda T, Haku T, Yamamoto T, Iwasaki M. Surgical complications of posterior lumbar interbody fusion with total facetectomy in 251 patients. J Neurosurg Spine. 2006;4(4):304–309. doi:10.3171/spi.2006.4.4.304
32. Choi KC, Lee JH, Kim JS, et al. Unsuccessful percutaneous endoscopic lumbar discectomy: a single-center experience of 10,228 cases. Neurosurgery. 2015;76(4):
33. Battié MC, Lazáry A, Fairbank J, et al. Disc degeneration-related clinical phenotypes. Eur Spine J. 2014;23(Suppl 3):S305–S314. doi:10.1007/s00586-013-2903-5
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.