Back to Journals » Drug Design, Development and Therapy » Volume 9
Treatment of alarming head and neck infantile hemangiomas with interferon-α2a: a clinical study in eleven consecutive patients
Authors Zhang L, Zheng JW, Yuan W
Received 13 May 2014
Accepted for publication 20 June 2014
Published 4 February 2015 Volume 2015:9 Pages 723—727
DOI https://doi.org/10.2147/DDDT.S67682
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 6
Ling Zhang,1,2 Jia Wei Zheng,1,2 Wei En Yuan1–3
1Department of Oral and Maxillofacial Surgery, College of Stomatology, Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, People’s Republic of China; 2Shanghai Key Laboratory of Stomatology, Shanghai, People’s Republic of China; 3School of Pharmacy, Shanghai Jiao Tong University, Shanghai, People’s Republic of China
Objective: To evaluate the efficacy and adverse effects of interferon-α2a in the treatment of alarming infantile hemangiomas in the head and neck region.
Patients and methods: From January 2009–December 2010, a subcutaneous injection of interferon-α2a was applied to eleven infants with giant multifocal or segmental hemangiomas at a dose of 3 million units/m² per day. All patients did not respond to propranolol or corticosteroids. The age at initiation of interferon-α2a therapy ranged from 3 days to 8 months (median: 4 months). The duration of therapy ranged from 2–4.5 months (median: 3 months). Eight patients received medication for 3 months, one patient for 4.5 months, and two patients for 2 months.
Results: Nine patients had a reduction in tumor mass of 95%; two patients’ tumors decreased in size by 75%. The overall response rate was 100%. The main adverse effects included fever, diarrhea, and anorexia, which resolved after stopping the medication. No serious adverse effect was observed.
Conclusion: Short-term treatment with interferon-α2a can be used as a safe and effective treatment for alarming infantile hemangiomas that are resistant to propranolol or corticosteroids, and that endanger the proper functioning of the affected organ or the patient’s life.
Keywords: hemangioma, interferon-α, head and neck, adverse effect
Introduction
Infantile hemangioma (IH) is the most common benign tumor of infancy,1 affecting nearly 10% of children under 1 year old and 30% of premature babies. Prematurity, older maternal age, preeclampsia, and multiple gestations have been suggested to be risk factors of the development of IH.2 Approximately 60% of hemangiomas are located at the head and neck regions,3 and they present with a 2.4:1 ratio of females to males.4 Their large size, facial location, and/or segmental morphology are the major risk factors associated with the development of complications including ulceration, functional impairment, and permanent disfigurement.5 As most IHs can involute spontaneously, there is still great controversy over the management of IH. However, consensus was achieved in that when the tumor gives rise to infection, ulceration, necrosis and bleeding resulting in serious disfigurement, dysfunctions and even threatening life, treatment is urgently required. The lesions that potentially impair vital function or cause life-threatening complications are described as “alarming hemangiomas”.6 There is no gold standard for alarming hemangioma. Recombinant interferon alpha (IFN-α), an inhibitor of angiogenesis, has been used successfully in treating complicated IH, particularly in hemangiomas that have failed to respond to oral propranolol.7,8 However, concerns of irreversible neurotoxicity, especially spastic diplegia, handicap the application of IFN-α.9–11 Here, we present a study where we treated eleven Chinese cases of alarming hemangioma with IFN-α2a.
Patients and methods
The study was approved by the ethics committee at the Ninth People’s Hospital, Shanghai Jiao Tong University (Shanghai, People’s Republic of China). All parents of the participants provided written informed consent.
Patients
Eleven consecutive infants (nine girls and two boys) with alarming hemangiomas in the head and neck region who failed to respond to a 2-week course of propranolol or corticosteroid therapy, and who received a subcutaneous injection of IFN-α in our institution between January 2009 and December 2010 were enrolled in this study. All patients had large (>5 cm in diameter) or multiple hemangiomas (≥2 sites) in the head and neck region; in three patients, the hemangioma caused airway obstruction. The demographic data regarding each patient’s age, sex, site of lesion, and duration of treatment are shown in Table 1. The diagnosis of hemangiomas was established mainly by obtaining the patient’s history, noting clinical manifestations, and performing a physical examination. All patients underwent ultrasonography, and four patients had a magnetic resonance imaging (MRI) examination to obtain more details of the lesion (when the diagnosis was uncertain, MRI was performed). After informed consent had been obtained from the parents according to institutional guidelines, the patients were treated with IFN-α2a for a period of 2–4.5 months. IFN-α2a was administrated subcutaneously once daily at a dose of 3×106 units/m2 per day. Periodical baseline electrocardiogram, neurologic examination, and laboratory evaluations of hepatic transaminases were performed.
  | Table 1 Demographic data of eleven patients with alarming hemangiomas in the study |
Outcome measurement
The size and extent of the deep-seated lesions were monitored by serial color Doppler ultrasonography; the percent change of the superficial lesions compared the pre- and posttreatment tumor size using photographs. The patients were observed at an interval of 3 weeks during the therapy course; follow-up visits were continued for up to 2 years. The final results were assessed by two other physicians from the Department of Oral Oncology, depending on the clinical examinations, photographs, and Doppler ultrasonography taken before and after treatment; the response to IFN-α2a therapy was evaluated according to the following scale (that was modified following Achauer et al12): scale 1, poor response (shrinkage 0%–25%); scale 2, fair response (shrinkage 26%–50%); scale 3, good response (shrinkage 51%–75%); scale 4, excellent response (shrinkage 76%–100%). The criterion for discontinuing IFN-α2a therapy was tumor shrinkage >75%.
Results
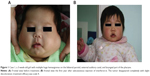
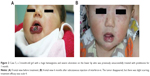
The overall response rate was 100%. All eleven patients received the treatment for a duration of 2–4.5 months (median: 3 months). Eight patients had received medication for 3 months, one for 4.5 months, and two for 2 months. The age at initiation of IFN-α2a therapy ranged from 3 days to 8 months (median: 4 months). It was observed that the most apparent regression of tumors happened at 1 month after medication administration; the tumor shrank further with the red color fading. Nine patients (82%) achieved scale 4 (Figures 1A, B, 2A, and B) and two patients (18%) achieved scale 3. The adverse effects included low fever (six cases; 54.5%), diarrhea (two cases; 18.2%), and anorexia (one case; 9.1%), as well as neutropenia and high levels of aminotransferases to varying degrees, which resolved after stopping the medication. No more than one symptom occurred to the same patient. Neurotoxicity such as spastic diplegia and other motor developmental disturbances were not found in this series. No patients experienced the rebound phenomenon, and no patients required the restarting of IFN or another therapy in the following 2 years.
Discussion
In this series of eleven infants with alarming hemangiomas treated with IFN-α2a, rapid and good response to treatment was obtained with few reversible adverse effects. There was no recurrence or worsening of the hemangiomas once the treatment was discontinued. In addition to its antiviral effect, IFN-α was also used as an inhibitor of angiogenesis given its antiangiogenic properties.13 Since its efficacy in pulmonary hemangiomatosis was first reported by White et al14 in 1989, IFN-α was chosen as a therapeutic modality for hemangiomas, especially for life-threatening cases or steroids-insensitive patients. According to other reports,15–17 the rate of response to IFN in hemangiomas varies from 80%–100%, which is very similar to our overall response rate of 100%; our result was as good as those found in these studies (P>0.05), and the duration time was shorter (P<0.05). The common adverse effects of IFN include influenza-like symptoms of fever, somnolence, anorexia, diarrhea or constipation, and a high level of aminotransferases; these symptoms are always slight and they can be reversed following the discontinuation of IFN.
Although exceptional, neurotoxicity is still the main concern in the treatment of IH with IFN. Epilepsy, spastic diplegia, and lower limb disability had been reported after IFN injection. Chang et al16 first reported in 1997 that one patient who received treatment with IFN-α2b developed mild gross motor delay, which improved 2 months after the cessation of therapy. The pathogenesis of IFN-related neurotoxic effects is unclear, but given its potential seriousness, many experts have limited the use of IFN-α for alarming hemangiomas. According to a meta-analysis of a large sample, which was carried out by Michaud et al9 in 2004, eleven of 441 children (2.5%) receiving IFN therapy for the treatment of vascular lesions developed spastic diplegia; the mean duration of IFN therapy was 11.2 (range: 4–30) months. Why had such neurotoxicity not been found in our patients? Perhaps there were not enough patients in our study to see this outcome, or it is also possible that the short duration of therapy decreased the risk for these children. Considering that the durations we used were only 2–4.5 months (median: 3 months), we suggest that the short-term use of IFN-α should be safe. The current treatments of IHs include drug therapy, cryosurgery, laser therapy, and surgical excision. Oral corticosteroids (for example, prednisolone, prednisone) used to be the mainstay of therapy for hemangiomas, but their clinical application was limited by the adverse effects such as Cushing’s syndrome and growth retardation. Since the impressive effect of propranolol in treating hemangioma was reported by Léauté-Labrèze et al18 in 2008, many studies (including our own experiences) have confirmed its effectiveness and have reported few side effects in hemangiomas.19–22 Nowadays, propranolol has been our first-line medicine for treating IH, but there are still some patients with alarming hemangiomas who may be concomitant with a contraindication for propranolol such as asthma, cardiac failure, or failure to respond to this medicine. For these cases, short-term use of IFN-α2a may be an effective alternative option.
Conclusion
The rapid and impressive responses, as well as the few side effects observed in this series without relapse after treatment with IFN-α2a prompts us to suggest that the short-term (<4.5 months) use of IFN-α2a can be chosen as an alternative option for alarming hemangiomas in the head and neck region, which are resistant to corticosteroid or propranolol treatment.
Acknowledgment
Supported by the National Natural Science Foundation of China (grant number 81070846).
Disclosure
The authors report no conflicts of interest in this work.
References
Garzon MC, Drolet BA, Baselga E, et al; Hemangioma Investigator Group. Comparison of infantile hemangiomas in preterm and term infants: a prospective study. Arch Dermatol. 2008;144(9):1231–1232. | ||
Drolet BA, Swanson EA, Frieden IJ; Hemangioma Investigator Group. Infantile hemangiomas: an emerging health issue linked to an increased rate of low birth weight infants. J Pediatr. 2008;153(5):712–715, 715.e1. | ||
Jinnin M, Ishihara T, Boye E, Olsen BR. Recent progress in studies of infantile hemangioma. J Dermatol. 2010;37(11):939–955. | ||
Haggstrom AN, Drolet BA, Baselga E, et al; Hemangioma Investigator Group. Prospective study of infantile hemangiomas: demographic, prenatal, and perinatal characteristics. J Pediatr. 2007;150(3):291–294. | ||
Haggstrom AN, Drolet BA, Baselga E, et al. Prospective study of infantile hemangiomas: clinical characteristics predicting complications and treatment. Pediatrics. 2006;118(3):882–887. | ||
Enjolras O, Riche MC, Merland JJ, Escande JP. Management of alarming hemangiomas in infancy: a review of 25 cases. Pediatrics. 1990; 85(4):491–498. | ||
Ezekowitz RA, Mulliken JB, Folkman J. Interferon alfa-2a therapy for life-threatening hemangiomas of infancy. N Engl J Med. 1992;326(22): 1456–1463. | ||
Chao YH, Liang DC, Chen SH, Wang LY, Yeh TC, Liu HC. Interferon-alpha for alarming hemangiomas in infants: experience of a single institution. Pediatr Int. 2009;51(4):469–473. | ||
Michaud AP, Bauman NM, Burke DK, Manaligod JM, Smith RJ. Spastic diplegia and other motor disturbances in infants receiving interferon-alpha. Laryngoscope. 2004;114(7):1231–1236. | ||
Barlow CF, Priebe CJ, Mulliken JB, et al. Spastic diplegia as a complication of interferon Alfa-2a treatment of hemangiomas of infancy. J Pediatr. 1998;132(3 Pt 1):527–530. | ||
Wörle H, Maass E, Köhler B, Treuner J. Interferon alpha-2a therapy in haemangiomas of infancy: spastic diplegia as a severe complication. Eur J Pediatr. 1999;158(4):344. | ||
Achauer BM, Chang CJ, Vander Kam VM. Management of hemangioma of infancy: review of 245 patients. Plast Reconstr Surg. 1997;99(5): 1301–1308. | ||
Lindner DJ. Interferons as antiangiogenic agents. Curr Oncol Rep. 2002;4(6):510–514. | ||
White CW, Sondheimer HM, Crouch EC, Wilson H, Fan LL. Treatment of pulmonary hemangiomatosis with recombinant interferon alfa-2a. N Engl J Med. 1989;320(18):1197–1200. | ||
Ricketts RR, Hatley RM, Corden BJ, Sabio H, Howell CG. Interferon-alpha-2a for the treatment of complex hemangiomas of infancy and childhood. Ann Surg. 1994;219(6):605–612; discussion 612–614. | ||
Chang E, Boyd A, Nelson CC, et al. Successful treatment of infantile hemangiomas with interferon-alpha-2b. J Pediatr Hematol Oncol. 1997; 19(3):237–244. | ||
Tamayo L, Ortiz DM, Orozco-Covarrubias L, et al. Therapeutic efficacy of interferon alfa-2b in infants with life-threatening giant hemangiomas. Arch Dermatol. 1997;133(12):1567–1571. | ||
Léauté-Labrèze C, Dumas de la Rogue E, Hubiche T, Boralevi F, Thambo JB, Taïeb A. Propanolol for severe hemangiomas of infancy. N Engl J Med. 2008;358(24):2649–2651. | ||
Bagazgoitia L, Torrelo A, Gutiérrez JC, et al. Propranolol for infantile hemangiomas. Pediatr Dermatol. 2011;28(2):108–114. | ||
Price CJ, Lattouf C, Baum B, et al. Propranolol vs corticosteroids for infantile hemangiomas: a multicenter retrospective analysis. Arch Dermatol. 2011;147(12):1371–1376. | ||
Hogeling M, Adams S, Wargon O. A randomized controlled trial of propranolol for infantile hemangiomas. Pediatrics. 2011;128(2):e259–e266. | ||
Qin ZP, Liu XJ, Li KL, Zhou Q, Yang XJ, Zheng JW. [Treatment of infantile hemangiomas with low-dose propranolol: evaluation of short-term efficacy and safety]. Zhonghua Yi Xue Za Zhi. 2009;89(44): 3130–3134. Chinese. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.