Back to Journals » Clinical Ophthalmology » Volume 15
Treatment of Advanced Retinoblastoma in Children Evacuated from Low-Income Countries: Experience from a National Referral Center in Portugal
Authors Castela G , Providência J , Monteiro M, Silva S, Brito M , Murta JN, Correa Z , Castelo Branco M
Received 12 October 2021
Accepted for publication 3 December 2021
Published 21 December 2021 Volume 2021:15 Pages 4765—4773
DOI https://doi.org/10.2147/OPTH.S343919
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Video abstract presented by Guilherme Castela.
Views: 195
Guilherme Castela,1,2 Joana Providência,1,2 Madalena Monteiro,1 Sónia Silva,3 Manuel Brito,3 Joaquim Neto Murta,1,2 Zélia Correa,4 Miguel Castelo Branco2
1Department of Ophthalmology, Centro Hospitalar e Universitário de Coimbra, Coimbra, Portugal; 2Faculty of Medicine, University of Coimbra, Coimbra, Portugal; 3Department of Pediatric Oncology, Centro Hospitalar e Universitário de Coimbra, Coimbra, Portugal; 4Bascon Palmer Eye Institute, Miami, FL, USA
Correspondence: Guilherme Castela
Department of Ophthalmology, Centro Hospitalar e Universitário de Coimbra, Avenida Dias da Silva 164, Coimbra, 3000, Portugal
Email [email protected]
Objective: The aim of our study was to characterize the evacuated African patients diagnosed with retinoblastoma and referred to the Portuguese national referral center (Centro Hospital e Universitário de Coimbra, University of Coimbra), identifying inequalities in the stage of diagnosis and prognostic results.
Design: Retrospective observational study of evacuated African patients diagnosed with retinoblastoma and referred to the Portuguese National Referral Center (Centro Hospital e Universitário de Coimbra, University of Coimbra).
Results: The study included 15 patients between October 2015 and October 2020 from Angola, Cape Verde, Guinea-Bissau and São Tomé and Príncipe. Seven (46.7%) children presented bilateral retinoblastoma. The median age at the time of diagnosis was 20.9 (interquartile range, 16– 41) months. The presenting symptoms were leukocoria (86.7%), strabism (53.3%) and buphthalmus (40%). In terms of tumor staging, five (33.3%) children presented with extraocular retinoblastoma and 10 (66.7%) children presented with intraocular retinoblastoma. At presentation, no pineal involvement was diagnosed but two (13.3%) children presented with central nervous system involvement at the time of the first observation. Children were treated with enucleation, exenteration, systemic chemotherapy, intra-arterial chemotherapy and/or supportive palliative care. During the follow-up period (mean 27.2 ± 18.2 months), the overall survival was 73.3%.
Conclusion: A small proportion of African children are being referred to our center, when considering the expected incidence of retinoblastoma in these countries, and referred children arrive at advanced stages of the disease, compromising treatment outcomes. Considering retinoblastoma is now a curable disease, national and international interventions are required to attempt a better management of children born in low-income countries.
Keywords: retinoblastoma, low income countries, treatment of advanced disease
Background
Retinoblastoma is the most prevalent intraocular tumor of infancy, with an estimated incidence of 1 case per 16,000 to 18,000 live births, with no differences between genders or races.1,2 Treatment strategies for retinoblastoma have dramatically evolved in the last decade, and nowadays successful treatment options are available, with survival rates superior to 90%, in most cases with globe and vision preservation, when the diagnosis is made precociously.3,4 With the development of new treatment strategies with excellent efficacy and safety outcomes, retinoblastoma has become a hallmark of discrepancies between high- and low-income countries, where neither diagnosis nor treatment are available due to lack of human and material differentiated resources. Recently, a study including more than half of all new retinoblastoma cases worldwide in 2017, described that patients from low-income countries were diagnosed at a significantly older age (median age of 30.5 months) and at more advanced stages (49.1% having extra-ocular retinoblastoma and 18.9% with metastasis) when compared with high-income countries.5
In 2015, a Portuguese National Referral Center for the treatment of retinoblastoma was created in Centro Hospitalar e Universitário de Coimbra. At the same time, protocols were established with Portuguese-Speaking African Countries (PALOP), namely Angola, Cape Verde, Guinea-Bissau and São Tomé and Príncipe, former colonies of the Portuguese empire, that have signed official agreements to promote the development of culture, education, and preservation of the Portuguese language. Children born in these four countries with lesions suspicious of retinoblastoma can be referred for definite diagnosis, staging, treatment and follow-up at our center, under a protocol supported by the Portuguese government.
The aim of this study is to report the retinoblastoma stage at diagnosis in African patients referred to our center and to investigate treatment outcomes of these patients.
Methods
Study Population
We performed a cohort retrospective observational study including children born in PALOP Countries consecutively diagnosed with retinoblastoma at the Portuguese National Referral Center of Intraocular Tumours, between October 2015 and October 2020. Our study was approved by the ethics committee of University of Coimbra and of Centro Hospitalar e Universitário de Coimbra and was performed according to the tenets of Declaration of Helsinki.
Inclusion criterion was the diagnosis of retinoblastoma in children evacuated from a PALOP Country, referred to the Portuguese National Reference Center of Intraocular Tumors.
At the diagnosis, the child’s complete medical history was obtained, and ophthalmological examination performed by a team specialized in pediatric ophthalmology and ocular oncology. Examination under anesthesia was performed for detailed fundus evaluation and to capture and record fundus images with RetCam3 (Clarity Medical Systems, Inc.). All the children were also observed by a team of pediatric oncologists for a complete comprehensive systemic evaluation. Staging was performed by the multidisciplinary team according to the International Classification of Retinoblastoma (ICRB) staging system and, in case of extra-ocular retinoblastoma, according to the American Joint Commission on Cancer (AJCC) staging system (8th edition).6,7 Blood samples were collected and were used to obtain a complete genetic testing for RB1 mutations. At baseline and periodically during the follow up (every 3–6 months), the included participants underwent cerebral magnetic resonance imaging (MRI) for evaluation of pineal involvement.
Patients with bilateral retinoblastoma, pineal involvement, positive family history of retinoblastoma or with a RB1 pathogenic variant found in the genetic testing were included in the hereditary retinoblastoma group. Remaining patients (unilateral retinoblastomas with negative family history and negative genetic testing) were included in the non-hereditary retinoblastoma group.
Statistical Analysis
Patients were characterized in terms of demographics (region of birth, sex, age at diagnosis, clinical signs at presentation and familial history of retinoblastoma) and tumor characteristics (eye stage according to ICRB and AJCC, multifocality and laterality). Information regarding time between first diagnosis of lesion suspicious of retinoblastoma in the home country and first observation at our center was also registered.
Epidemiological data, namely annual living births and population under 5 years old, were obtained from the databases of the United Nations Population Fund. These values were used to calculate the estimated expected annual incidence in each country considering 1 per 16,000–18,000 annual incidence of retinoblastoma.
All statistical analysis was performed using IBM SPSS Statistics 26 for Windows.
Results
Demographics and Clinical Presentation
During the 5 years of the study, 15 children (6 females and 9 males) referred to our center were diagnosed with retinoblastoma. Four (26.7%) children were born in Angola, 4 in Cape Verde, 6 (40%) in Guinea-Bissau and 1 (6.7%) in São Tomé and Príncipe (Table 1). The mean annual population under 5 years, calculated with data from the United Nations Population Fund, and the respective expected estimated annual incidence considering an incidence of 1 in 16,000–18,000 can be found in Table 1.
 |
Table 1 Mean Annual Population at Risk of Retinoblastoma and Estimated Retinoblastoma Incidence in Portuguese-Speaking African Countries |
None of the children presented with a known positive family history. Seven (46.7%) children presented with bilateral retinoblastoma. The median age at the time of diagnosis was 20.9 (interquartile range, 16–41) months. The presenting symptoms at the first observation at our center were leukocoria (present in 86.7% of children) and strabism (in 53.3%). Six (40%) children presented with buphthalmus, cellulitis or proptosis suggestive of orbital invasion. Two (13.4%) were enucleated at the hometown country before the referral, of which one was present with orbital recurrence at the time of first observation at our center.
The following RB1 pathologic variants were found in the referred children: c25020+1G>T, c1981C>T (pArg661Trp), c958C>T (pArg320*), c1891C>T (Gln631*) and c1945delinsAA (pLeu649Asnfs*4). Three children did not perform a genetic test due to progressive advanced disease at the time of arrival to our center.
Retinoblastoma Staging
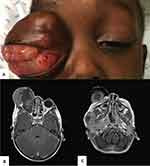
Five (33.3%) children presented with extraocular retinoblastoma at the time of first observation at our center, three with bilateral retinoblastoma (from which two had bilateral extraocular retinoblastoma) and two with unilateral extraocular retinoblastoma. Extraocular disease was clinically evident, with proptosis and/or orbital mass (cT4b), in four patients, and one patient had only radiologic evidence of bilateral retrobulbar optic nerve involvement (cT4a), according to the AJCC TNM Classification System (Figure 1).
The remaining 10 (66.7%) children with intraocular retinoblastoma presented the following classification according to the ICRB staging system: 6 (40%) unilateral retinoblastomas were classified as group E and 4 (26.8%), that presented bilateral retinoblastoma, had group E retinoblastoma in the worst eye and group B (1, 6.7%), group C (1, 6.7%) or group D (2, 13.4%) retinoblastoma in the best eye (Table 2).
 |
Table 2 Patients Diagnosed with Retinoblastoma Evacuated from Angola, Guinea-Bissau, Cape Verde and São Tomé and Príncipe |
At presentation, no pineal involvement was diagnosed. Two (13.3) children presented with central nervous system involvement at the time of the first observation at our center. Other distant metastasis (e.g. bone marrow, liver or other) was not found in our sample.
Treatment Outcomes
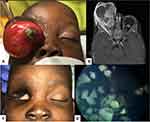
Considering the treatment of children diagnosed with unilateral retinoblastoma, two (13.3%) children were enucleated and as no pathological prognostic risk factors were found no systemic chemotherapy was necessary. Four (26.7%) children were submitted to enucleation complemented with systemic chemotherapy, namely six cycles of the association of vincristine, etoposide and cisplatin (VEC). One child had already been enucleated in their home country and no further treatment was necessary. Finally, one child presented with aggressive CNS involvement at the time of referral and only supportive palliative care was offered (Figure 2).
Considering the treatment of children diagnosed with bilateral retinoblastoma, one child was submitted to bilateral enucleation with pre-chiasmatic bilateral optic nerve section, in an intervention with the collaboration of the neurosurgery department (Figure 1). One child was submitted to eyelid sparing orbital exenteration and the fellow eye was treated with systemic chemotherapy (6 cycles of VEC) complemented with transpupillary thermotherapy (TTT) (Figure 3). One child presented with aggressive CNS involvement at the time of referral and only supportive palliative care was offered. The remaining 4 children were submitted to enucleation of the worst eye and the fellow eye was treated with a combination of systemic chemotherapy (6 cycles of VEC) complemented with intra-arterial chemotherapy (one child), intravitreal chemotherapy (one child) or TTT (one child) (Figure 4).
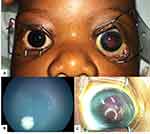 |
Figure 4 Bilateral Retinoblastoma presenting with buphthalmos of the left eye (A and C) and an intraocular lesion in the right eye (B). |
During the follow-up period (mean 27.2 ± 18.2 months), the overall survival was 73.3%. Two children died soon after referral due to advanced metastatic retinoblastoma at presentation, one died 9 months after bilateral enucleation due to leptomeningeal invasion during the follow up and one presented with pineal involvement 12 months after the initial treatment. Two children, both with bilateral retinoblastoma, both presented recurrence of retinoblastoma activity in the best eye at 6 months of follow up and were successfully treated with intra-arterial chemotherapy.
Discussion
We present a comprehensive analysis of children born in low-income African countries, referred to a reference center for the management of retinoblastoma, during a period of 5 years. The median age at diagnosis in our sample was 20.9 months which was in accordance with the data reported recently by the Global National Retinoblastoma Group. In a cross-sectional analysis of retinoblastoma presentation by national income level, the group reported a median age at diagnosis of 30.5 months in children born in low-income countries, which contrasted enormously to the median age of 14.1 months found in children born in high-income countries.5 However, the proportion of extra-ocular retinoblastoma was slightly lower in our sample (33.3% vs 49.1%, respectively).5 As reported before, we found a smaller proportion of familial history of retinoblastoma. It is believed that in low-income countries the incidence of hereditary retinoblastoma is lower compared with high-income countries due to higher mortality and lower reproduction in the affected patients.
For all the countries (Angola, Cape Verde, São Tomé and Príncipe and Guinea-Bissau) the number of referred children was substantially lower than the estimated incidence based on the annual birth rates of these countries. A recently published paper analyzing 1024 patients from 43 African countries also found that fewer than half of the expected number of patients with retinoblastomas were referred to specialized centers.8 This can be explained by the high mortality in the first years of life, low access to healthcare facilities and other barriers in these countries. The proportion of referred children born in Angola is considerably lower when compared with the other countries. This can be explained by the fact that Angola provides cancer treatment at Instituto Angolano de Controlo de Cancer, where enucleation and systemic chemotherapy can be offered to the children. Furthermore, Angola has a higher immigration rate and possibly some children can be treated in other foreign countries. Nevertheless, it must be a priority to improve health education about retinoblastoma in Portuguese-speaking African countries, in order to increase the rate of referred children, at early disease stages, to achieve in the future better outcomes in the treatment of these children.
Enucleation performed in the home country can be lifesaving in children with unilateral retinoblastoma, as there is a significant delay between the referral and the definite time of travel to our center, which consequently delays treatment. However, it is important to guarantee the safety of the procedure and that pathology analysis is performed to guide the adequate subsequent treatment. In the two cases of children who were enucleated in their home country, both by Portuguese ophthalmologists in humanitarian missions in the respective countries, only one had access to cerebral imaging before the procedure (computed tomography scan or nuclear magnetic resonance imaging) and to pathology analysis, that was performed to the enucleated specimen posteriorly sent to Portugal. The other case presented aggressive orbital invasion when first observed at our center and died shortly after arriving.
Considering retinoblastoma is a curable disease, national and international interventions are required to attempt a better management of children born in low-income countries. The information in our report helps to better understand current gaps in the process of referral of children with ocular lesions suspicious of retinoblastoma.
Strengths and Limitations
- First results of retinoblastoma-diagnosed children born in Portuguese Speaking African Countries, providing epidemiological information and survival rates in these low-income countries.
- Experience of a national reference center in the management of advanced cases of retinoblastoma, in which definite treatment protocols are still lacking.
- Alert for the importance of an early diagnosis and referral of children with retinoblastoma born in low income countries.
- Our limitations include a small sample and a selection bias of the children arriving in our center.
Conclusion
In summary, we report our center’s experience in the treatment of African children diagnosed with retinoblastoma and evacuated from their home country for treatment at a referral center. We found that a small proportion of children are being referred, when considering the expected incidence of retinoblastoma in these countries. Referred children arrive at our center with advanced stages of the disease, compromising treatment outcomes and survival. In the future, we hope to improve the local awareness in PALOP countries regarding early retinoblastoma diagnosis and of the possibility of evacuation for treatment at our National Referral Center to ultimately remove the discrepancies in the access to healthcare facilities for children born in these low-income countries.
Patient and Public Involvement
It was not appropriate or possible to involve patients or the public in the design, or conduct, or reporting, or dissemination plans of our research.
Data Sharing Statement
Data are provided in the tables. Any additional data can be requested from the corresponding author.
Ethics Statement
This study was approved by the Ethics committee of Centro Hospitalar e Universitário de Coimbra, Coimbra, Portugal and of Faculty of Medicine of University of Coimbra. A patient parental informed consent was obtained to review the medical clinical records. Patient data were reviewed assuring patient confidentiality and in compliance with the Declaration of Helsinki. A parent of the patient provided informed consent for the figures published.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This research received no specific grant from any funding agency in the public, commercial or not-for- profit sectors.
Disclosure
The authors report no conflicts of interest for this work.
References
1. Seregard S, Lundell G, Svedberg H, Kivelä T. Incidence of retinoblastoma from 1958 to 1998 in Northern Europe: advantages of birth cohort analysis. Ophthalmology. 2004;111(6):1228–1232. doi:10.1016/j.ophtha.2003.10.023
2. Pendergrass TW, Davis S. Incidence of Retinoblastoma in the United States. Arch Ophthalmol. 1980;98(7):1204–1210. doi:10.1001/archopht.1980.01020040056003
3. Manjandavida FP, Stathopoulos C, Zhang J, Honavar SG, Shields CL. Intra-arterial chemotherapy in retinoblastoma – a paradigm change. Indian J Ophthalmol. 2019;67(6):740–754. doi:10.4103/ijo.IJO_866_19
4. Gobin YP, Dunkel IJ, Marr BP, Brodie SE, Abramson DH. Intra-arterial chemotherapy for the management of retinoblastoma four-year experience. Arch Ophthalmol. 2011;129(6):732–737. doi:10.1001/archophthalmol.2011.5
5. Fabian ID, Abdallah E, Abdullahi SU, et al. Global retinoblastoma presentation and analysis by national income level. JAMA Oncol. 2020:1–11. doi:10.1001/jamaoncol.2019.6716
6. Shields CL, Mashayekhi A, Au AK, et al. The International Classification of Retinoblastoma Predicts Chemoreduction Success. Ophthalmology. 2006;113(12):2276–2280. doi:10.1016/j.ophtha.2006.06.018
7. TNM8. The updated TNM classification for retinoblastoma. Community Eye Health. 2018;31(101):34.
8. Fabian ID, Stacey AW, Foster A, et al. Travel burden and clinical presentation of retinoblastoma: analysis of 1024 patients from 43 African countries and 518 patients from 40 European countries. Br J Ophthalmol. 2020:1–9. doi:10.1136/bjophthalmol-2020-316613
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.