Back to Journals » Neuropsychiatric Disease and Treatment » Volume 15
Treatment of acute thromboembolic complication after stent-assisted coil embolization of ruptured intracranial aneurysm: a case report
Authors Xu N, Meng H, Liu T, Feng Y, Qi Y, Wang H
Received 17 August 2018
Accepted for publication 17 November 2018
Published 21 December 2018 Volume 2019:15 Pages 69—74
DOI https://doi.org/10.2147/NDT.S184372
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Yuping Ning
Ning Xu, Hao Meng, Tianyi Liu, Yingli Feng, Yuan Qi, Honglei Wang
Department of Neurosurgery, The First Hospital of Jilin University, Changchun 130021, China
Abstract: A 45-year-old Chinese man presented with acute severe headache for 2 days. He was diagnosed as subarachnoid hemorrhage. Head CT and subsequent head digital subtraction angiography (DSA) showed left internal carotid artery (ICA) aneurysm in the supraclinoid segment. Stent-assisted coil embolization of aneurysm was performed. Three hours after the surgery, the patient was found to be drowsy and with paralysis of the right limb and slurred speech. Urgent head CT examination ruled out acute hemorrhage; however, DSA showed acute thrombosis in the left ICA between the branches of the ophthalmic artery and middle cerebral artery, which was probably from an acute in-stent thrombosis. Urokinase (100,000 units) was given through a micro-tube but failed to dissolve the thrombus; thus, stent embolectomy was performed, which successfully removed the thrombus. Repeat angiography showed that the left ICA was completely recanalized. Postoperatively, the patient regained consciousness and was well-limbed and fluent in speech. No neurological symptoms or signs were found at 6-, 12-, and 24-month follow-up.
Keywords: acute thromboembolic complication, intracranial aneurysm, stent-assisted coil embolization, re-canalized
Introduction
Stent placement is generally considered a safe and effective treatment for craniocervical aneurysms.1,2 However, thromboembolism is a common complication, leading to permanent disability. The incidence of thromboembolic complication was reported to be between 2% and 15% after endovascular treatment of intracranial aneurysms.3,4 The onset of thromboembolic complications can be acute, subacute, or delayed.5 Its prognosis depends on a number of factors such as location of occluded blood vessel, the status of collateral circulation, and timely revascularization.
Herein we report a patient who failed stent-assisted coil embolization of aneurysm due to acute in-stent thrombosis and suffered postoperative thromboembolic complication. The occluded artery was successfully recanalized through stent embolectomy and the patient completely recovered.
Case report
The study protocol was approved by the ethics committee of the First Hospital of Jilin University. The patient provided written informed consent to surgery and to the publication of the current case report, and the patient data were anonymized.
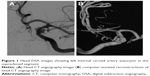
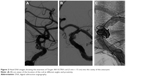
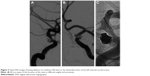
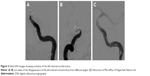
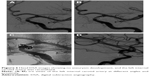
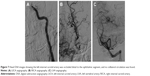
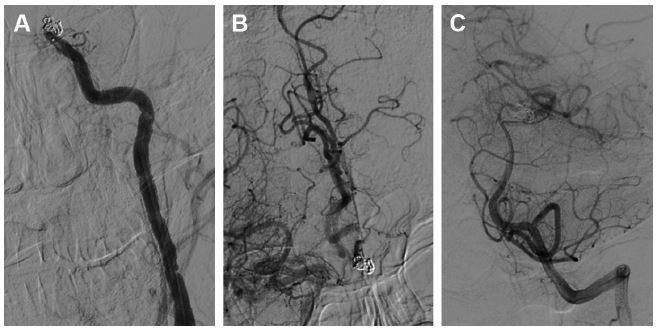
A 45-year-old Chinese male was hospitalized on March 6, 2015 due to sudden severe headache for 2 days. The patient was previously healthy; he denied history of hypertension, diabetes or cardiac disease. He had no history of drug or food allergy. Physical examination showed that the patient was awake, alert, and oriented, with neck stiffness but no limb paralysis. Head CT showed subarachnoid hemorrhage (SAH) (data not shown) and head digital subtraction angiography (DSA) demonstrated left internal carotid artery (ICA) aneurysm in the supraclinoid segment. The aneurysm was irregular in shape and appeared leaf-lobulated with a size of 5.2×3.7 mm2 (Figure 1). Stent-assisted aneurysm embolization was performed on March 10, 2015. Clopidogrel 300 mg and aspirin 300 mg were given orally 2 hours postoperatively. After general anesthesia, the right femoral artery was punctured with Seldinger technique, and a 6F arterial sheath was inserted. A 6F guidance catheter (Chaperon 6F; MicroVention, Tustin, CA, USA) was delivered to the petrous segment of the left ICA. An Echelon-10 microcatheter (ev3 Neurovascular, Irvine, CA, USA) was delivered into the aneurysm in the supraclinoid segment of the left ICA; meanwhile, a Rebar-27 microcatheter (ev3 Neurovascular) was delivered to the M1 segment of the left middle cerebral artery (MCA) for later use. Then, a Target 360 ULTRA coil (5 mm × 15 cm; Stryker Corporation, Fremont, CA, USA) was delivered into the aneurysm cavity via the Echelon-10 microcatheter, and the basket-forming of the coil was good (Figure 2). A Solitaire AB stent (6 × 20 mm; ev3 Neurovascular) was delivered to the distal bifurcation of the left ICA through the Rebar-27 microcatheter (Figure 3). The stent was released half open to partially block the opening of the aneurysm in the supraclinoid segment of the left ICA (Figure 4A and B). Then, a Helix ev3 Axium (3 mm × 8 cm), a MicroPlex 10 HyperSoft Helical (3 mm × 6 cm), and a Helix ev3 Axium (2 mm × 2 cm) coil were delivered into the aneurysm cavity via Echelon-10 microcatheter. Angiography showed a complete embolization of the aneurysm, but the anterior communicating artery was not developed (Figure 4C). As a result, 10 mL tirofiban was injected through the microcatheter (Figure 4D), and the Helix ev3 Axium (2 mm × 2 cm) coil was withdrawn, but the left ICA was poorly developed (Figure 5A and B). So the Solitaire AB stent was retracted, during which the MicroPlex 10 HyperSoft Helical coil was removed from the lumen of the aneurysm (Figure 5C). Repeat DSA showed no aneurysm development, and the left ICA was unobstructed (Figure 6A–D). The operation was then terminated. The patient was awake after the operation, and the language and physical activity were normal.
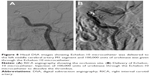
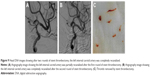
Three hours after the operation, the patient became drowsy, the right limb was paralyzed, and his speech became slurred. Urgent head CT did not reveal any further intracranial bleeding. Acute occlusion of the left ICA was suspected. The patient was sent to the operation room immediately, and urgent brain angiography was performed. The left ICA was found to be occluded distal to the ophthalmic segment, and there was no collateral circulation from either the anterior communicating artery or the posterior communicating artery (Figure 7). The Echelon-10 microcatheter was delivered to the left MCA M1 segment across the occluded segment of the left ICA, and angiography showed that the occlusion was mainly localized between the ophthalmic segment of the left ICA and the M1 segment of the left MCA. About 100,000 units of urokinase was given through the Echelon-10 microcatheter but failed to dissolve the thrombus (Figure 8). Thus, a Rebar-18 microcatheter (ev3 Neurovascular) was delivered to the M1 segment of left MCA and a Solitaire AB stent (4 × 20 mm) was also delivered to the left MCA M1 segment beyond the thrombus formation site; then, the Rebar-18 microcatheter was retracted, the Solitaire AB stent was open fully and also retracted to remove the thrombus. After two rounds of stent thrombectomy, the left ICA was completely re-canalized (Figure 9). No histological examination of the thrombus specimen was undertaken. There was no development of left ICA supraclinoid segment aneurysm on repeat angiography. The ICA was observed for 30 minutes, and it remained patent. Postoperatively, the patient regained his consciousness and was well-limbed and fluent in speech. The patient was maintained on aspirin 100 mg daily after the operation. The patient was followed up and at 24 months after discharge was symptom free.
Discussion
Thromboembolic complications usually occur during endovascular operation and 48 hours postoperatively, but can be as late as 9 weeks postoperatively. Early thromboembolic complications are mainly caused by plaques falling off intraoperatively, while late thromboembolic complications are probably associated with long-term thrombus organization or shedding of unstable thrombus. As for the type of thrombus, early stage thrombus is mostly platelet thrombus, while late-stage thrombus is mainly fibrin thrombus.
Many factors contribute to the development of thromboembolic complications, including hypercoagulation state, inadequate intraoperative anticoagulation, plaque falloff, and the coil protruding into the parent artery.6 Though stent placement poses a risk for thromboembolic complications, postoperative thrombus formation following coiling is also possible without using stents, especially in cases of SAH. Such thrombus formation might be stimulated by the injuries of the vascular endothelium during procedures and facilitated by SAH-related hypercoagulative state. The clinical manifestations of thromboembolic complications include transient ischemia attack, asymptomatic or symptomatic cerebral infarction, and even death.7 However, if a patient has adequate collateral flow via the ICA-terminus, in-stent thrombosis in the ICA does not always cause ischemia.
For the treatment of thromboembolic complications, general approaches include antiplatelet medication, intravenous fluid, and medication to boost blood pressure to promote collateral circulation. Thrombolytic therapy includes arterial and mechanical thrombolysis and combined thrombolysis with urokinase, and mechanical thrombolysis has a higher complete recanalization rate than simple drug thrombolysis.8 Combination of two medications, such as tirofiban plus urokinase, was also reported to be an effective and safe treatment for thromboembolism during coil treatment of ruptured cerebral aneurysms.9
To prevent thromboembolic complications, some general guidelines recommend heparin solution rinse (3,000–6,000 U/L), whole body heparinization (first dose 100 U/kg, maintenance dose 1,000 U/h), postoperative anticoagulation and platelet inhibition with tirofiban, adjustment of heparin dose with activated clotting time, improvement of technical factors such as placement of balloon and stent, closer observation such as more frequent neural function monitoring, and more careful history taking such as a history of antiphospholipid antibody syndrome. Although some recent reports have favored the use of stents for treatment of acutely ruptured aneurysms, it is considered controversial by most operators due to concerns about the risk of using dual antiplatelet therapy in the setting of acute SAH. The risk of ischemic complications seems higher compared to the intervention to unruptured one. However, in the setting of full platelet inhibition with tirofiban, the use of stent is safe.
Among all the complications associated with stent-assisted embolization of aneurysm, acute thrombosis is not uncommon. Especially when the stent was half released, the stent was not fully open, and its interference with blood flow was more obvious, so the chance of thrombus formation was greater. However, after complete withdrawal of the stent and three hours after the procedure, a second round of thrombus formation was extremely rare, which prompted us to report this case. In our case, after second acute thrombus formation, thrombectomy was performed using a Solitaire stent and eventually the blood vessel was completely recanalized. Literature review did not reveal any similar case reported. Our patient was found to have normal coagulation parameters, so we propose that the cause of intraoperative thrombus formation was due to the half-release stent technique used, that is, the stent was only half open, which might interfere with local blood flow, leading to thrombosis. A second reason may be that, when the coils were packed, due to a wide aneurysm neck, more coils were exposed in the parent artery, which could stimulate thrombus formation since coils were foreign bodies. As for the cause of postoperative thrombosis, we suspect that it was due to prolonged operation caused by failed stent-assisted coil embolization of aneurysm followed by acute intra-stent thrombosis. Prolonged operation is one of the risk factors leading to thromboembolic complications. Nevertheless, we successfully treated the postoperative thrombosis by a combination of arterial thrombolysis (urokinase rinse) followed by mechanic thrombolysis (stent assisted thrombectomy) which has been recommended to be the most effective approach.
Conclusion
Although rare, acute in-stent thrombosis and acute postoperative thrombosis can happen in patients after stent-assisted coil embolization of aneurysm. Accurate diagnosis with angiography in a timely manner is key to survival. Combination of arterial thrombolysis with mechanic thrombolysis could be an effective approach in treating these complications.
Disclosure
The authors report no conflicts of interest in this work.
References
Zhang T, Deng J, Chen H, et al. Treatment of cranial-cervical aneurysms with stent-graft: 20 cases report with short-term follow-up. Zhonghua Wai Ke Za Zhi. 2016;54(5):346–351. Chinese. | ||
McLaughlin N, McArthur DL, Martin NA. Use of stent-assisted coil embolization for the treatment of wide-necked aneurysms: a systematic review. Surg Neurol Int. 2013;4:43. | ||
Ries T, Siemonsen S, Grzyska U, Zeumer H, Fiehler J. Abciximab is a safe rescue therapy in thromboembolic events complicating cerebral aneurysm coil embolization: single center experience in 42 cases and review of the literature. Stroke. 2009;40(5):1750–1757. | ||
Brinjikji W, McDonald JS, Kallmes DF, Cloft HJ. Rescue treatment of thromboembolic complications during endovascular treatment of cerebral aneurysms. Stroke. 2013;44(5):1343–1347. | ||
Kanaan H, Jankowitz B, Aleu A, et al. In-stent thrombosis and stenosis after neck-remodeling device-assisted coil embolization of intracranial aneurysms. Neurosurgery. 2010;67(6):1523–1533. | ||
Itrat A, Toth G, Min D, Hussain MS. Extensive stretching of intracranial aneurysm coil causing TIAs. Neurology. 2015;85(18):1635. | ||
Qureshi AI, Luft AR, Sharma M, Guterman LR, Hopkins LN. Prevention and treatment of thromboembolic and ischemic complications associated with endovascular procedures: Part II – Clinical aspects and recommendations. Neurosurgery. 2000;46(6):1360–1375. | ||
Cronqvist M, Pierot L, Boulin A, Cognard C, Castaings L, Moret J. Local intraarterial fibrinolysis of thromboemboli occurring during endovascular treatment of intracerebral aneurysm: a comparison of anatomic results and clinical outcome. AJNR Am J Neuroradiol. 1998;19(1):157–165. | ||
Feng L, Chen J, Lv CF, Liu J. Intra-arterial infusion of tirofiban and urokinase for thromboembolic complications during coil embolization of ruptured intracranial aneurysms. Turk Neurosurg. 2014;24(6):929–936. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.