Back to Journals » Journal of Pain Research » Volume 13
Treatment of a Large Cohort of Veterans Experiencing Musculoskeletal Disorders with Spinal Cord Stimulation in the Veterans Health Administration: Veteran Characteristics and Outcomes
Authors Wandner LD, Fenton BT, Goulet JL , Carroll CM, Heapy A, Higgins DM , Bair MJ, Sandbrink F, Kerns RD
Received 9 December 2019
Accepted for publication 7 May 2020
Published 7 July 2020 Volume 2020:13 Pages 1687—1697
DOI https://doi.org/10.2147/JPR.S241567
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor E Alfonso Romero-Sandoval
Laura D Wandner,1,2 Brenda T Fenton,3 Joseph L Goulet,3,4 Constance M Carroll,5 Alicia Heapy,3,6 Diana M Higgins,7,8 Matthew J Bair,9– 11 Friedhelm Sandbrink,12,13 Robert D Kerns3,6,14,15
1National Institute of Neurological Disorders and Stroke (NINDS), National Institutes of Health (NIH), Bethesda, MD, USA; 2Department of Anesthesiology, Walter Reed National Military Medical Center, Bethesda, MD, USA; 3Pain Research, Informatics, Multimorbidities and Education (PRIME) Center, VA Connecticut Healthcare System, West Haven, CT, USA; 4Department of Emergency Medicine, Yale School of Medicine, New Haven, CT, USA; 5Yale University AIDS Program, New Haven, CT, USA; 6Department of Psychiatry, Yale School of Medicine, New Haven, CT, USA; 7VA Boston Healthcare System, Boston, MA, USA; 8Department of Psychiatry, Boston University School of Medicine, Boston, MA, USA; 9VA HSR&D Center for Health Information and Communication, Roudebush VA Medical Center, Indianapolis, IN, USA; 10Division of General Internal Medicine and Geriatrics, Department of Medicine, Indiana University School of Medicine, Indianapolis, IN, USA; 11Regenstrief Institute, Indianapolis, IN, USA; 12Department of Neurology, VA Medical Center, Washington, DC, USA; 13Department of Neurology, Georgetown University, Washington, DC, USA; 14Department of Neurology, Yale School of Medicine, New Haven, CT, USA; 15Department of Psychology, Yale University, New Haven, CT, USA
Correspondence: Laura D Wandner
National Institutes of Health (NIH), 10 Center Dr, Bethesda, MD 20814, USA
Email [email protected]
Objective: Spinal cord stimulator (SCS) implantation is used to treat chronic pain, including painful musculoskeletal disorders (MSDs). This study examined the characteristics and outcomes of veterans receiving SCSs in Veterans Health Administration (VHA) facilities.
Methods: The sample was drawn from the MSD Cohort and limited to three MSDs with the highest number of implants (N=815,475). There were 1490 veterans with these conditions who received SCS implants from 2000 to 2012, of which 95% (n=1414) had pain intensity numeric rating scale (NRS) data both pre- and post-implant.
Results: Veterans who were 35– 44 years old, White, and married reported higher pain NRS ratings, had comorbid inclusion diagnoses, had no medical comorbidities, had a BMI 25– 29.9, or had a depressive disorder diagnosis were more likely to receive an SCS. Veterans 55+ years old or with an alcohol or substance use disorder were less likely to receive an SCS. Over 90% of those receiving an SCS were prescribed opioids in the year prior to implant. Veterans who had a presurgical pain score ≥ 4 had a clinically meaningful decrease in their pain score in the year following their 90-day recovery period (Day 91– 456) greater than expected by chance alone. Similarly, there was a significant decrease in the percent of veterans receiving opioid therapy (92.4% vs 86.6%, p< 0.0001) and a significant overall decrease in opioid dose [morphine equivalent dose per day (MEDD) =26.48 vs MEDD=22.59, p< 0.0003].
Conclusion: Results offer evidence of benefit for some veterans with the examined conditions. Given known risks of opioid therapy, the reduction is an important potential benefit of SCS implants.
Keywords: spinal cord stimulator, musculoskeletal disorders, veterans, outcomes, opioids
Introduction
The Veterans Health Administration (VHA) is the largest healthcare organization in the United States, treating over 9 million veterans annually in recent years.1 Veterans are more likely than non-veterans to report pain and severe pain compared to non-veterans.2 Estimates suggest that approximately 50% of male veterans, and as many as 75% of female veterans, report pain when presenting to VHA primary care settings.3,4 Painful musculoskeletal disorders (MSD) represent the largest cluster of medical conditions among veterans from the Afghan and Iraqi wars.5 Since 2000, over 5 million veterans receiving VHA care were diagnosed with one or more MSDs; 25% of those were diagnosed with back conditions.6
Since the 1960s, spinal cord stimulator (SCS) implantation has been used to treat severe pain conditions, including some MSDs. Since that time, there have been numerous reports on the effectiveness of SCS implantation as well as systematic reviews of the published literature.7,13 Previous research, however, documents variable effects of SCS implantation, particularly modest effects in reducing pain intensity, with evidence of dissipating effects over time. The large variation in SCS efficacy has been attributed to poor patient selection.7,9,12,14,15 Although no nationally accepted guidelines exist to determine appropriate candidates for SCSs, the efficacy of SCSs is better for patients with specific diagnoses (eg, lumbar post-laminectomy syndrome, radiculopathy, polyneuropathy, complex regional pain syndrome, failed back surgery, back pain, and limb pain).7,9,12,14,16,19 Furthermore, efficacy is higher after a successful SCS trial, and also when patients are selected for permanent implantation using stringent criteria (eg, using rule-outs for specific psychological conditions such as somatization and substance use disorders).9,12 Research also suggests that SCS implants should be reserved for patients with medically indicated conditions, who remain refractory to more conservative pain management interventions, or who are at particularly high risk of harm related to high dose long-term opioid therapy.10,17,20 However, some new research suggests that SCS implants may be useful to manage chronic untreatable pain.21,23 Overall, there are only limited samples that recent or large enough to study outcomes such as reductions in: pain intensity ratings, use and dosage of opioid therapy, improvement in functioning, and/or improvement in quality of life ratings.19,24,32
Our aims in this study were to describe the demographic and clinical characteristics of veterans receiving SCS implants in the VHA and their one-year post-implant outcomes. We focused on SCS implants for three high prevalence MSDs for which SCS may be indicated, namely post-laminectomy syndrome of the lumbar region, thoracic or lumbosacral neuritis or radiculitis, and lumbago. For these analyses, we compared veterans with these three MSDs who either received an SCS implant or did not during the observation period. For this cohort of veterans, we examined changes in pain intensity ratings and changes in opioid therapy receipt and dosing following SCS implantation.
Methods
Creation of the MSD Cohort
The MSD cohort is described in detail elsewhere.33 Briefly, it was created to identify veterans with MSD diagnoses from 2000 to 2012.33 In order to be included in the MSD cohort, veterans must have had at least two outpatient visits occurring within 18 months of one another or one recorded inpatient MSD diagnosis.34 The index date for entry into the cohort was the date of the veteran’s first outpatient or inpatient MSD diagnosis. A veteran could have more than one MSD diagnosis on the index date. The MSD cohort was approved by the Institutional Review Boards of the VA Connecticut Healthcare System and the Yale School of Medicine and was granted a HIPAA waiver and waiver of informed consent.33
For veterans identified with an MSD, additional information from other VHA electronic data sources was collected, such as demographic characteristics (ie, age, gender, race/ethnicity, and marital status) at or near the index date. Veterans’ pain intensity numeric rating scale (NRS) scores were collected from vital signs data recorded in their electronic health records (EHRs). The NRS is used in routine clinical care to screen for the presence and intensity of pain by asking veterans, “On a scale of 0 to 10, where 0 means no pain and 10 means the worst possible pain, what is your current pain level?” Pain intensity ratings at MSD index date are the highest pain intensity ratings collected on the index date. Pain intensity ratings were categorized as none (0); mild (1,2,3); moderate (4,5,6); or severe (7,8,9,10).35,36
Medical and mental health diagnoses were collected from the EHR and considered comorbid with an MSD diagnosis if they occurred up to one year prior to the MSD diagnosis or up to six months after. All comorbid medical and mental health conditions were collected in the same manner as MSD diagnoses (ie, required two or more outpatient codes within 18 months, or one or more inpatient codes). The Charlson Comorbidity Index (CCI) was calculated based on medical diagnoses that were current in the year prior and up to six months after the MSD index date.37 Higher scores on the CCI suggest greater comorbidity, with patients who scored a 5 or greater on the CCI at increased risk of mortality within one year. Body Mass Index (BMI) was calculated using height and weight at the time closest to the veteran’s entry into the MSD cohort. Mental health conditions examined in the current study include: depressive disorders (ie, major depressive disorder, depressive disorder NOS, dysthymia), anxiety disorders (ie, anxiety disorder NOS, panic disorder, generalized anxiety disorder, agoraphobia with and without panic), post-traumatic stress disorder [PTSD]), serious mental illness (SMI; bipolar disorder, schizophrenia, other psychosis), alcohol use disorder, and drug use disorder. Drug and alcohol use disorders were combined as substance use disorders in multivariable models given their common negative relationship with SCS implantation and small case size in the SCS group. Pain intensity ratings and the Charlson Comorbidity Index categories were collapsed due to small sample sizes within the SCS implant group (for example, minimal pain was combined with the no pain group).
Data on MSD cohort members were collected until the end of 2012 to allow for follow-up. Demographic data, except for marital status, were collected on the date of the first MSD diagnosis. Marital status is the most current status available at the time of the most recent cohort update.
Identification of SCS Implants and Creation of the SCS Analytic Sample
Potential SCS implantations were identified using current procedural terminology (CPT) codes of: 63,650 (lead insertion), 63,655 (neuro-stimulator spinal procedure), or 63,658 (placement of a spinal neuro-stimulator) at any VHA facility from January 2000 to December 2012. Three diagnoses (post-laminectomy syndrome of the lumbar region, thoracic or lumbosacral neuritis or radiculitis, and lumbago; ICD-9CM codes 724.2, 724.4 and 722.83) accounted for 72.26% of SCS implants during this period. An analytic sample was created as a subsample of the MSD cohort, including persons with at least one of those three diagnoses regardless of implant status (n=815,475). This subsample allowed comparison of veterans with similar diagnoses who either did or did not receive an SCS implant. Of the 815,475 veterans with one or more of the conditions of interest, there were 1490 (0.18%) veterans with one of these diagnoses who received an SCS implant. To validate these codes, two members of the research team (LDW and CMC) reviewed the procedure descriptions for each veteran in the VHA surgical tables. Procedures labeled “placement of a permanent SCS,” “stage or Phase II SCS,” “dorsal column stimulator,” “spinal neuro-modular implant,” “completion of a laminectomy,” “insertion or replacement of an SCS,” or “implantable pulse generator (IPG)” were included as SCS procedures. The date of the veteran’s first SCS was recorded. If it could be determined that a veteran had a trial SCS and a permanent SCS implanted, the recorded date was the date of receiving his/her first permanent SCS.
Independent Variables of Interest: Veteran-Level Data Associated with Implant and Follow-Up Outcomes
We examined pain intensity ratings and opioid therapy in the year prior to SCS as predictors of SCS implantation and changes in these measures as outcomes of implantation. Pain intensity ratings prior to implant were operationalized as the mean of all pain intensity ratings reported in the year prior to receiving an SCS implant. Mean pain intensity ratings were also calculated for the 90-day period following implant and the period 91–456 days following implant (one year following the post-SCS 90-day recovery period). VHA pharmacy data on all opioid prescription dispensed in the year prior to receiving an SCS implant were extracted. Opioid doses were standardized to a morphine equivalency (MEQ) using established weighting factors.38,39 Buprenorphine and methadone were excluded, since they are used primarily in the treatment of opioid use disorder. Using the same time-periods as for average pain intensity, morphine equivalent doses per day (or MEDDs) were calculated for three time periods: the year prior to implant, 90 days post-SCS implant, and the year following the post-SCS 90-day recovery period (Day 91–456). There were no missing data on opioids; there were 76 pain intensity ratings missing (N for analyses with pain intensity ratings =1414).
Statistical Analyses
Descriptive statistics were used to examine demographic and clinical characteristics between the SCS-implant and no-implant groups. Chi-square and t-tests were used to test between-group differences. The association of SCS implantation with demographic and clinical characteristics selected based upon their association with pain and/or being contraindications for SCS from the literature was examined using logistic regression. Finally, to look at clinically meaningful changes in both pain intensity ratings and opioid prescription (at least 20%, 30%, and 50% for each outcome) among those with SCS implantation, we used a chi-square test. The timeframe of the SCS implant also was examined in multinomial models containing the three individual diagnoses. Outcomes were no change (reference group), decrease, and increase in pain intensity ratings and in separate multinomial models no change (reference group), decrease, and increase in MEDDs. In order to examine clinically meaningful changes in these outcomes, cutoffs for meaningful differences were established as ≥20%, ≥30% and ≥50% change.
Results
Characteristics of Veterans Receiving SCS Implants
There were 815,475 veterans with any of the three conditions examined. In this analytic sample, there were 1490 veterans (0.2%) who received SCS implants for lumbago, post-laminectomy syndrome of the lumbar region, or thoracic or lumbosacral neuritis or radiculitis between 2000 and 2012. Less than 30 veterans received SCS implants in the first year examined (2000), but the number of implants increased over time, peaking in 2011 (n = 217).
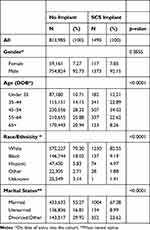
Table 1 compares the demographic and clinical characteristics of those veterans who received SCS implants versus those who did not. Those receiving SCS implants were more likely to be White, under the age of 55 years, and married; differences were all significant at p<0.0001. There were several important differences between groups in pain intensity ratings and comorbidity measures. The pain intensity ratings on the MSD index date were significantly higher among veterans who received SCS implants (Categorical pain intensity 0–10 Chi-square test, chi-square= 133.4, 3 df, p< 0.0001). In the year prior to SCS implantation, 77.4% of veterans had pain intensity ratings in the moderate or severe category (data not shown). The pain intensity ratings at MSD baseline and the year prior to SCS implantation were significantly correlated (Spearman correlation=0.2, p<0.0001) (Data not shown). There was a large percentage of veterans for whom pain intensity ratings were missing (28%). This was also observed in the larger MSD cohort and is likely to be the case for older dates of service as the proportion of veterans missing pain intensity ratings has decreased over time.33
Veterans with depressive disorders were more likely to receive an SCS implant than veterans with alcohol and/or drug use disorders. Veterans with higher Charlson Comorbidity Index (CCI) scores were less likely to receive an SCS implant than those with lower CCI scores. Seventy-two percent of veterans with one or more CCI comorbid condition received an SCS implant compared to 81% of veterans without comorbidities (chi-square test=65.1, 3 df, p<0.0001). Those who were normal or underweight were less likely to receive an implant (Chi-square test = 13.6, 2 df, p=0.02). Anxiety disorders, PTSD and SMI, were not significantly related to SCS implant. Most veterans (92.3%) who received an implant were prescribed opioids during the year prior to that surgery, with a median MEDD of 26.5 mg.
In logistic models adjusting for demographic and clinical characteristics (Table 2), findings remained similar; there was a significantly increased odds for SCS implantation in the 35–44 age group and a significantly decreased odds in the 55+ group compared to the under 35-year-old veterans. Veterans with either moderate or severe pain intensity ratings were more than twice as likely to receive an SCS implant than those with mild pain intensity ratings. The multivariable model revealed that veterans with one or more Charlson comorbid conditions had lower odds of receiving an SCS implant, with the higher comorbidity group failing to reach statistical significance. These two categories were collapsed and the comparison was made between any veterans with any comorbidity and those with no comorbid conditions. The number of MSD diagnoses was not a significant predictor of SCS implant and was removed from the final model.
 |
Table 2 Bivariate Analysis of Implant Status and Clinical Characteristics from Entry into the MSD Cohort |
Examining the Odds of an SCS Implant by Inclusion of Diagnosis
Three MSDs were included in the SCS analytic sample (“inclusion diagnoses,” see Methods); these diagnoses (post-laminectomy syndrome of the lumbar region, thoracic or lumbosacral neuritis or radiculitis, and lumbago) were examined individually to determine the contribution of each group to the SCS outcomes. The disorders are not mutually exclusive (7% of the analytic sample and 47% of the veterans who had an SCS implant had at least 2 MSD inclusion diagnoses). For each of the inclusion diagnoses, there was overinclusion in the SCS sample for veterans with more than one diagnosis. Veterans with a diagnosis of lumbago (either alone or concurrent with one of the other inclusion diagnoses) comprised 96.2% of the analytic sample and 93.6% of the sample receiving SCS. However, among those veterans receiving an implant, only 47.4% had a diagnosis of lumbago alone. Similarly, veterans with post-laminectomy syndrome of the lumbar region (either alone or concurrent with at least one other inclusion diagnosis) accounted for only 1.2% of the analytic sample but comprised 25.0% of the veterans receiving implants. Veterans with thoracic or lumbosacral neuritis or radiculitis (alone or concurrent with one of the other two diagnoses) examined accounted for 9.7% of the analytic sample and 41.2% of implants.
In our full logistic regression models with SCS implant as the outcome, the odds ratio of receiving an SCS for post-laminectomy syndrome of the lumbar region was very high (OR=17.6, 95% CI 14.9, 20.8), followed by thoracic or lumbosacral neuritis or radiculitis (OR=5.2, 95% CI 4.5, 6.0), and for lumbago (OR=2.0, 1.5, 2.6).
There were significant differences in baseline pain intensity ratings among the diagnostic groups in the analytic sample (Baseline mean pain intensity ratings=4.04, ANOVA, Chi-square (6 df) = 329.21, p<0.0001) with all post-laminectomy groups (ie, those with post-laminectomy alone or concurrent with one or more of the inclusion diagnoses) having higher pain intensity ratings. This same pattern was observed in the implant subsample but did not reach statistical significance (Baseline mean pain=5.25, ANOVA, Chi-square (6 df), F=1.67, p=0.1249).
Examining SCS by Time as the Intervention Has Changed Over Time
The nature of SCS technology has changed over time, and there is a widely held belief that the effectiveness of the approach has improved. To investigate if SCS outcomes have improved, a dichotomous variable was created (SCS implant date ≤ 2006, SCS implant date > 2006). The choice of 2006 as the cut-off point was based on expert opinion on the lag time for VHA integration of new procedures. Roughly one-quarter of spinal cord implants (N=407, 27.3%) were conducted before or during 2006. The early and later implant groups differed on four characteristics: the later implant group contained larger proportions of Black veterans (10.7% vs 5.16%, p<0.0129), higher pain intensity ratings pre-implant (p<0.0001) but not at baseline, veterans with PTSD (14.22% vs 9.09%, p<0.0083), and differences in the proportion of the specific diagnoses that comprised the sample (Chi-square, 6 df =32.58, p<0.0001).
Clinical Outcomes
Pain Intensity
Collected at the time of entry into the cohort, the average baseline pain intensity rating of veterans who went on to have an SCS was significantly higher than those who did not (Kruskal–Wallis test conclusions same as t-test; Chi-square=114.57, 1 df, p<0.0001). Examining the pain rating closest to the implant among veterans receiving one (“pre-implant”), the average pre-implant pain intensity rating was 5.16± 1.7 (mean±SD, n=1481). Pain intensity ratings were higher in the 90-day post-operative period than pre-implant [average rating=5.29±2.2 (mean±SD), sign test M=65.5, P<0.0005]. In the year following the post-operative period (Days 91–456), the average pain intensity rating was 5.11±2.1.
There was no statistically significant difference in the pain intensity rating in the year following the 90-day post-operative period (Days 91–456) compared to the pre-implant rating (sign test, M=14.5, p<0.45). Changes in pre- to post-SCS pain intensity ratings in Days 91–456 of ≥20%, ≥30%, and ≥50% were used as thresholds for identifying clinically meaningful improvements in pain intensity.40 Low proportions of veterans had clinically meaningful decreases in pain intensity (27.0%, 18.8%, and 9.3% for ≥20%, ≥30% and ≥50%, respectively) (Figure 1 illustrates changes in pain intensity where a clinically meaningful change was set at ≥30% change; we see a small proportion of post-SCS veterans had a meaningful decrease in pain in the year following the 90-day recovery period.
 |
Figure 1 Proportions of veterans in each pain intensity rating change category. |
Inclusion diagnosis did not differentially impact changes in pain intensity ratings (Chi-square (6 df) =11.00, p=0.0855) or in MEDDs (Chi-square (6 df) =10.07, p=0.1217) among those who had an SCS implant. However, the level of pre-implant pain did impact changes. Stratification of pain intensity groups (mild, moderate, severe; 5 persons removed for “no pain”) revealed significant differences in pain changes across pre-implant severity groups at each clinical cutoff point (≥20%, ≥30%, ≥50%) after implant. It is important to note that large proportions of patients with mild pain showed significant increases in their pain ratings after implant (range 32.9155.27% of patients). In patients who had pain (≥4) prior to their SCS implant (N=1098), more had a clinically meaningful decrease in their pain score in the year following their 90-day recovery period (Day 91–456) than expected by chance alone (t-test −4.39, p<0.0001). We had set a clinically meaningful change to be ≥30%.
SCS implants after 2006 were associated with a significantly lower odds of substantial increases in pain intensity ratings in the year following the post-SCS 90-day recovery period (Definitions of substantial increase: ≥30% pain increase OR=0.66, 95% CI: 0.49–0.90, p<0.0077; and ≥50% OR=0.60, 95% CI: 0.42–0.86, p<0.0057). While the estimates and significance of implant timeframe remained significant with inclusion diagnoses, age, race/ethnicity, and PTSD in the models, they changed and became nonsignificant once pre-implant pain intensity rating was added to the multinomial model.
Opioid Therapy
As previously noted, 92.4% of veterans receiving an SCS implant were prescribed opioids during the year prior to implant. The median MEDD was 26.48mg (Interquartile range: 1st quartile 6.58, 3rd quartile 70.14 mg). In the 90-day post-operative period, the proportion remained the same (92.3%) as did the median MEDD (28.03 mg, Interquartile range 1st quarter: 7.50, 3rd quarter: 73.56 mg). However, the proportion of veterans dispensed any opioids significantly decreased from 92% to 86.6% in the year following the 90-day post-operative window compared to the year prior to implant (Chi-square=163.30, 1df, p<0.0001). The median MEDD in the year after the 90-day post-operative period was 22.59 mg (Interquartile range 1st quarter: 3.62, 3rd quarter: 64.90 mg), a reduction of 0.49 mg from the pre-implant dosage (Sign test M = −68.5, p<0.0003; Interquartile range 1st quarter: −12.74, 3rd quarter: 8.75). When pre-specified clinically meaningful decreases in MEDD were examined, moderate proportions of veterans reached these thresholds (41.41%, 36.71%, and 28.05% for ≥20%, ≥30% and ≥50%, respectively) (Figure 2 illustrates changes in MEDD where a clinically meaningful change was set at ≥20% change).
 |
Figure 2 Proportions of veterans in each change category for morphine equivalent daily dose. |
The odds of being prescribed opioids in the year following the 90-day post-operative period was significantly higher for those who had been prescribed opioids during the year prior to their implant than those who had not (89.83% vs 47.37%, Chi-square test=163.30, 1 df, p<0.0001). A total of 200 veterans who had received an implant had no opioids dispensed to them after their post-operative period.
Stratification of the pre-implant pain group (mild, moderate, severe; 3 persons removed for “no pain”) revealed no significant differences in opioid changes between pre-implant severity groups at each clinical cutoff point (≥20%, ≥30%, ≥50%) after implant. Later implants (>2006) were associated with significantly lower odds of substantial MEDD increases in that year (Definitions of substantial increase: ≥20% MEDD increase OR=0.59, 95% CI: 0.42–0.80, p<0.0006; ≥30% MEDD increase OR=0.63, 95% CI: 0.48–0.84, p<0.0017; ≥50% MEDD increase OR=0.57, 95% CI: 0.43–0.75, p<0.0001). When age, race/ethnicity, PTSD and pre-implant pain rating were added to these models, the estimates and significance of timeframe of implant remained significant. Having an implant after 2006 was associated with significantly lower odds of MEDD increases (≥20%, ≥30% and ≥50%). There were no significant associations between the timeframe of implant and decreases in pain or MEDDs at any of the clinically meaningful ranges.
Discussion
SCS implants are one option for the management of refractory chronic pain. For many years, the VHA has been performing these implants to help manage veterans’ pain. This is the first study, however, to examine the characteristics of veterans who have received SCS implants as well as the outcomes associated with this therapy.
Consistent with the guidelines and recommendations for the conservative use of SCS for chronic pain, between 2000 and 2012, only a small percentage of veterans with MSD received an SCS in VHA settings. For that reason, the SCS analytic sample was a subset of veterans with any of three diagnoses with relatively high rates of SCS implantation (per our MSD data), accounting for over 72% of all SCS implants in the period of observation. The findings from this study are consistent with the relatively small number of SCSs implanted worldwide compared to the prevalence estimates of chronic pain. The increasing number of SCSs implanted through 2011 is likely consistent with VHA’s National Pain Management Strategy Stepped Care Model of Pain Management and other efforts to promote access to advanced pain medicine diagnostics and interventions.41 It is important to note that VHA facilities that lack the capacity to perform SCSs may authorize receipt of an implant at another VHA facility or a non-VHA facility. Implants obtained outside VHA are not captured in this study.
The current study describes the demographic and clinical characteristics of veterans who received SCS implants in VHA settings during the study period. Among the three diagnoses examined, the likelihood of receiving an SCS particularly increased with diagnoses of either post-laminectomy syndrome of the lumbar region and/or thoracic or lumbosacral neuritis or radiculitis. Many veterans had more than one MSD inclusion diagnosis which increased their odds for an SCS implant. This suggests more serious and/or complex cases were more likely to get this more invasive treatment option. In particular, all post-laminectomy groups had high rates of SCS implants, relative to their inclusion rate in the analytic sample.
White, aged 35–44 years, and married veterans with the selected diagnoses of interest had greater odds of receiving an SCS implant. It is possible that the difference in SCS implant receipt reflects an age- or race-related difference in access to this pain management treatment.42 An alternative explanation for the observed age difference is that older veterans may have received an SCS implant prior to the starting date of the MSD cohort, and, as a result, would be less likely to need an SCS during the study time period. Younger veterans also may have been in better health and/or more willing to accept newer technology or surgical interventions. Race differences could reflect differences in veteran preferences or potentially provider bias.
Veterans in receipt of SCS implants were more likely to have higher pain intensity ratings at the time of their diagnosed MSD than those who did not receive this therapy. In persons who had actionable pain (≥4) prior to their SCS implant, participants had a clinically meaningful decrease in their pain score in the year following their 90-day recovery period. Lower rates of medical comorbidity and substance use disorders among those receiving SCS implants are consistent with the contraindications for SCS use.12 Previous research suggests that the presence of mood and anxiety disorders or PTSD is not a contraindication for implantation.43,45 This prior research is important because mood and anxiety disorders are known to be particularly prevalent among individuals with chronic pain and may be associated with heightened pain intensity, increased likelihood of receiving opioid therapy, and greater overall distress.46,48 In fact, this study suggests that veterans with complex chronic pain marked by comorbid depressive disorder are more likely to receive SCS implants than those without this disorder. It may be that providers’ recommendations for SCSs reflect their efforts to address the heightened distress and suffering of veterans with painful MSDs. As expected, given that presence of active substance use disorders is a relative contraindication for many medical procedures, the presence of these disorders was associated with lower odds of SCS implantation.
Ninety-two percent of veterans receiving SCS implants received opioid therapy in the year prior to implantation, and they had a median pain intensity rating in the moderate or severe range. These data may suggest that veterans who were considered for SCS implantation were among those whose pain was not optimally managed despite opioid therapy. There was a significant decrease in the percent of veterans receiving opioid therapy and a significant overall decrease in opioid dose. The results offer evidence of benefit for some veterans with the targeted conditions in the year following receipt of SCS therapy. Given the risks associated with opioids, a reduction in prescribed opioids is an important benefit of SCS implants. Of course, SCS is not without risks and is relatively expensive and invasive. A limitation of our study is the lack of available data in the EHR regarding physical and emotional functioning and quality of life. Future research that examines these important veteran-centered outcomes (eg pain-related interference) is encouraged to determine if it is a better indicator of positive outcomes among veterans who have had an SCS.
Serious concerns have been raised about the escalating rates of adverse health consequences from long-term opioid therapy, including overdose and death.49,50 For more than a decade, the VHA has promoted policy, evidence-based guidelines, and quality improvement initiatives to address this concern.51 Thus, it is particularly important for veterans with chronic pain to have access to additional pain management strategies, such as SCSs, that can help manage pain. Further research is needed to understand the effect of SCS implantation on pharmacological treatments for pain.
Strengths, Limitations, and Future Directions
This study has multiple strengths. First, the availability of comprehensive EHR and administrative data allowed many variables to be analyzed to better describe veterans receiving SCS implants. Second, the comprehensive EHR enabled the examination of two particularly important veterans’ outcomes, namely pain intensity ratings and opioid dosage outcomes from pre- to post-SCS implant. Third, this is the only known study focused on veterans, a particularly vulnerable subgroup, who received SCS implants within the VHA.46 The subset was limited to the three diagnoses most associated with receipt of an SCS implant; this limitation precludes our ability to generalize to all veterans receiving SCS implants. This information can inform the VHA’s leadership about its use of this pain management approach as well as shape future public policy about the use of SCS implants. Although there are many important differences between the VHA and other integrated healthcare systems – and especially private, fee-for-service healthcare settings – given the paucity of large epidemiologic observational studies of SCSs, the results of the present study may have broader implications for non-VHA care of veterans and civilians, as well.
There also are limitations to this study. First, because this is a retrospective study, only variables that were readily available in the EHR were examined. Pain intensity ratings and opioids are currently the only two pain-relevant measures that were available from clinical data. Additional demographic or clinical characteristics about the veteran such as pain duration and pain interference were not available. Second, additional outcome variables (eg, disability variables, work status, and quality of life) could not be examined in this study because the data were not collected in the EHR. Third, because this study only examined veterans with SCSs who were members of the MSD cohort and had one of the three designated diagnoses upon entry into the cohort, we do not know how many other veterans received SCS implants but were not included in this study or if they developed additional MSDs following entry into the cohort. We also do not know how many veterans received an SCS implant outside of the VHA, even if the procedure was authorized by VHA. Fourth, we could not reliably distinguish between the coding of SCS trials vs implants. Fifth, the data from this study are approximately 7 years old due to the limits of the MSD cohort used for this study.33 Finally, advances in SCS technology continue to be made, as well as the claims of improved effectiveness of SCSs.23
Future research can extend these analyses by further examining additional outcome variables available in the EHR of veterans with SCS implants (eg, depressive disorder, use of non-opioid analgesics, use of the healthcare system). For example, a future study could examine the hypothesis of reduced healthcare utilization, improved pain trajectory and associated costs of care following SCS implantation. With the increasing use of EHR in other public and private healthcare settings, the methods used in this observational study could be applied to examine similar questions in other healthcare settings. It also would be interesting to compare veterans, active duty service members, and civilians to determine whether demographic and clinical characteristics, facility characteristics, and/or clinical outcomes differ among those groups.
Acknowledgments
This study was funded by grants from the Department of Veterans Affairs Health Services Research and Development Service (CIN 13-407, CRE 12-012, and CIN 13-416.
Disclosure
Laura D Wandner and Brenda T Fenton are co-primary authors.The views expressed in this article are those of the authors and do not necessarily reflect the official policy of the Department of Veterans Affairs, the National Institutes of Health, or the US Government.
Alicia Heapy reports grants from VA HSR&D during the conduct of the study and personal fees from Magellan Health outside the submitted work. Robert D Kerns reports grants from Department of Veterans Affairs during the conduct of the study and grants from NIH, PCORI, and AAPM outside the submitted work. The authors report no other possible conflicts of interest in this work.
References
1. Department of Veterans Affairs. National center for veteran analysis and statistics veterans health administration; 2015 [
2. Nahin RL. Severe pain in Veterans: the effect of age and sex, and comparisons with the general population. J Pain. 2017;18(3):247–254. doi:10.1016/j.jpain.2016.10.021
3. Kerns RD, Otis JD, Rosenberg R, Reid MC. Veterans’ reports of pain and associations with ratings of health, health-risk behaviors, affective distress, and use of the healthcare system. J Rehabil Res Dev. 2003;40(5):371–379. doi:10.1682/JRRD.2003.09.0371
4. Haskell SG, Heapy AA, Reid MC, Papas RK, Kerns RD. The prevalence and age-related characteristics of pain in a sample of women veterans receiving primary care. J Women’s Health. 2006;15(7):862–869. doi:10.1089/jwh.2006.15.862
5. Affairs D, Analysis of VA Healthcare Utilization Among Operation Enduring Freedom (OEF), Operation Iraqi Freedom (OIF), and Operation New Dawn (OND) Veterans 2014. Health V, editor. 2014.
6. Goulet JL, Kerns RD, Bair M, et al. The musculoskeletal diagnosis cohort: examining pain and pain care among veterans. Pain. 2016;157:1696–1703. doi:10.1097/j.pain.0000000000000567
7. Mailis Gagon FA, Sandoval TJ. Spinal cord stimulation for chronic pain. Cochran Collab. 2004;3:1–17.
8. Verrills P, Sinclair C, Barnard A. A review of spinal cord stimulation systems or chronic pain. J Pain Res. 2016;9:481–492. doi:10.2147/JPR.S108884
9. Wolter T. Spinal cord stimulation for neuropathic pain: current perspectives. J Pain Res. 2014;7:651–663. doi:10.2147/JPR.S37589
10. McRoberts PW, Doleys DM, Cairns KD. Spinal cord stimulation. In: Comprehensive Treatment of Chronic Pain by Medical, Interventional, and Integrated Approaches. New York: Springer; 2013:601–622.
11. North RB, Wetzel TF. Spinal cord stimulation for chronic pain of spinal origin: A valuable long-term solution. Spine. 2002;27(22):2584–2591. doi:10.1097/00007632-200211150-00035
12. Cameron T. Safety and efficacy of spinal cord stimulation for the treatment of chronic pain: a 20-year literature review. J Neurosurg Spine. 2004;100:254–267. doi:10.3171/spi.2004.100.3.0254
13. Flacco ME, Manzoli L, Boccia S, et al. Head-to-head randomized trials are mostly industry sponsored and almost always favor the industry sponsor. J Clin Epidemiol. 2015;68(7):811–820. doi:10.1016/j.jclinepi.2014.12.016
14. Stojanovic MP, Abdi S. Spinal cord stimulation. Pain Physician. 2002;5(2):156–166.
15. Zucco F, Ciampichini R, Lavano A, et al. Cost-effectiveness and cost-utility analysis of spinal cord stimulation in patients with failed back surgery syndrome: results from the PRECISE study. Neuromodulation. 2015;18(4):266–276. doi:10.1111/ner.12292
16. Boswell MV, Shah RV, Everett CR, et al. Interventional techniques in the management of chronic spinal pain: evidence-based practice guidelines. Pain Physician. 2005;8:1–47.
17. Chou R, Atlas SJ, Stanos SP, Rosenquist RW. Nonsurgical interventional therapies for low back pain: a review of the evidence for an American pain society clinical practice guidelines. Spine. 2009;34(10):1078–1093. doi:10.1097/BRS.0b013e3181a103b1
18. Lee AW, Piltsis JG. Spinal cord stimulation: indications and outcomes. Neurosurgery Focus. 2006;21(6):1–6. doi:10.3171/foc.2006.21.6.6
19. Kapural L, Yu C, Doust MW, et al. Novel 10-kHz high-frequency therapy (HF10 Therapy) is superior to traditional low-frequency spinal cord stimulation for the treatment of chronic back and leg pain: the SENZA-RCT randomized controlled trial. Anesthesiology. 2015;123(4):851–860. doi:10.1097/ALN.0000000000000774
20. Prager J, Jacobs M. Evaluation of patients for implantable pain modalities: medical and behavioral assessment. Clin J Pain. 2001;17:206–214. doi:10.1097/00002508-200109000-00004
21. Floridia D, Cerra F, Guzzo G, et al. Treatment of pain post-brachial plexus injury using high-frequency spinal cord stimulation. J Pain Res. 2018;11:2997–3002. doi:10.2147/JPR.S168031
22. Benyamin R, Grider JS, Motejunas MW, et al. Spinal cord stimulation: principles and applications. In: Davis SF, Kaye AD, editors. Principles of Neuropsychological Assessment, Mapping, and Monitoring.
23. Sdrulla AD, Guan Y, Raja SN. Spinal cord stimulation: clinical efficacy and potential mechanisms. Pain Practice. 2018;18(8):1048–1067. doi:10.1111/papr.12692
24. Harke H, Gretenkort P, Ulrich Ladleif H, Rahman S. Spinal cord stimulation in sympathetically maintained complex regional pain syndrome type I with severe disability. A prospective study. Eur J Pain. 2005;9:363–373. doi:10.1016/j.ejpain.2004.09.003
25. Kemler MA, De Vet HCW, Barendse GAM, Van Den Wildenberg FAJM, Van Kleef M. The effect of spinal cord stimulation in patients with chronic reflex sympathetic dystrophy: two years’ follow-up of the randomized controlled trials. Ann Neurol. 2004;55:13–18. doi:10.1002/ana.10996
26. Kemler MA, De Vet HCW, Barendse GAM, Van Den Wildenberg FAJM, Van Kleef M. Effect of spinal cord stimulation for chronic complex regional pain syndrome type I: five-year final follow-up of patients in a randomized controlled trial. J Neurosurg. 2008;108:292–298. doi:10.3171/JNS/2008/108/2/0292
27. Kemler MA, Barendse GAM, Van Kleef M, et al. Spinal cord stimulation in patients with chronic reflex sympathetic dystrophy. N Engl J Med. 2000;343:618–624. doi:10.1056/NEJM200008313430904
28. Kumar K, Nath R, Wyant GM. Treatment of chronic pain by epidural spinal cord stimulation: a 10-year experience. J Neurosurg. 1991;75:402–407. doi:10.3171/jns.1991.75.3.0402
29. Kumar K, Taylor RS, Jacques L, et al. Spinal cord stimulation versus conventional medical management for neuropathic pain: a multicentre randomised controlled trial in patients with failed back surgery syndrome. Pain. 2007;132:179–188. doi:10.1016/j.pain.2007.07.028
30. North RB, Ewend MG, Lawton MT, Kidd DH, Piantadosi S. Failed back surgery syndrome: 5-year follow-up after spinal cord stimulator implantation. Neurosurgery. 1991;28(5):692–699. doi:10.1227/00006123-199105000-00009
31. North RB, Kidd DH, Farrokhi F, Piantadosi SA. Spinal cord stimulation versus repeated lumbosacral spine surgery for chronic pain: a randomized, controlled trial. Neurosurgery. 2005;56:98–107. doi:10.1227/01.NEU.0000144839.65524.E0
32. Provenzano DA, Williams JR, Jarzabek G, DeRiggi LA, Scott TF. Treatment of neuropathic pain and functional limitations associated with multiple sclerosis using an MRI-compatible spinal cord stimulator: a case report with two year follow-up and literature review. Neuromodulation. 2016;19:406–413. doi:10.1111/ner.12409
33. Goulet JL, Kerns RD, Bair M, et al. The musculoskeletal diagnosis cohort: examining pain and pain care among veterans. JAMA. 2016;157(8):1696–1703.
34. Justice AC, Dombrowski E, Conigliaro J, et al. Veterans Aging Cohort Study (VACS): overview and description. Med Care. 2006;44(Suppl–24):S13S24. doi:10.1097/01.mlr.0000223741.02074.66
35. Cleeland CS, Reyes-Gibby CC, Schall M, et al. Rapid improvement in pain management: the Veterans Health Administration and the Institute for Healthcare Improvement Collaborative. Clin J Pain. 2003;19(5):298–305. doi:10.1097/00002508-200309000-00003
36. Goulet JL, Brandt C, Crystal S, et al. Agreement between electronic medical record-based and self-administered pain numeric rating scale: clinical and research implications. Med Care. 2013;51(3):245–250. doi:10.1097/MLR.0b013e318277f1ad
37. Romano PS, Roos LL, Jollis JG. Adapting a clinical comorbidity index for use with ICD-9-CM administrative data. J Clin Epidemiol. 1993;46(10):1075–1079. doi:10.1016/0895-4356(93)90103-8
38. Korff MV, Saunders KW, Thomas Ray G, et al. De facto long-term opioid therapy for noncancer pain. Clin J Pain. 2008;24(6):521–527. doi:10.1097/AJP.0b013e318169d03b
39. Center for Disease Control and Prevention. Opioid Morphine Equivalent Conversion Factors. Atlanta, GA; 2014.
40. Dworkin RH, Turk DC, Wyrwich KW, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: impact recommendations. J Pain. 2008;9:105–121. doi:10.1016/j.jpain.2007.09.005
41. Kerns RD, Philip EJ, Lee AW, Rosenberger PH. Implementation of the Veterans Health Administration National pain management strategy. Transl Behav Med. 2011;1(4):635–643. doi:10.1007/s13142-011-0094-3
42. Hausmann LRM, Brandt CA, Carroll CM, et al. Racial and ethnic differences in total knee arthroplasty in the Veternas Affairs Health Care System, 20012013. Arthritis Care Res. 2017;69(8):1171–1178. doi:10.1002/acr.23137
43. Beltrutti D, Lamberto A, Barolat G, et al. The psychological assessment of candidates for spinal cord stimulation for chronic pain management. Pain Practice. 2004;4:204–221. doi:10.1111/j.1533-2500.2004.04305.x
44. Celestin J, Edwards RR, Jamison RN. Pretreatment psychosocial variables as predictors of outcomes following lumbar surgery and spinal cord stimulation: a systematic review and literature synthesis. Pain Med. 2009;10(4):639–653. doi:10.1111/j.1526-4637.2009.00632.x
45. Sparkes E, Raphael JH, Duarte RV, LeMarchand K, Jackson C, Ashford RL. A systematic literature review of psychological characteristics as determinants of outcome for spinal cord stimulation therapy. Pain. 2010;150:284–289. doi:10.1016/j.pain.2010.05.001
46. IOM. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Medicine I, editor. Washington, DC: The National Academies Press; 2011.
47. Gatchel RJ. Comorbidity of chronic pain and mental health disorders: the biopsychosocial perspective. Am Psychol. 2004;59(8):795–805. doi:10.1037/0003-066X.59.8.795
48. Otis JD, Keane TM, Kerns RD, Monson C, Scioli E. The development of an integrated treatment for veterans with chronic pain and posttraumatic stress disorder. Pain Med. 2009;10(7):1300–1311. doi:10.1111/j.1526-4637.2009.00715.x
49. Dunn KM, Saunders KW, Rutter CM, et al. Overdose and prescribed opioids: associations among chronic non-cancer pain patients. Ann Intern Med. 2010;152(2):85–92. doi:10.7326/0003-4819-152-2-201001190-00006
50. Starrels JL, Becker WC, Alford DP, Kapoor A, Williams AR, Turner BJ. Systematic review: treatment agreements and urine drug testing to reduce opioid misuse in patients with chronic pain. Ann Intern Med. 2010;152:712–720. doi:10.7326/0003-4819-152-11-201006010-00004
51. Seal KH, Shi Y, Cohen G, et al. Association of mental health disorders with prescription opioids and high-risk opioid use in U.S. Veterans of Iraq and Afghanistan. J Am Med Assoc. 2012;307(9):940–947. doi:10.1001/jama.2012.234
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.