Back to Journals » OncoTargets and Therapy » Volume 13
Treatment for Liver Tumor Using Combined Transarterial Embolization and Interaarterial Transfecting HIF-1α shRNA in a Rabbit VX2 Model
Authors Guo C , Wang C, Zhang J, Chen X
Received 21 May 2020
Accepted for publication 31 July 2020
Published 24 August 2020 Volume 2020:13 Pages 8511—8519
DOI https://doi.org/10.2147/OTT.S262434
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Takuya Aoki
Chuangen Guo,1 Cheng Wang,2 Jingfeng Zhang,1,3 Xiao Chen4
1Department of Radiology, The First Affiliated Hospital, College of Medicine, Zhejiang University, Hangzhou, Zhejiang Province 310003, People’s Republic of China; 2Department of Radiology, Nanjing Drum Tower Hospital, Nanjing University, Nanjing, Jiangsu Province 210029, People’s Republic of China; 3Department of Radiology, Hwa Mei Hospital, University of Chinese Academy of Sciences, Ningbo, Zhejiang Province 315010, People’s Republic of China; 4Department of Radiology, The Affiliated Hospital of Nanjing University of Chinese Medicine, Nanjing, Jiangsu Province 210029, People’s Republic of China
Correspondence: Jingfeng Zhang Email [email protected]
Background: Hypoxia-inducible factor-1α (HIF-1α) has been selected as therapeutic gene in gene therapy. The aim of this study was to explore the treatment effect of combined transarterial embolization using microsphere treatment (MD) and intraarterial transfecting HIF-1α shRNA on hepatocellular carcinoma (HCC).
Materials and Methods: Rabbit skin fibroblast was transfected with HIF-1α shRNA to evaluate the knocking down efficiency. Sixteen rabbit VX2 liver tumor models were randomly divided into four groups: the control group without any treatment, the MD group, the shRNA group (HIF-1α shRNA transfection by transcatheter intraarterial infusion), and the shRNA+MD group. The necrotic score, mitotic count and expression of HIF-1α, vascular endothelial growth factor (VEGF), CD34 and periodic acid-Schiff (PAS) stain were evaluated at the 14th and 28th day after treatment. The expression of HIF-1α and VEGF of VX2 tumors was also evaluated by real-time polymerase chain reaction on the 28th day.
Results: The expression of HIF-1α-mRNA was lower in HIF-1α shRNA group than the control (p < 0.01). The tumor size was smaller in the shRNA + MD group than the shRNA group and the MD group (p < 0.05) on the 28th day. The growth rate of tumors in the shRNA + MD group was also lower than in other groups. The gene and protein expressions of both HIF-1α and VEGF in the shRNA + MD group were lower than the MD group, shRNA group and control group on the 28th day (p < 0.05). The necrotic score was higher in the shRNA + MD group than the MD group and control group (p < 0.05). The mitotic count and PAS-positive cells in shRNA + MD group were lower and CD34 was higher than the other three groups (p < 0.05).
Conclusion: Compared to therapy with MD or HIF-1α shRNA with transcatheter intraarterial transfection alone, the combined treatment has a better effect on HCC.
Keywords: hepatocellular carcinoma, transarterial embolization, hypoxia-inducible factor-1α, shRNA
Introduction
Hepatocellular carcinoma (HCC) is one of the most common types of malignant liver tumor and is the third most common cause of cancer worldwide. HCC incidence is rising faster than that of any other cancers in both men and women.1–3
Transcatheter arterial embolization (TAE) is a widely used palliative treatment for patients with nonsurgical HCC. After blocking the blood supply with TAE, cancer cells that are in an intense state lack the necessary oxygen, which will cause necrosis. Those will induce a high expression of hypoxia-inducible factor-1α (HIF-1α), which is believed to amplify tumor aggressiveness, cause tumor recurrence and metastasis after initial treatment. HIF-1α which can stimulate the formation of new blood vessels and alleviate cell hypoxia, can be higher in hepatoma cells and enhance the survival of cancer cells.4–6 Current research shows that the HIF-1α pathway is a master regulator of angiogenesis. HIF-1 seems to play an important role in the signal transduction pathway of vascular endothelial growth factor (VEGF) under hypoxic condition.7,8 It can increase gene expression and enhance protein translation for VEGF.9 VEGF is the primary mediator of angiogenesis in primary liver tumors.10,11 Previous studies selected HIF-1α as therapeutic gene for gene therapy. The interference gene can reduce HIF-1α expression in liver cancer cells under the condition of hypoxia, which effectively restrain the formation of new blood vessels in HCC tissue after TAE. Additional gene therapy will increase the treatment effect of TAE and the recurrence of liver cancer will be greatly reduced.8,12 Due to the lack of support for the new blood vessels and due to the decreased expression of HIF-1α, the combined TAE treatment and gene therapy would be a great boon for liver cancer patients. Intraarterial (IA) therapy for liver tumors demonstrated great potential in cancer treatment because it achieves high anticancer drug concentrations in tumor tissues.13–15
The VX2 tumor originated from human papilloma-derived squamous cell carcinoma.16 The rabbit VX2 hepatic tumor grows rapidly, and the tumor is hypervascular in the arterial phase, which is similar to that of hepatocellular carcinoma. In addition, its size is large enough to be observed by clinical imaging.17 Hence, the rabbit VX2 hepatic tumor model has been widely used to investigate various aspects of tumor behavior in the field of interventional radiology.18–21 In this study, we evaluated the effect of TAE and HIF-1α related gene therapy on HCC using a rabbit VX2 tumor model.
Materials and Methods
Animal Model
The animal experiments were approved by and conducted in accordance with the guidelines of the animal care committee of the First Affiliated Hospital of College of Medicine, Zhejiang University (ID: 14033). All New Zealand white rabbits, aged from 2 to 3 months and weighing from 2 to 2.5 kg, were purchased from the Zhejiang Academy of Agricultural Sciences (China). Rabbits were kept at a temperature of 20–25°C and a humidity of 40–70%. All rabbits were kept at this condition for a week for acclimation. VX2 tumors were harvested from carrier rabbits, and the solid areas of the tumor were extracted and cut into 1 mm3 pieces; then, the VX2 tumor was transplanted into the left lobe of the liver at a depth of 1 cm using surgical orthotopic transplantation. A magnetic resonance imaging (MRI) was performed at 14 days after tumor inoculation, when the tumor reached 2 cm in diameter, and the necrotic lesions were smaller than 50% of the tumor diameter, the rabbit model was grouped for further analyses.
Construction of Plasmid and Adenovirus Acquisition
pCD316-ZsGreen-shRNA was chosen as the Cloning Vector, and the plasmid of hypoxia-inducible factor-1α short hairpin RNA (HIF-1α shRNA) was successfully constructed. The primer sequences were as follows: (HIF-1α-shRNA-1-F: gTGAAGGCACAGATGAACTGTTCAAGAGACAGTTCATCTGTGCCTTCATTTTTTg, R: gatccAAAAAATGAAGGCACAGATGAACTGTCTCTTGAACAGTTCATCTGTGCCTTCACTGCA; HIF-1α-shRNA-2-F: gGGAGCCTGATGCTTTAACTTTCAAGAGAAGTTAAAGCATCAGGCTCCTTTTTTg, 2R: gatccAAAAAAGGAGCCTGATGCTTTAACTTCTCTTGAAAGTTAAAGCATCAGGCTCCCTGCA; HIF-1α-shRNA-3-F: gGCAACGGTCATATATAATATTCAAGAGATATTATATATGACCGTTGCTTTTTTg, 3R: gatccAAAAAAGCAACGGTCATATATAATATCTCTTGAATATTATATATGACCGTTGCCTGCA). In order to collect the adenovirus for the subsequent experiments, the 293a cell (Type Culture Collection of the Chinese Academy of Sciences, Shanghai, China) was transfected with the HIF-1α shRNA plasmid. When the cell confluence reached 70%, the HIF-1α shRNA was concentrated and purified, and stored at −80°C until further use. The titers of HIF-1α shRNA were determined by calculating PFUs of the adenovirus. The titer was 2×1010 PFU/mL.
Primary Rabbit Skin Fibroblast Culture and Virus Infection
The fresh rabbit skin tissues were collected. After removing the adipose tissue, the remaining tissue was cut into small pieces of about 0.5 × 0.5 mm. Six to eight pieces of tissue were infiltrated with serum and cultured in flasks. When a small quantity of cells were observed around the adhered tissue, and 5 mL of complete culture medium was added to infiltrate the tissue block. After culturing for 72 h, the tissue was removed, and the remaining adherent cells were cultured to a density of about 80% and passed to P3 or P4 for further experiments. The primary culture of fibroblast were approved by the ethical committee of the First Affiliated Hospital of Zhejiang College of Medicine, Zhejiang University (ID: 14033).
Real-Time Polymerase Chain Reaction (RT-PCR)
The P3 rabbit skin fibroblasts were transfected with the HIF-1α shRNAs. The blank control group (CK) and negative control group (NC) were also set. The NC group was transfected with empty vectors. An inverted biological fluorescence microscope (IX73, Olympus, Japan) was used to evaluate the expression of GFP protein at 24 and 48 h after transfecting HIF-1α shRNA. The content of HIF-1α mRNAs in rabbit skin fibroblasts was determined by RT-PCR in order to evaluate the efficiency of shRNA at 48 h.
Total RNA was isolated using TRIzol reagent (Generay, Shanghai, China) and cDNA was synthesized using PrimeScript™ RT reagent Kit (Takara, Tokyo, Japan). RT-PCR was performed with a CFX connect Real-Time PCR Detection System (Bio-Rad, Hercules, USA). The expression of RNA in each group was normalized by the internal control of rabbit glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Primers used in the PCR reaction are listed as follows: GAPDH-For: TGCCGCCTGGAGAAAGC, GAPDH-Rev: CGACCTGGTCCTCGG-TGTAG; HIF-1α-For: ACAACATCACCACCATACAGGG, HIF-1α-Rev: ACAGCTAACACGTTAGGGCTTC. VEGF-For: TTCCTGCG-TGCCTCTGGTGC; VEGF-Rev: ACGTTGAACTCCTCGGTGGG. The relative expression of HIF-1α and VEGF were calculated using the 2-∆∆Ct method.23
Western Blot
The protein expression of HIF-1α was evaluated by Western blot. Briefly, the fibroblast transfected with the HIF-1α shRNA-1. Total protein was obtained, separated by 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) gels, and transferred to PVDF films (Millipore, USA). The PVDF films were blocked with 5% BSA, and then incubated in specific primary antibodies (anti-HIF-1α, 1:1000 dilution, Abcam, USA) at 4°C overnight and horseradish-peroxidase-conjugated secondary antibodies (1:2000) at room temperature, respectively. The target proteins were demonstrated with enhanced chemiluminescence reagent (Beyotime) and were detected using a Bio-Rad imaging system (USA).
Grouping
Sixteen male New Zealand white rabbits were randomly divided into four groups (four rabbits per group). One group was regarded as the control group without any treatment. The second group was only treated with intraarterial administration of drug-free microspheres (transarterial embolization with drug-free microspheres group, MD group). The shRNA group was only treated with HIF-1α shRNA transfection by transcatheter intra-arterial infusion. The fourth group was treated with HIF-1α shRNA transfection by transcatheter intraarterial infusion and then transarterial embolization with drug-free microspheres (shRNA + MD group). Transarterial embolization (TAE) was performed under fluoroscopic guidance as that described in our previous study.22 Briefly, a 3-Fr dilator was inserted into the right femoral artery. Angiography was performed with a microcatheter to show the hepatic arterial anatomy. The adenovirus and/or microspheres were injected into the tumor arteries.
Magnetic Resonance Imaging (MRI)
MRI scan was performed to evaluate the tumor status at the 0 and 14th day. The scan protocol was as follows: t2-weighted/fat suppression sequence (TR/TE: 2840ms/74) with 2.0 mm slice thickness, 1.0 mm interslice gap, 384×256 matrix and 10.0 cm field-of-view.
Histopathology
On the 14th day and 28th day after treatment, the VX2 tumor samples were harvested from euthanized rabbits (2 rabbits per group), and the gross tumor size was measured. The tumor samples were stained with hematoxylin and eosin (H&E), and an immunohistochemistry (IHC) assay was performed. The necrotic score and mitotic count were evaluated by H&E. The tissue was used for immunostaining of HIF-1α and VEGF. The tissues were fixed in 4% paraformaldehyde for 4 days, and then embedded in paraffin to permit observation of pathological changes under a light microscope. For IHC, the paraffin sections were treated with 3% hydrogen peroxide for 10 min and then treated with antigen retrieval at 90°C for 15 min. In addition, the sections were incubated with first antibodies (Abcam, USA, 1:50) for 30 min at room temperature and secondary antibodies for 15 min at 37°C. The pathological changes were evaluated under an optical microscope (three fields per slide).
CD34-Periodic Acid-Schiff (PAS) stain double staining was performed to detect vasculogenic mimicry (VM). IHC staining with CD34 (Thermo Fisher, USA) was performed on the sections as described above prior to PAS staining. The slides were treated with periodic acid solution for 10 min, washed with distilled water for 5 min. The slides were submerged in Schiff solution at room temperature in the dark for 15 min. VM channels surrounded by HCC cells were positive for PAS staining, but negative for CD34.
The expressions of HIF-1α and VEGF in the tissues of the VX2 tumors from different groups at the 28th day were also determined by RT-PCR.
Statistical Analyses
All statistical analyses were performed with the commercially available software (SPSS 22.0 Chicago, IL, USA). One way ANOVA or two-tailed independent t-test was performed for statistical analysis. P-values <0.05 were regarded as statistical significant.
Results
Primary Rabbit Skin Fibroblast Culture and Virus Infection
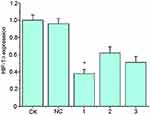
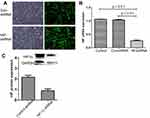
HIF-1α-shRNA-1 had the highest knockdown efficiency (Figure 1). The fluorescence microscope showed that the NC group and the HIF-1α group both expressed the GFP protein at 24 and 48 h after HIF-1α shRNA or empty vector treatment (Figure 2A). The expression of HIF-1α-mRNA was significantly lower in HIF-1α shRNA than in the NC group and the CK group (p < 0.01, Figure 2B). The expression of HIF-1α protein in HIF-1α shRNA was also lower than that in NC group (Figure 2C).
The Tumor Growth After Treatment
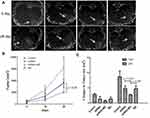
MR images showed the tumor size at 0 and 14th days (Figure 3A). Tumor size was measured from euthanized rabbits on the 14th day and 28th day after treatment (Figure 3B). The growth rate in the four groups was statistically significant (p < 0.05), and the growth rate of siRNA + MD group was lower than the other groups. The change of tumor size was significantly smaller in the shRNA + MD group than the shRNA group and the MD group (p < 0.05, respectively) (Figure 3C).
The Expression of HIF-1α and VEGF
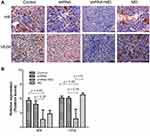
The expressions of HIF-1α and VEGF were evaluated by IHC (Figure 4A). The relative expression (positive score) of HIF-1α in the shRNA + MD group (1.67 ± 1.97) was significantly lower than the MD group (2.83 ± 0.75), shRNA group (4.83 ± 0.75) and control group (5.67 ± 0.52) at the 28th day (p < 0.05). Similar results were found in VEGF (Figure 4B).
RT-PCR was performed to evaluate the gene expression of HIF-1α and VEGF in the fresh tumor tissues on the 28th day. The expression of both HIF-1α and VEGF in shRNA + MD group (0.39 and 0.72) was significantly lower than the MD group (1.05 and 1.00), shRNA group (0.86 and 0.86) and control group (0.71 and 1.83) at the 28th day (p < 0.05, respectively).
Histologic Findings
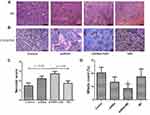
The necrotic score and mitotic count were evaluated by H&E (Figure 5A and C). The necrotic score was higher in the shRNA + MD group than the MD group and control group (p < 0.05, respectively), but no significant difference was found between the shRNA + MD and shRNA group (Figure 5A and C). The mitotic count in shRNA+MD group (9.33 ± 9.65) was significantly lower than the MD group (17.50 ± 5.92), shRNA group (13.42 ± 6.96) and control group (20.50 ± 4.18) (Figure 5D, p < 0.05).
The positive rate of CD34 in the shRNA + MD group was significantly lower than the other three groups (Figure 5B), but the PAS staining showed a lower positive rate in the shRNA + MD group compared to the other three groups.
Discussion
Blocking the blood supply is a common nonsurgical treatment of hypervascular tumor. TAE is a widely used palliative therapy in blocking the vessels for HCC. In addition, reducing the probability of vascular growth at the molecular level is also a new therapeutic method.24,25 The previous report showed that HIF-1 plays an important role in the signal transduction pathway of vascular endothelial growth factor (VEGF) under hypoxic conditions.7 Chen et al found that RNAi of HIF-1α could significantly reduce the expression of HIF-1α and VEGF and also inhibit the growth of tumors.26 In our study, HIF-1α shRNA was imported into tumors by transcatheter intraarterial infusion and then transarterial embolization with drug-free microspheres. The result indicated that the combination treatment has a better effect than any singular treatment.
As is commonly known, the concentrations of HIF-1α shRNA in the common gene transfection methods (such as virus and liposome) are low in the tumor. Intraarterial therapy for liver tumors demonstrated great potential in cancer treatment because it enhances high drug concentrations in tumor tissues.15,27 In our study, HIF-1α shRNA was imported into tumors by transcatheter intraarterial infusion directly in order to increase the concentration, which is one of the strength in our study.
Current research shows that the HIF-1α pathway is a master regulator of angiogenesis. The low expression of HIF-1α plays an important role in preventing tumor recurrence and metastasis. VEGF can promote the formation of blood vessels in tumor tissues. It is the biological basis of tumor growth and an important factor in promoting tumor metastasis.10,11 The low expression of VEGF decreases the formation of blood vessels at the molecular level. Recently the VEGF-targeted therapy pathway has shown promising results in the treatment of advanced HCC.28 The expressions of HIF-1α and VEGF in the shRNA + MD group was the lowest, which indicated that this therapy can decrease the formation of blood vessels, slow tumor growth, reduce tumor size and promote tumor cell apoptosis. The necrotic score and mitotic count showed that the combined treatment was the most effective in promoting tumor cell apoptosis and preventing tumor cell proliferation.
VM is defined as a pattern of blood supply in carcinoma without participation of endothelial cells. VM in HCC cells was positive for PAS staining, but negative for CD34.29 As shown in our study, the tissues in shRNA + MD group had the least VM compared to the other three groups. Previous studies reported that the VM formation is inhibited by transfecting HIF-1α shRNA in HCC, which is similar to our results.4,8,30 In addition, tumor growth and size can reflect the effect of treatment intuitively. The results indicated that the combined treatment was the most effective in slowing the growth of the tumor, but the size of the tumor did not decrease. We believe that this finding may be due to the slightly short treatment time, which was only 28 days.
There were several limitations, including the relatively small sample size. Second, we did not have any data for survival time because of small sample size. Third, the fibroblast was used in vitro study. Finally, VX2 tumors were used in the study. However, the VX2 hepatic tumor model has been widely recognized for its effectiveness in HCC inoculation.
In summary, the expression of HIF-1α and VEGF, the formation of VM and the mitotic count were lower and the necrotic score was higher in the shRNA + MD group than the other groups. Compared to therapy with MD or HIF-1α shRNA transfection alone, the combination treatment has a better effect on inhibiting HCC growth.
Disclosure
The authors have no conflicts of interest for this work.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34. doi:10.3322/caac.21551
2. El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365(12):1118–1127. doi:10.1056/NEJMra1001683
3. He J, Gu D, Wu X, et al. Major causes of death among men and women in China. N Engl J Med. 2005;353(11):1124–1134. doi:10.1056/NEJMsa050467
4. Wang M, Zhao X, Zhu D, et al. HIF-1α promoted vasculogenic mimicry formation in hepatocellular carcinoma through LOXL2 up-regulation in hypoxic tumor microenvironment. J Exp Clin Cancer Res. 2017;36(1):60. doi:10.1186/s13046-017-0533-1
5. Semenza GL. HIF-1 and human disease: one highly involved factor. Genes Dev. 2000;14(16):1983.
6. Wang D, Zhang X, Lu Y, Wang X, Zhu L. Hypoxia inducible factor 1α in hepatocellular carcinoma with cirrhosis: association with prognosis. Pathol Res Pract. 2018;214(12):1987. doi:10.1016/j.prp.2018.09.007
7. Mizukami Y, Kohgo Y, Chung DC. Hypoxia inducible factor-1 independent pathways in tumor angiogenesis. Clin Cancer Res. 2007;13(19):5670–5674. doi:10.1158/1078-0432.CCR-07-0111
8. Zhang JG, Zhou HM, Zhang X, et al. Hypoxic induction of vasculogenic mimicry in hepatocellular carcinoma: role of HIF-1 α, RhoA/ROCK and Rac1/PAK signaling. BMC Cancer. 2020;20(1):32. doi:10.1186/s12885-019-6501-8
9. Zhang D, Lv FL, Wang GH. Effects of HIF-1α on diabetic retinopathy angiogenesis and VEGF expression. Eur Rev Med Pharmacol Sci. 2018;22(16):5071–5076. doi:10.26355/eurrev_201808_15699
10. Moon WS, Rhyu KH, Kang MJ, et al. Overexpression of VEGF and angiopoietin 2: a key to high vascularity of hepatocellular carcinoma? Mod Pathol. 2003;16(6):552–557. doi:10.1097/01.MP.0000071841.17900.69
11. Buijs N, Oosterink JE, Jessup M, et al. A new key player in VEGF-dependent angiogenesis in human hepatocellular carcinoma: dimethylarginine dimethylaminohydrolase 1. Angiogenesis. 2017;20(4):557–565. doi:10.1007/s10456-017-9567-4
12. Liao Y, Luo H, He Z, et al. A Combination of UTMD-Mediated HIF-1α shRNA Transfection and TAE in the Treatment of Hepatic Cancer. Biomed Res Int. 2019;2019:1937460. doi:10.1155/2019/1937460
13. Brandi G, Biasco G, Mirarchi MG, et al. A Phase I study of continuous hepatic arterial infusion of Irinotecan in patients with locally advanced hepatocellular carcinoma. Dig Liver Dis. 2011;43(12):1015–1021. doi:10.1016/j.dld.2011.08.005
14. van Riel JM, van Groeningen CJ, Kedde MA, et al. Continuous administration of irinotecan by hepatic arterial infusion: a phase I and pharmacokinetic study. Clin Cancer Res. 2002;8(2):405–412.
15. Czejka M, Kiss A, Koessner C, Terkola R, Ettlinger D, Schueller J. Metabolic activation of irinotecan during intra-arterial chemotherapy of metastatic colorectal cancer. Anticancer Res. 2011;31(10):3573–3878.
16. Georges E, Breitburd F, Jibard N, Orth G. Two Shope papillomavirus-associated VX2 carcinoma cell lines with different levels of keratinocyte differentiation and transplantability. J Virol. 1985;55(1):246–250. doi:10.1128/JVI.55.1.246-250.1985
17. Li C, Wang W, Ding H, et al. Value of contrast-enhanced sonography in the diagnosis of peripheral intrahepatic cholangiocarcinoma. J Clin Ultrasound. 2011;39(8):447–453. doi:10.1002/jcu.20797
18. Hu S, Sun C, Wang B, et al. Diffusion-weighted MR imaging to evaluate immediate response to irreversible electroporation in a rabbit VX2 liver tumor model. J Vasc Interv Radiol. 2019;30(11):1863–1869. doi:10.1016/j.jvir.2019.05.030
19. Gupta T, Virmani S, Neidt TM, et al. MR tracking of iron-labeled glass radioembolization microspheres during transcatheter delivery to rabbit VX2 liver tumors: feasibility study. Radiology. 2008;249(3):845–854. doi:10.1148/radiol.2491072027
20. Keller S, Chapiro J, Brangsch J, et al. Quantitative MRI for assessment of treatment outcomes in a rabbit VX2 hepatic tumor model. J Magn Reson Imaging. 2019. doi:10.1002/jmri.26968
21. Khabbaz RC, Huang YH, Smith AA, Garcia KD, Lokken RP, Gaba RC. Development and angiographic use of the rabbit VX2 model for liver cancer. J Vis Exp. 2019;143.
22. Zou Y, Guo CG, Yang ZG, Sun JH, Zhang MM, Fu CY. A small interfering RNA targeting vascular endothelial growth factor efficiently inhibits growth of VX2 cells and VX2 tumor model of hepatocellular carcinoma in rabbit by transarterial embolization-mediated siRNA delivery. Drug Des Devel Ther. 2016;10:1243–1255. doi:10.2147/DDDT.S94122
23. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25(4):402–408. doi:10.1006/meth.2001.1262
24. Facciorusso A. Drug-eluting beads transarterial chemoembolization for hepatocellular carcinoma: current state of the art. World J Gastroenterol. 2018;24(2):161–169. doi:10.3748/wjg.v24.i2.161
25. Mauri G, Varano GM, Orsi F. TAE for HCC: when the old way is better than the new ones!!! Cardiovasc Intervent Radiol. 2016;39(6):799–800. doi:10.1007/s00270-016-1340-3
26. Chen C, Wang J, Liu R, Qian S. RNA interference of hypoxia-inducible factor-1 alpha improves the effects of transcatheter arterial embolization in rat liver tumors. Tumour Biol. 2012;33(4):1095–1103. doi:10.1007/s13277-012-0349-8
27. Fiorentini G, Lucchi SR, Giovanis P, Cantore M, Guadagni S, Papiani G. Irinotecan hepatic arterial infusion chemotherapy for hepatic metastases from colorectal cancer: results of a phase I clinical study. Tumori J. 2001;87(6):388–890. doi:10.1177/030089160108700606
28. El Shorbagy S, AbuTaleb F, Labib HA, et al. Prognostic significance of VEGF and HIF-1 α in hepatocellular carcinoma patients receiving sorafenib versus metformin sorafenib combination. J Gastrointest Cancer. 2020. doi:10.1007/s12029-020-00389-w
29. Xu Y, Li Q, Li XY, Yang QY, Xu WW, Liu GL. Short-term anti-vascular endothelial growth factor treatment elicits vasculogenic mimicry formation of tumors to accelerate metastasis. J Exp Clin Cancer Res. 2012;31(1):16. doi:10.1186/1756-9966-31-16
30. Liu WB, Xu GL, Jia WD, et al. Prognostic significance and mechanisms of patterned matrix vasculogenic mimicry in hepatocellular carcinoma. Med Oncol. 2011;28(Suppl 1):S228–s238. doi:10.1007/s12032-010-9706-x
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.