Back to Journals » Clinical Ophthalmology » Volume 14
Treatment Course of Patients with Exudative Age-Related Macular Degeneration Using Ocular Hypotensives
Authors Modjtahedi BS, Luong TQ , Chiu S, van Zyl T, Lin JC, Fong DS
Received 25 August 2019
Accepted for publication 5 November 2019
Published 22 January 2020 Volume 2020:14 Pages 187—195
DOI https://doi.org/10.2147/OPTH.S228618
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Bobeck S Modjtahedi, 1–3 Tiffany Q Luong, 3 Stephan Chiu, 2 Tavé van Zyl, 4 Jane C Lin, 3 Donald S Fong 1–3
1Eye Monitoring Center, Kaiser Permanente Southern California, Baldwin Park, CA, USA; 2Department of Ophthalmology, Southern California Permanente Medical Group, Baldwin Park, CA, USA; 3Department of Research and Evaluation, Southern California Permanente Medical Group, Pasadena, CA, USA; 4Glaucoma Service, Massachusetts Eye and Ear Infirmary, Boston, MA, USA
Correspondence: Bobeck S Modjtahedi
Eye Monitoring Center, Kaiser Permanente Southern California, 1011 Baldwin Park Blvd, Baldwin Park, CA 91706, USA
Email [email protected]
Purpose: To characterize the visual outcomes and the treatment course of patients with exudative age-related macular degeneration (AMD) based on ocular hypotensive use.
Design: A matched retrospective cohort study of patients enrolled in Kaiser Permanente Southern California health plan was conducted. Patients taking ocular hypotensives were identified using pharmacy dispensing data and were matched to controls to compare visual acuity, number of anti-VEGF injections, and conversation to secondary anti-VEGF agents in the first year of treatment. Subgroup analysis was performed based on the number of ocular hypotensive agents (0, 1, 2 or 3+ agents) and drug class (aqueous suppressants and prostaglandin analogs).
Results: A total of 234 patients patients were examined. Baseline and final visual acuity did not significantly differ between drop users and controls, including on subgroup analysis. The average number of anti-VEGF injections did not differ between drop users and controls (6.1 vs 6.2, p=0.97), nor did the percentage of patients who were switched to a second-line anti-VEGF agent (23.9% vs 17.9%, p=0.26). Subgroup analysis did not reveal significant differences in the number of anti-VEGF injections and the percentage of patients switched to secondary agents, with patients receiving 6 ± 1 injections across regardless of the number or class of ocular hypotensive agents used.
Conclusion: Patients with concurrent glaucoma and exudative AMD have similar visual outcomes and treatment courses compared to those not taking ocular hypotensives. Although aqueous suppressants have been suggested to prolong anti-VEGF residence time, patients using these agents did not demonstrate visual benefit or a reduced injection burden in this series.
Keywords: age-related macular degeneration, bevacizumab, glaucoma, aqueous suppressant, pharmacology, pharmacokinetics, vascular endothelial growth factor, intravitreal injection
Introduction
Exudative age-related macular degeneration (AMD) and glaucoma represent important causes of visual morbidity. Given that both diseases are associated with aging, they often coexist, and many patients require concurrent treatment regimens. Whereas the impact of intravitreal injections on intraocular pressure (IOP) and glaucoma risk is an area of active investigation,1 less is known about the effect of topical ocular hypotensive medications on the treatment course of patients with both glaucoma and exudative AMD.
It has been suggested that ocular hypotensives may hold potential therapeutic benefit in the treatment of exudative AMD by altering disease activity (e.g., direct effect on choroidal neovascular membranes) or via their IOP-lowering effects (e.g., altering anti-VEGF pharmacokinetics). Carbonic anhydrase inhibitors (CAI) have been observed to reduce macular edema, typically in degenerative conditions such as retinitis pigmentosa or X-linked retinoschisis, by increasing fluid outflow from the retina by modulating Müller and retinal pigment epithelium cell activity.2 Animal models have suggested that beta-adrenergic blockade can reduce the expression of VEGF and induce neovascular regression.3 Conversely, prostaglandin analogs (PGA) have been reported to increase the risk of macular edema and inflammation, especially following intraocular surgery.4 There have been mixed results as to whether systemic beta-blockers (BB) alter the risk of developing exudative AMD or affect the injection burden of patients with exudative AMD, although the weight of recent evidence suggests there is no meaningful alteration of disease course from these agents.5–8
Although the pharmacokinetics of intravitreal anti-VEGF agents are incompletely understood, the predominant route of clearance is believed to be via the anterior chamber.9–14 If true, then a pharmacologic reduction of aqueous flow should theoretically prolong intraocular anti-VEGF residence time with a resultant increase in drug activity and duration.15–17 This principle was invoked by both Sridhar et al16 (n=10) and Lee et al15 (n=15), who independently demonstrated some improvements in anatomic response when dorzolamide–timolol was added to anti-VEGF therapy in patients with persistent signs of exudation in small, non-controlled, non-blinded prospective series. In a secondary analysis study, Rahimy et al examined the treatment outcomes of patients enrolled in the Comparison of AMD Treatment Trial (CATT) based on their use of ocular hypotensives. Although a trend towards better anatomic and visual outcomes was identified among patients taking aqueous suppressants, most of these differences did not reach statistical significance.17
The current study sought to determine whether treatment courses differed during the first year of therapy among patients with exudative AMD based on their use of ocular hypotensive agents.
Methods
This matched retrospective cohort study was approved by the Institutional Review Board (IRB) of Kaiser Permanente Southern California (KPSC) and adhered to the Declaration of Helsinki. The IRB waived the need for individual patient consent given the retrospective nature of this study. Patients with a new diagnosis of exudative AMD [identified using International Classification of Disease codes (ICD-9 code 362.52, ICD-10 code H35.32)] who received an intravitreal injection of bevacizumab [identified from Current Procedural Terminology (CPT) codes] within 30 days of diagnosis were included in this cohort. Patients were excluded from the study if their initial treatment for exudative AMD was not bevacizumab (which is the first-line agent in our medical group); if they did not have KPSC pharmacy benefits; if they had less than 1 year of follow-up; if they did not have at least 1-year prior membership before diagnosis date; or if they had a prior history of exudative AMD, diabetic macular edema, retinal vein occlusion, or retinal artery occlusion.
The KPSC pharmacy dispensing database was used to identify which patients within the cohort had filled a prescription and obtained subsequent refills for BB, CAI, alpha agonists (AA), and PGA. Patients were defined as drop users if they had at least 275 days of medication coverage (i.e., they had picked up an initial prescription and subsequent refills which covered at least a 275-day supply) during their first year of exudative AMD treatment. Non-users were defined as patients with no dispensing record of any ocular hypotensive medications during their first year of exudative AMD treatment. Patients with 1–274 days of medication supply were not included for analyses. Drop users with exudative AMD were then matched to non-drop users with exudative AMD based on age (±1 year), gender, race, and baseline smoking status. Manual chart review was conducted to confirm that the eye receiving intravitreal bevacizumab was also receiving ocular hypotensives. In patients with simultaneous bilateral exudative AMD, the study eye was selected by simple randomization. Primary outcome measures included visual acuity, number of intravitreal anti-VEGF injections received, and the percentage of patients who were switched to a second-line anti-VEGF agent (ranibizumab or aflibercept) during the first year of treatment.
Given the heterogeneous nature of the cohort’s ocular hypotensive medication usage and the frequent use of multiple agents, it was not possible to investigate the effects of each individual medication on outcomes during subgroup analysis. Instead, subgroup analysis was conducted based on medication class and was limited to patients taking exclusively aqueous suppressants (BB and/or CAI) or outflow enhancers (PGA). Patients on alpha agonists were excluded from this comparison because of its mixed mechanism of action. Subgroup analysis was also conducted based on the number of ocular hypotensive agents used among those patients who maintained the same number of medications in the first year of exudative AMD treatment. Additional patient characteristics were examined, including body mass index (BMI), Charlson comorbidity index, hyperlipidemia, sleep apnea, history of myocardial infarction or stroke, hemoglobin A1c, use of oral diabetes medications, insulin use, angiotensin-converting enzyme inhibitor use, angiotensin II receptor blocker use, systemic beta-blocker use, and calcium channel blocker use. Snellen visual acuity was converted to Early Treatment Diabetic Retinopathy Study (ETDRS) letters for the purpose of statistical analysis. Comparisons were performed using Wilcoxon rank-sum test for continuous variables and the chi-squared test for categorical variables. The Fisher’s exact test was applied for categorical variables where >20% of the cells had an expected count of <5.
Results
A total of 234 patients were included in this analysis, with 117 in each group (defined as users of ocular hypotensives and non-users). The average age in each group was 81.7 years at the time of exudative AMD diagnosis: 75.2% of all patients were White (non-Hispanic), 56.4% were female, and 95.7% were non-smokers. Table 1 shows the characteristics of the entire study population and when stratified by the number of ocular hypotensive agents used. Table 2 outlines which ocular hypotensive medications were used.
 |
Table 1 Demographic Characteristics of Patients Using Ocular Hypotensives and Matcheda Controls, Stratified by the Number of Medication Classesb |
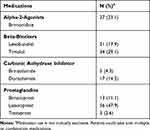 |
Table 2 Ocular Hypotensive Regimen at Time of Exudative Age-Related Macular Degeneration Diagnosis (N=117) |
Outcomes among patients using ocular hypotensive agents compared to those not taking any drops are summarized in Table 3. Baseline vision, baseline IOP, vision at final follow-up, and IOP at final follow-up were similar between the groups. Patients in the ocular hypotensive group received an average of 6.1 injections in the first year compared to 6.2 injections in the control group (p=0.97). A similar proportion of patients taking ocular hypotensives were switched to second-line anti-VEGF agents when compared to controls (23.9% vs 17.9%, p=0.26). Results were comparable across different sub-types of ocular hypotensive agents (Table 4). Final visual acuity was similar in patients taking aqueous suppressants (n=18) when compared to controls (46.8 EDTRS letters vs 54.2, p=0.47) as was the total number of injections (6.9 vs 6.1, p=0.20) and percentage of patients switched to second-line agents (33.3% vs 22.2%, p=0.71). Patients on PGA monotherapy (n=20) did not demonstrate a significantly different number of total anti-VEGF injections (5.4 vs 5.5, p=0.83) or rate of conversion to a second-line agent (15% vs 20%, p=1.00) compared to their matched controls. Patients taking 1 ocular hypotensive, 2 ocular hypotensives, 3+ ocular hypotensives, or no ocular hypotensives all had a similar number of average anti-VEGF injections in the first year of exudative AMD treatment (6 ±1 injections). The final visual acuity and percentage of patients switched to second-line anti-VEGF treatments were similar across all groups (Table 3). The number of patients on 3+ ocular hypotensives who were switched to second-line therapy was small, but the significance of this observation was limited by the overall small sample size.
 |
Table 3 Outcomes and Treatment Course, Stratified by Number of Ocular Hypotensive Agentsa |
 |
Table 4 Outcomes and Treatment Course Stratified by Ocular Hypotensive Class |
Baseline vision, baseline IOP, final vision, and final IOP were similar in all analyses: all ocular hypotensive users vs controls (Table 3), subgroup analysis based on the number of drops (Table 3), and subgroup analysis based on the types of drops (Table 4).
Discussion
This study sought to compare the treatment course of patients during their first year of anti-VEGF therapy for exudative AMD based on the usage of ocular hypotensive agents. These results indicate that visual acuity, treatment course, and injection burden are similar between users and non-users of ocular hypotensive agents. Specifically, baseline and final visual acuity were similar in all subgroups, and patients received approximately six intravitreal injections (± 1) in the first year of exudative AMD treatment, a number consistent across all subgroups regardless of the number and type of ocular hypotensive agents used. Although six injections in the first year is fewer than the number of injections patients received in the seminal anti-VEGF clinical trials, it is in-line with the other real-world analyses spanning several countries.18 Approximately, 15–30% of patients across all groups were switched to second-line anti-VEGF therapy. Using the number of drops as a surrogate for glaucoma severity, this study indicates that glaucoma patients (regardless of severity) have similar exudative AMD treatment courses and visual outcomes compared to those without glaucoma. The number of drops was used as an indirect measure of glaucoma severity because visual field and OCT retinal nerve fiber layer data were not available.
If pharmacological reduction of aqueous outflow prolonged intraocular anti-VEGF residence time, and by extension its duration of action, it would be expected that aqueous suppressants would enhance anti-VEGF efficacy in contrast to PGAs (which increase outflow). Two series have reported improved anatomic response in exudative AMD patients taking aqueous suppressants who have persistent signs of exudation despite anti-VEGF therapy; however, these studies were limited by small sample sizes and the lack of control groups.15,16 The implications of these studies must also be tempered by observations that quantification of changes to macular anatomy can be susceptible to segmentation errors, especially when examining patients with exudative AMD, and visual outcomes do not always correlate with morphologic changes. Of note, neither of the aforementioned series demonstrated improvement in visual acuity after patients started dorzolamide-timolol despite the presence of anatomic improvement on optical coherence tomography (OCT). Similar investigations have examined whether there is a supplemental benefit to adding dorzolamide–timolol in the treatment of retinal vein occlusions. Obeid et al reported improved anatomic and functional gains in patients with persistent macular edema despite anti-VEGF treatment after the addition of dorzolamide–timolol to their treatment regimen (n=8) although this study also lacked a control group.19 Byeon et al compared the treatment response in patients with retinal vein occlusions based on whether they were randomized to receive dorzolamide–timolol, and they did not find sustained benefit in those taking drops.11 Rahimy et al’s secondary analysis of patients enrolled in CATT demonstrated a trend towards improved OCT measures and visual acuity after 2 years of anti-VEGF treatment in patients taking aqueous suppressants (n=19) compared to patients not taking ocular hypotensives (n=857)—a trend that was not seen in those using PGAs (n=28).17 Aqueous suppressant users gained 2.6 more letters [95% confidence interval (CI): −3.4 to +8.5 letters, p=0.39], experienced a greater reduction in retinal thickness on OCT compared to baseline (−17.9 µm, 95% CI: −36.5 to −0.7 µm, p=0.06), and demonstrated a greater reduction in total retinal thickness on OCT from baseline (mean difference −54.7 µm, 95% CI: −103 to- 6.2 µm, p=0.03) compared those not taking ocular hypotensives on adjusted analysis.17 The authors did not find a significant difference in the number of injections that patients received among those in the pro re nata arms of CATT (15.7 injections in the control group, 13.9 injections in aqueous suppressant users, and 16.7 injections in PGA users). Although the authors point towards a trend supporting a therapeutic benefit from aqueous suppressants, it is important to note that most differences were not statistically significant, the absolute changes between groups were modest, and the confidence intervals covered a large range. The lack of significant differences in the present study between aqueous suppressant users, PGA users, and their matched controls indicates that modulating aqueous outflow may not substantially impact bevacizumab’s efficacy.
The present study did not demonstrate a difference in final visual acuity or treatment course between matched controls and ocular hypotensive users regardless of the number and class of agents used. One limitation of this study was that data on OCT morphology was not available. This study is limited by the small sample size, as were the prior studies investigating the possible therapeutic benefit of aqueous suppressants.15–17 Similar to Rahimy et al,17 the present study is limited by its retrospective design which creates the risk of unaccounted for confounders; however, this study utilized a matched cohort to limit the differences between study groups and only examined the patients initially treated with bevacizumab to eliminate the possibility of different ocular hypotensive–anti-VEGF interactions. A strength of the present study was that pharmacy dispensing data were used to monitor ocular hypotensive use, instead of relying on patients’ self-reported utilization of drops, which allows for greater confidence in correctly identifying patients’ medication use. Although pharmacy dispensing data is an imperfect measure of drop compliance, it likely correlates with actual medicine use by the patients (i.e., patients who pick up refills are more likely to be using their drops than those who do not). It is possible that patients’ underlying glaucoma may have offset the possible beneficial effect of ocular hypotensive agents and that their clinical histories influenced physicians’ treatment patterns due to concerns surrounding IOP fluctuations following intravitreal injections. Although physicians may be less aggressive with intravitreal injections in patients with glaucoma, there is typically less tolerance for macular fluid in the treatment of exudative AMD compared to other diseases and therefore less room for physician discretion in treatment course. The purpose of this study was to examine anti-VEGF treatment course and how it is affected by ocular hypotensive drugs; therefore, evaluations of glaucomatous progression (which has been studied extensively in previous papers) were not included in this analysis. Further prospective studies are necessary to better elucidate what role, if any, ocular hypotensives may play in augmenting anti-VEGF’s therapeutic effect and whether the presence of underlying glaucoma influences any such effect.
Disclosure
Dr Fong has received grant support outside the submitted work from Allergan, Regeneron, and Santen. Ms Luong has received grant support outside the submitted work from Allergan and Santen. The authors report no other conflicts of interest in this work.
References
1. Bracha P, Moore NA, Ciulla TA, WuDunn D, Cantor LB. The acute and chronic effects of intravitreal anti-vascular endothelial growth factor injections on intraocular pressure: a review. Surv Ophthalmol. 2018;63(3):281–295. doi:10.1016/j.survophthal.2017.08.008
2. Huang Q, Chen R, Lin X, Xiang Z. Efficacy of carbonic anhydrase inhibitors in management of cystoid macular edema in retinitis pigmentosa: a meta-analysis. PLoS One. 2017;12(10):e0186180. doi:10.1371/journal.pone.0186180
3. Lavine JA, Sang Y, Wang S, Ip MS, Sheibani N. Attenuation of choroidal neovascularization by beta(2)-adrenoreceptor antagonism. JAMA Ophthalmol. 2013;131(3):376–382. doi:10.1001/jamaophthalmol.2013.1476
4. Razeghinejad MR. The effect of latanaprost on intraocular inflammation and macular edema. Ocul Immunol Inflamm. 2017;27(2):1–8.
5. Kolomeyer AM, Maguire MG, Pan W, VanderBeek BL. Systemic beta-blockers and risk of progression to neovascular age-related macular degeneration. Retina. 2018;39(5):918–925.
6. Montero JA, Ruiz-Moreno JM, Sanchis-Merino E, Perez-Martin S. Systemic beta-blockers may reduce the need for repeated intravitreal injections in patients with wet age-related macular degeneration treated by bevacizumab. Retina. 2013;33(3):508–512. doi:10.1097/IAE.0b013e3182695ba0
7. Thomas AS, Redd T, Hwang T. Effect of systemic beta-blockers, ace inhibitors, and angiotensin receptor blockers on development of choroidal neovascularization in patients with age-related macular degeneration. Retina. 2015;35(10):1964–1968. doi:10.1097/IAE.0000000000000603
8. Traband A, Shaffer JA, VanderBeek BL. Systemic beta-blockers in neovascular age-related macular degeneration. Retina. 2017;37(1):41–46. doi:10.1097/IAE.0000000000001226
9. Ahn J, Kim H, Woo SJ, et al. Pharmacokinetics of intravitreally injected bevacizumab in vitrectomized eyes. J Ocul Pharmacol Ther. 2013;29(7):612–618. doi:10.1089/jop.2013.0009
10. Bakri SJ, Snyder MR, Reid JM, Pulido JS, Singh RJ. Pharmacokinetics of intravitreal bevacizumab (Avastin). Ophthalmology. 2007;114(5):855–859. doi:10.1016/j.ophtha.2007.01.017
11. Byeon SH, Kwon OW, Song JH, Kim SE, Park YS. Prolongation of activity of single intravitreal bevacizumab by adjuvant topical aqueous depressant (Timolol-Dorzolamide). Graefes Arch Clin Exp Ophthalmol. 2009;247(1):35–42. doi:10.1007/s00417-008-0917-1
12. Gaudreault J, Fei D, Rusit J, Suboc P, Shiu V. Preclinical pharmacokinetics of Ranibizumab (rhuFabV2) after a single intravitreal administration. Invest Ophthalmol Vis Sci. 2005;46(2):726–733. doi:10.1167/iovs.04-0601
13. Stewart MW. Predicted biologic activity of intravitreal bevacizumab. Retina. 2007;27(9):1196–1200. doi:10.1097/IAE.0b013e318158ea28
14. Park SJ, Oh J, Kim YK, et al. Intraocular pharmacokinetics of intravitreal vascular endothelial growth factor-Trap in a rabbit model. Eye. 2015;29(4):561–568. doi:10.1038/eye.2014.329
15. Lee JH, Lee SC, Byeon SH, Koh HJ, Kim SS, Lee CS. Efficacy of adjuvant topical dorzolamide-timolol in patients with neovascular age-related macular degeneration refractory to anti-vascular endothelial growth factor therapy. Retina. 2018.
16. Sridhar J, Hsu J, Shahlaee A, et al. Topical dorzolamide-timolol with intravitreous anti-vascular endothelial growth factor for neovascular age-related macular degeneration. JAMA Ophthalmol. 2016;134(4):437–443. doi:10.1001/jamaophthalmol.2016.0045
17. Rahimy E, Ying GS, Pan W, Hsu J. Effect of intraocular pressure-lowering medications on neovascular age-related macular degeneration treatment outcomes in the comparison of age-related macular degeneration treatment trials. Retina. 2018.
18. Holz FG, Tadayoni R, Beatty S, et al. Multi-country real-life experience of anti-vascular endothelial growth factor therapy for wet age-related macular degeneration. Br J Ophthalmol. 2015;99(2):220–226. doi:10.1136/bjophthalmol-2014-305327
19. Obeid A, Hsu J, Ehmann D. et al. Topical dorzolamide-timolol with intravitreous anti-vascular endothelial growth factor for retinal vein occlusion: a pilot study. Retin Cases Brief Rep;2018. 1. doi:10.1097/ICB.0000000000000752
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
