Back to Archived Journals » Comparative Effectiveness Research » Volume 5
Treatment characteristics and mortality of a large insured female population with advanced or metastatic breast cancer by receipt of HER2-targeted agents
Authors Hao Y, Meyer N, Landsman-Blumberg P, Johnson W, Willemann Rogerio J
Received 25 August 2014
Accepted for publication 18 October 2014
Published 28 April 2015 Volume 2015:5 Pages 35—47
DOI https://doi.org/10.2147/CER.S73220
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Corrine I Voils
Yanni Hao,1 Nicole Meyer,2 Pamela Landsman-Blumberg,2 William Johnson,2 Jaqueline Willemann Rogerio1
1Novartis Pharmaceuticals Corporation, East Hanover, NJ, USA; 2Truven Health Analytics, Cambridge, MA, USA
Purpose: This retrospective administrative claims study of women diagnosed with advanced or metastatic breast cancer compared treatment characteristics and mortality by receipt of human epithelial growth factor receptor 2 (HER2)-targeted agents and by disease stage and age group among patients using HER2-targeted agents.
Patients and methods: Adult women diagnosed with stage III or IV breast cancer (index date) between 2008 and 2012 were identified from MarketScan® databases containing medical and pharmacy claims for >40 million enrollees insured with >100 US health plans. Patients were followed until the earlier of the following: end of enrollment, inpatient death, or December 31, 2012. Study cohorts were women ± HER2-targeted agent use, HER2-targeted agent users' subgroups of stages III and IV, and age group. Pre- and postindex breast cancer treatments were compared among study cohorts. Overall survival was compared using log-rank tests. Cox proportional-hazards models were used to study the predictors of overall survival.
Results: Of 30,660 eligible women, 14.4% received HER2-targeted agents. HER2-targeted agent users received more aggressive pre- and postindex cancer treatments compared to those with no HER2-targeted agents. HER2-targeted agents had higher rates of pre- and postindex breast cancer surgery, adjuvant/neoadjuvant chemotherapy, radiation therapy, chemotherapy, and biologics-based therapy. Among HER2-targeted agent users, younger women and those with stage III breast cancer received more aggressive treatments. After adjusting for clinically relevant patient characteristics, women receiving HER2-targeted agents had a 20% reduced risk of death compared to patients not receiving HER2-targeted agents. Among all patients and the subset of HER2-targeted agent users, stage IV patients and those in the oldest age group had a higher risk of death.
Conclusion: Among patients with advanced or metastatic breast cancer, receipt of HER2-targeted agents was associated with more aggressive treatment and longer survival. Patients with higher stage breast cancer and older patients received less aggressive therapies and had a higher risk of death.
Keywords: metastatic breast cancer, HER2, treatment, survival, human epidermal growth factor receptor 2, therapy
Introduction
In 2009, over 200,000 women in the US were diagnosed with breast cancer,1 approximately 20% of whom have human epithelial growth factor receptor 2 (HER2)-positive tumors.2 The absolute lifetime risk of developing HER2-positive breast cancer is 1.8% and it increases with age, with the majority of women diagnosed at ages 40–69 years.3 Women with HER2-positive breast cancer have a more aggressive disease, greater likelihood of recurrence, poorer prognosis, and decreased survival compared to women with HER2-negative breast cancer.4 HER2-targeted therapies have significantly improved outcomes for HER2-positive patients with both early and metastatic breast cancer (mBC).5 There are currently four HER2-targeted agents approved in the US (trastuzumab, lapatinib, pertuzumab, and ado-trastuzumab emtansine), and additional targeted agents are in development.6,7
While clinical outcomes in HER2-positive patients have improved significantly, inconsistency in therapy and patient outcomes have been reported in recent studies of HER2-targeted patients.4,8–10 Factors influencing patient therapy and prognosis include age at diagnosis, disease severity, hormone receptor status, and comorbidity profile. Furthermore, while the National Comprehensive Cancer Network guidelines recommend first-line regimens of HER2-targeted agents combined with chemotherapy in the treatment of metastatic disease, studies have shown that older patients may be less likely to receive such treatments.8 Although numerous clinical trials have assessed the efficacy of HER2-targeted agents, there is limited information about the use of HER2-targeted agents and the characteristics of patients with locally advanced or metastatic disease receiving these treatments in real-world settings.
The objectives of this analysis were to examine treatment patterns and mortality among women with advanced or mBC stratified by HER2 status and to describe these patterns among patients receiving HER2-targeted agents stratified by disease stage and age group.
Methods
Data sources
Paid medical and prescription claims from 2007 through 2012 were sourced from the Truven Health MarketScan® Commercial and Medicare Supplemental databases, which contain the health care experience of privately insured individuals and those with Medicare Supplemental insurance. Enrollees include the primary insurance holder as well as their spouses and dependents. Dates of death for a subgroup of patients were sourced from a database of MarketScan claims linked to the Social Security Administration death files. Linked claims are available only for employed primary insurance enrollees, not dependents. Dates of death were available from January 1, 2007, through December 31, 2012.
Data are fully compliant with the Health Insurance Portability and Accountability Act of 1996. Because the study used only deidentified patient records and did not involve the collection, use, or transmittal of individually identifiable data, Institutional Review Board approval was not required.
Study design
A retrospective longitudinal observational study design was used to follow patients over time from their initial diagnosis of either locally advanced (stage III) or metastatic (stage IV) breast cancer to the end of their available follow-up: disenrollment from an eligible health plan, inpatient death, or end of the database December 31, 2012, whichever occurred first. For each patient, the date of the first claim for stage III or stage IV metastases was defined as the index date. Patients were required to have a minimum of 12 months of continuous enrollment prior to their diagnosis of locally advanced or metastatic disease (pre-index period).
The initial sample was divided into two mutually exclusive cohorts based on the presence or absence of claims for HER2-targeted agents (trastuzumab or lapatinib) during the entire study period (ie, pre-index period through follow-up); patients without medical or pharmacy claim for any HER2-targeted agent were assigned to the “No HER2-targeted agents” cohort, while patients with any such claims were assigned to the “HER2-targeted agents” cohort, likely representing HER2-positive patients. Patients with HER2-targeted agents were further stratified by disease stage (III or IV) and age (18–44 years, 45–64 years, and ≥65 years) at index.
Demographic and clinical histories for all study patients were measured at index and during the 12-month pre-index period, respectively, and treatment characteristics were examined during the variable-length follow-up period that included the index date. A minimum of 3 months of continuous enrollment was required solely for the analysis of treatment patterns. Pre-index period and follow-up measures were compared across the study cohorts: no HER2-targeted vs HER2-targeted agent users; stage III vs stage IV among HER2-targeted agent users; and ages 18–44 years vs 45–64 years vs ≥65 years, also among HER2-targeted agent users.
Patient selection
The study population consisted of women, aged 18 years or older, who were newly diagnosed with stage III or stage IV breast cancer from January 1, 2008, through December 31, 2011; the time frame of 2008–2011 allowed all study patients to have 12 months of preindex and postindex data, if available. Breast cancer was defined as the occurrence of at least one inpatient or two nondiagnostic outpatient claims at least 30 days apart with a diagnosis code of breast cancer in any position (International Classification of Diseases, 9th Revision, Clinical Modification [ICD-9-CM]: 174.xx). Cancer stage as defined by the American Joint Committee on Cancer is not available in administrative claims data;11 therefore, ICD-9-CM diagnosis codes for secondary malignant neoplasms were used to proxy the corresponding American Joint Committee on Cancer listed location of metastases. At least one inpatient or outpatient nondiagnostic claim within 60 days before or subsequent to any breast cancer claim for stage III (ICD-9-CM diagnosis codes: stage III 196.0, 196.3, or 198.2) or stage IV (196.1, 196.2, 196.5–196.6, 196.8–196.9, 197.0–197.8, 198.0–198.1, 198.3–198.8, 198.82, 198.89, or 199.0–199.1) was required for inclusion. The date of service of the first metastasis claim was assigned as the index date and disease stage at index was determined. Furthermore, patients had to have 12 months of continuous medical and prescription coverage prior to the index date (pre-index period), ≥18 years of age as of the index date, and be newly diagnosed with stage III or stage IV breast cancer (no metastases claims in the pre-index period). Patients were excluded based on pre-index period claims for a primary cancer other than breast cancer or study period claims for human immunodeficiency virus infection/acquired immune deficiency syndrome or pregnancy.
Final study patients were first stratified according to HER2-targeted agent use anytime during the study period as defined earlier. Use was defined as at least one pharmacy or medical claim for trastuzumab or lapatinib, defined by National Drug Codes or Healthcare Common Procedure Coding System codes, respectively. Patients receiving HER2-targeted agents were further stratified by disease stage and age group at index.
Study measures
Demographic variables were identified relative to the study index date and included age, US Census geographic region population density, primary payer, and plan type. Clinical history included sites of metastases at diagnosis; diagnosis of earlier-stage breast cancer; and where found, breast cancer-related surgical treatment (lumpectomy or mastectomy), radiation therapy, and hormonal, biologics-based, or chemotherapy treatments. Comorbid conditions (anemia, anxiety/depression, cardiac arrhythmia, cerebrovascular disease, congestive heart failure, coronary artery disease, chronic obstructive pulmonary disorder, diabetes, and hypertension), and the National Cancer Institute modification of the Charlson Comorbidity Index (NCI–CCI)12 were included as measures of illness burden. The NCI–CCI is an aggregate measure of comorbidity burden specific to cancer, reflecting both the Deyo and Romano adaptations of the Charlson index and excluding all cancer-related diagnoses. Breast cancer-related treatment was measured in the follow-up period only for those with at least 3 months of continuous postindex enrollment. Possible treatments included surgery (lumpectomy or mastectomy), radiation therapy, and antineoplastic mono- or combination hormonal, biologics-based, or chemotherapy. Mortality status was available for 10,086 (33%) of all study patients using the MarketScan Death Denominator File. Dates of death are available through December 31, 2012.
Statistical analysis
Categorical variables were summarized by frequency and percentage. Continuous variables were reported by means and standard deviations. Statistical comparisons were evaluated using chi-square or exact tests for categorical measures and analysis of variance or parametric tests for continuous measures depending on the distributional properties of the specific measure evaluated.
Kaplan–Meier product limit estimates of the probability of survival were plotted as a function of time to provide a visual comparison of the survival patterns among the study cohorts.
Hazard ratios (HRs) for survival were adjusted for the influence of covariates using Cox proportional-hazards models. Covariates included receipt of HER2-targeted agents at any time, disease stage at index (stage III vs stage IV), age group (18–44 years, 45–64 years, and ≥65 years), plan type (comprehensive, point of service, health maintenance organizations, consumer-driven/high-deductible health plans, preferred provider/exclusive provider organizations, and other/unknown), US census region (Northeast, North Central, South, West, and unknown), urban vs rural residence, preindex NCI–CCI, and preindex comorbidities (anemia, anxiety, cardiac arrhythmia, coronary artery disease, congestive heart failure, chronic obstructive pulmonary disorder, cerebrovascular disease, depression, diabetes, and hypertension), as well as preindex breast cancer treatments including surgery, radiation therapy, chemotherapy, non-HER2 biologics, and hormonal therapy. Two models were analyzed, one with all patients and one using the subgroup of HER2-targeted agent users. The aptness of the proportional-hazards assumption was assessed visually with plots of the Schoenfeld residuals for each covariate used in the model.13 The Cox proportional-hazards model afforded direct inference about the influence of each covariate on the probability of survival and provided estimates of HRs. HRs and their 95% confidence intervals (CIs) are reported.
Results
Study samples
Of the 509,035 women with a diagnosis of breast cancer from January 1, 2008, through December 31, 2011, 88,334 (17.3%) had a stage III or stage IV metastases diagnosis (Figure 1). After screening for age (n=88,326), continuous enrollment (n=40,249), evidence of new diagnoses for stage III or stage IV metastases (N=35,205), and exclusionary diagnoses (n=30,660), a total of 30,660 women (6.0%) were eligible for inclusion in the study. Of the eligible population, 4,405 (14.4%) received HER2-targeted agents at some point during the study period. Of these 4,405 patients, 57.0% indexed with stage III and 43.0% with stage IV disease; 17.7% of these were in the age group of 18–44 years, 67.8% were in the 45- to 64-year range, and 14.6% were aged ≥65 years.
  | Figure 1 Sample selection. |
Patients with and without receipt of HER2-targeted agents
Compared to patients without HER2-targeted agents, those with HER2-targeted agents were younger (mean age [standard deviation], 55 [11] vs 59 [13] years; P<0.001) and were less likely to have Medicare as the primary payer (15% vs 27%; P<0.001) (Table 1). Patients were enrolled predominantly in preferred provider organization (PPO), exclusive provider organization (EPO), and health maintenance organization (HMO) plans and resided primarily in the southern and north central regions of the US.
Similar proportions of patients with HER2-targeted agents and no HER2-targeted agents had evidence of earlier-stage breast cancer in the pre-index period (48% each). A larger percentage of patients in the HER2-targeted group underwent surgery for breast cancer in the pre-index period compared to patients in the no HER2-targeted group (20% vs 17%; P<0.001). HER2-targeted patients had higher rates of preindex adjuvant/neoadjuvant chemotherapy (74% vs 53%), radiation treatment (12% vs 10%), and lower use of non-HER2 biologics (0% vs 3%) and hormones (30% vs 41%); all P<0.005. Those not receiving HER2-targeted agents had a greater proportion of patients in the higher NCI–CCI comorbidity score groups compared to those receiving HER2-targeted agents (P<0.001) during the pre-index period. The no HER2-targeted patients were more likely to be at stage IV (45% vs 43%; P=0.005) but had fewer metastasis sites at index (1.26 vs 1.30; P<0.001). As expected, fewer HER2-targeted agent patients had pre-index period comorbid conditions of cardiac arrhythmia (6% vs 9%, P<0.001), cerebrovascular disease (2% vs 4%, P<0.001), coronary artery disease (4% vs 6%, P<0.001), and hypertension (29% vs 36%, P<0.001).
HER2-targeted agent recipients by disease stage and age group
Among HER2-targeted patients, stage IV patients were older than stage III patients (age 56 years vs 54 years; P<0.001) and were more likely to be covered by Medicare (18% vs 13%, P<0.001) (Table 2). The proportion of patients with evidence of breast cancer in the pre-index period was higher among stage IV patients compared to those with stage III (73% vs 30%; P<0.001) and increased with age (18–44 years: 44%; 45–64 years: 48%; and ≥65 years: 54%; P<0.001). Similarly, the percentage of patients with NCI–CCI comorbidity scores ≥2 was higher among stage IV patients and those of older ages (P=0.015 for number of patients with NCI–CCI scores of 0, 1, 2, and 3+). Stage IV patients with breast cancer in the pre-index period were more likely to receive preindex radiation therapy (14% vs 8%; P<0.001) and antineoplastics compared to stage III patients, with the exception of chemotherapy (54% vs 40%; P<0.001). Among stage III patients, the locations of index metastatic occurrence were lymph nodes of the axilla and the upper limb for 96% and lymph nodes of the head, face, and neck for 3% of patients. At index, 20% of stage IV patients had liver metastases, 14% lung metastases, 41% bone metastases, and 15% brain metastases. As age increased, the proportion of patients with stage IV disease at index increased (ages 18–44 years: 36%; 45–64 years: 43%; ≥65 years: 51%; P<0.001). Accordingly, liver, lung, bone, and brain metastases were found in increasing proportions of patients by age.
Treatment characteristics
Significant differences were found in the mean length of follow-up between HER2-targeted and no HER2-targeted patients (702 [445] days vs 659 [449]; P<0.001) (Table 3). Inpatient death was the reason for end of follow-up in 7% of HER2-targeted patients and 8% of patients without HER2-targeted agents. Of those with at least 3 months of follow-up, a greater proportion of those with HER2-targeted agents received postindex breast cancer surgery (53% vs 47%), radiation (65% vs 54%), and non-HER2 antineoplastic therapies (89% vs 83%) than patients with no HER2-targeted agents. Compared to patients without HER2-targeted agents, patients receiving HER2-targeted agents had lower levels of hormone therapy (56% vs 78%) and non-HER2 biologics (5% vs 8%), both P<0.001. Chemotherapy use was higher among HER2-targeted patients (85% vs 58%, P<0.001), presumably reflecting the high use of HER2-targeted agents that are usually combined with chemotherapy. Notably, among the HER2-targeted agent users, 58% used HER2 biologics in the pre-index period and 95% in the follow-up, indicating that some patients discontinued the use of HER2 biologics after their index metastases, potentially due to disease progression or adverse reactions.
Among users of HER2-targeted agents, the mean length of follow-up for stage III patients was 100 days longer than for stage IV patients (745 vs 645, P<0.001) and declined by approximately 20 days with each increasing age group but did not reach statistical significance (Table 4). Among HER2-targeted patients with at least 3 months follow-up, postindex breast cancer surgery was greater among stage III patients than among stage IV patients (78% vs 19%) and decreased with age (62% for age group 18–44 years, 53% for age group 45–64 years, 42% for the group aged ≥65 years); both P<0.001. Postindex, stage III patients were more likely (P<0.001) to have radiation therapy (72% vs 56%) and be treated with HER2-targeted agents (97% vs 91%) and other antineoplastics (92% vs 84%) compared to stage IV patients. Radiation therapy declined in the HER2-targeted patients postindex with increasing age (18–44 years: 70%; 45–64 years: 65%; ≥65 years: 61%; P<0.001). Use of HER2-targeted agents and antineoplastics also declined with increasing age, but these did not reach statistical significance.
Survival analysis models
Survival analysis was performed for the subset of patients who could be linked to the Social Security Administration death files (N=10,086). Figure 2 shows the unadjusted Kaplan–Meier product limit estimates of the probability of survival 1) for patients with and without HER2-targeted agents and 2) for disease stage and age group stratifications among HER2-targeted agent users.
Two multivariate models were analyzed for survival, adjusting for patient demographics and clinical characteristics: one based on all patients and one based on the subgroup of HER2-targeted agent users. Across both multivariate models, consistent patterns were seen for predictors of survival. Older age, advanced disease stage, and preindex use of chemotherapy and radiation therapy were associated with shorter survival, while preindex breast cancer surgery reduced the risk of death. Preindex hormone use was not statistically significant.
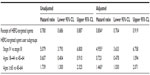
After adjusting for patient characteristics, multivariate results suggested that patients receiving HER2-targeted agents (as a proxy for HER2-positive status) had a significantly decreased adjusted hazard of death compared to patients without HER2-targeted agents (HR =0.804; 95% CI: 0.704–0.919; P=0.001) (Table 5). In the model based on all patients, the predictors of longer survival were preindex breast cancer surgery and residence in the western portion of the US. Predictors of shorter survival included older age, advanced disease stage, preindex radiation therapy, preindex use of chemotherapy or non-HER2 biologics, comorbid anemia, congestive heart failure, chronic obstructive pulmonary disease, and enrollment in comprehensive health plans (P<0.05). The adjusted hazard was statistically significantly increased for women with stage IV disease compared to those with stage III disease (HR =4.204; 95% CI: 3.790–4.663).
Among those women who received HER2-targeted agents, older age and advanced disease stage were significantly associated with the hazard of death (Table 6). Women with stage IV disease at index had 4.95 times the risk of death compared to women with stage III disease at index (95% CI: 3.633–6.758). Compared with women aged 45–64 years, those of age ≥65 years had an increased risk of death (HR =1.460; 95% CI: 1.030–2.071). Preindex surgery and residence in regions of the US other than the South were predictors of longer survival (P<0.05) Predictors of shorter survival included preindex radiation therapy, preindex use of chemotherapy or non-HER2 biologics, and comorbid cardiac arrhythmia (P<0.001).
Discussion
Recent data indicate that the introduction of HER2-targeted agents in standard therapy has significantly improved the prognostic outcomes for patients with HER2-positive mBC. Using a large national administrative claims database, this study examined real-world evidence on treatment characteristics and predictors of survival in women newly diagnosed with stage III and stage IV breast cancer by receipt of HER2-targeted agents.
Results from our study indicate that receipt of HER2-targeted agents is significantly associated with longer survival. After adjusting for clinically relevant patient characteristics, women with receipt of HER2-targeted agents had a 20% reduced risk of death compared to patients not receiving HER2-targeted agents (Table 7). Among all patients and the subset of HER2-targeted agent users, stage IV patients and those in the oldest age group had a higher adjusted risk of death; preindex breast cancer surgery reduced the risk of death, while preindex radiation therapy, chemotherapy, and non-HER2 biologics increased this risk. These findings are consistent with those from several recent studies that have shown better survival outcomes in HER2-positive patients compared to HER2-negative patients. Using a cohort of 2,091 women with metastatic stage IV breast cancer from a single institution, Dawood et al10 found that women with HER2/neu-positive disease who received trastuzumab as first-line therapy had a 44% decreased risk of death compared with women with HER2/neu-negative disease. In a study of 273 mBC patients starting first-line chemotherapy at a single oncology clinic, Thientosapol et al14 reported that patients with HER2-positive tumors lived significantly longer than those with HER2-negative tumors (HR =0.49; 95% CI: 0.34–0.72; P<0.001) due to favorable responses to HER2-targeted therapy.
Several studies8,10,15,16 have also reported a range of prognostic factors for women with metastatic breast cancer, including factors such as age at diagnosis, hormone receptor status, human HER2 status, and site of metastases, for predicting survival from the time of metastases. In a cohort of 1,038 women with relapsed mBC, Largillier et al16 reported that age at initial diagnosis, site of metastasis, and hormone receptor status were independent prognostic factors for survival following the development of metastatic disease. Dawood et al10 found that factors significantly associated with improved survival included younger age at diagnosis, positive hormone receptor status, lower grade of disease, and absence of visceral metastasis. However, due to large amounts of missing data, neither of these studies was able to adjust for HER2 status. Other studies have reported that age and hormone status are not clear predictors of survival. In a recent study of women with early-stage HER2-positive breast cancer, Partridge et al8 found that age was not strongly associated with improved survival but noted that future research should examine whether age is a predictor of later recurrence.
Compared to patients with no HER2-targeted agents, our study found that patients with HER2-targeted agents received more aggressive pre- and postindex cancer treatments compared to patients without HER2-targeted agents. Although those with HER2-targeted agents were younger and had significantly lower comorbidity, they had higher rates of preindex breast cancer surgery, adjuvant/neoadjuvant chemotherapy, radiation treatment, and antineoplastic treatments (except for hormone therapy). Similarly, among patients with at least 3 months of follow-up, patients with HER2-targeted agents had higher rates of postindex breast cancer surgery, radiation therapy, and non-HER2 antineoplastic treatments, compared to no HER2-targeted agent patients. Of those receiving any antineoplastics during the follow-up period, HER2-targeted agent users had a higher rate of chemotherapy use, but lower rates of therapy with hormones and non-HER2 biologics. The clinical history and breast cancer treatment differences between these cohorts partly reflect differential treatment patterns of HER2-positive and HER2-negative breast cancer patients. National Comprehensive Cancer Network guidelines recommend regimens containing trastuzumab and chemotherapy as preferred first-line agents in the treatment of HER2-positive mBC. Higher rates of pre- and postindex chemotherapy compared to patients with no HER2-targeted therapy are likely to be due to combined treatment of HER2-targeted therapy and chemotherapy regimens. More aggressive treatments may also be explained by the differences in mean age and disease stage between the cohorts. Users of HER2-targeted agents were younger and comprised a larger proportion of patients with stage III disease, factors that have been associated with more aggressive treatments.
A recent survey17 of surgeons and oncologists found that comorbidity and age were the factors most likely to affect an oncologist’s treatment decision (34% and 25%, respectively) on the use of trastuzumab in HER2-positive breast cancer patients. The study17 found that as age and comorbidity increased, the likelihood of oncologists prescribing chemotherapy in addition to trastuzumab decreased. These results are consistent with pre- and postindex treatments observed in patients with HER2-targeted agents. Pre- and postindex use of chemotherapy was significantly lower for stage IV patients compared to that for stage III patients. Similarly, patients aged ≥65 had significantly lower rates of pre- and postindex chemotherapy compared to younger patients (P<0.05). A study4 of elderly patients with HER2-positive mBC found that compared to younger patients, elderly patients were the least likely to receive trastuzumab in combination with chemotherapy and were most likely to receive trastuzumab alone or in combination with hormonal therapy, consistent with the findings of our study.
Several limitations to this study should be noted. This was a retrospective, observational study using administrative claims data. In addition to the limitations inherent in any retrospective analysis, administrative claims are collected for payment purposes and the determination of breast cancer or any clinical outcomes is dependent on the completeness and accuracy of medical coding, which is subject to data coding restrictions and data entry error. Diagnoses for metastases may be undercoded in administrative claims. Therefore, the study may have excluded breast cancer patients who should have been included or may have incorrectly classified patients as having stage III or stage IV breast cancer. Death occurring outside of an inpatient setting is not identifiable from the claims data, and patients who die in nonhospital settings will appear as disenrolled in the data. Due to changes in the availability of death information reported by the Social Security Administration, mortality rates were potentially underestimated for deaths occurring after November 2011. Another limitation is that there might be characteristics that are not observable in claims data and may also be confounding factors which cannot be controlled for in this study. Finally, because this study was limited to individuals with commercial health coverage or private Medicare supplemental coverage, results may not be generalizable to breast cancer patients with other insurance or without health insurance coverage.
Conclusion
Receipt of HER2-targeted agents was significantly associated with younger age and receipt of pre- and postindex breast cancer treatments. Clinical history and differences in breast cancer treatment between these groups partly reflect differential treatment patterns of HER2-positive and HER2-negative breast cancer patients. Among patients receiving HER2-targeted agents, treatment characteristics for the HER2-targeted agent cohort differ by disease stage and age group.
Disclosure
This study was funded by Novartis Pharmaceuticals Corporation. The authors report no other conflicts of interest in this work.
References
US Cancer Statistics Working Group. United States Cancer Statistics: 1999–2009 Incidence and Mortality Web-based Report. Atlanta, GA: Department of Health and Human Services, Centers for Disease Control and Prevention, and National Cancer Institute; 2013. Available from: http://apps.nccd.cdc.gov/uscs/. Accessed May 14, 2014. | |
Jones SE. Metastatic breast cancer: the treatment challenge. Clin Breast Cancer. 2008;8:224–233. | |
Kurian AW, Fish K, Shema SJ, Clarke CA. Lifetime risks of specific breast cancer subtypes among women in four racial/ethnic groups. Breast Cancer Res. 2010;12(6):R99. | |
Kaufman PA, Brufsky AM, Mayer M, et al. Treatment patterns and clinical outcomes in elderly patients with HER2-positive metastatic breast cancer from the registHER observational study. Breast Cancer Res Treat. 2012;135:875–883. | |
National Cancer Institute. Targeted cancer therapies. National Cancer Institute at the National Institutes of Health. Available from: http://www.cancer.gov/cancertopics/factsheet/Therapy/targeted. Accessed February 13, 2015. | |
American Cancer Society. Breast Cancer Facts and Figures 2011–2012. Atlanta: American Cancer Society; 2012. Available from: http://www.cancer.org/acs/groups/content/@epidemiologysurveilance/documents/document/acspc-030975.pdf. Accessed May 14, 2014. | |
Incorvati JA, Shah S, Mu Y, Lu J. Targeted therapy for HER2 positive breast cancer. J Hematol Oncol. 2013;6:38. | |
Partridge AH, Gelber S, Piccart-Gebhart MJ, et al. Effect of age on breast cancer outcomes in women with human epidermal growth factor receptor 2-positive breast cancer: results from a herceptin adjuvant trial. J Clin Oncol. 2013;31(21):2692–2698. | |
Olson EM, Najita JS, Sohl J, et al. Clinical outcomes and treatment practice patterns of patients with HER2-positive metastatic breast cancer in the post-trastuzumab era. Breast. 2013;22(4):525–531. | |
Dawood S, Broglio K, Buzdar AU, Hortobagyi GN, Giordano SH. Prognosis of women with metastatic breast cancer by HER2 status and trastuzumab treatment: an institutional-based review. J Clin Oncol. 2010;28(1):92–98. | |
AJCC. Breast. In: Edge SB, Byrd DR, Compton CC, et al, editors. AJCC Cancer Staging Manual. 7th ed. New York, NY: Springer; 2010:347–376. | |
Hines RB, Chatla C, Harvey L. Predictive capacity of three comorbidity indices in estimating mortality after surgery for colon cancer. J Clin Oncol. 2009;27(26):4339–4345. | |
Hess KR. Graphical methods for assessing violations of the proportional hazards assumption in a Cox regression. Stat Med. 1995;14:1707–1723. | |
Thientosapol ES, Tran TT, Della-Fiorentina SA, et al. Survival times of women with metastatic breast cancer starting first-line chemotherapy in routine clinical practice versus contemporary randomised trials. Intern Med J. 2013;43(8):883–888. | |
Dawood S, Broglio K, Ensor J, Hortobagyi GN, Giordano SH. Survival differences among women with de novo stage IV and relapsed breast cancer. Ann Oncol. 2010;21(11):2169–2174. | |
Largillier R, Ferrero JM, Doyen J, et al. Prognostic factors in 1,038 women with metastatic breast cancer. Ann Oncol. 2008;19(12):2012–2019. | |
Ring A. The influences of age and co-morbidities on treatment decisions for patients with HER2-positive early breast cancer. Crit Rev Oncol Hematol. 2010;76(2):127–132. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.