Back to Journals » Infection and Drug Resistance » Volume 14
Treatment and Clinical Outcomes of Osteoarticular Infections Among Pediatrics Admitted to Jimma University Medical Center, Ethiopia: A Prospective Observational Study
Authors Mamo MD , Daba FB , Beshir Jnr M, Fanta K
Received 14 June 2021
Accepted for publication 22 July 2021
Published 27 July 2021 Volume 2021:14 Pages 2933—2941
DOI https://doi.org/10.2147/IDR.S323490
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Suresh Antony
Mekonnen Damessa Mamo,1 Fekede Bekele Daba,1 Mohammed Beshir Jnr,2 Korinan Fanta1
1Department of Clinical Pharmacy, Institute of Health, Jimma University, Jimma, Oromia, Ethiopia; 2Department of Pediatrics, Institute of Health, Jimma University, Jimma, Oromia, Ethiopia
Correspondence: Mekonnen Damessa Mamo; Korinan Fanta
Department of Clinical Pharmacy, Institute of Health, Jimma University, P.O.Box: 378, Jimma, Oromia, Ethiopia
Tel +251924100153
; +251911598485
Fax +251576617980
Email [email protected]; [email protected]
Background: Despite the high burden of osteoarticular infections in sub-Saharan Africa, there is a paucity of data regarding the osteoarticular infections management approach and clinical outcomes in the region. Hence, this study aimed to evaluate a management protocol, clinical outcomes, and its determinants among pediatric patients with osteoarticular infections.
Methods: A pediatric patient admitted to Jimma University Medical Center (JUMC), Ethiopia, with a diagnosis of osteoarticular infections was enrolled prospectively from April 30 to October 30, 2019. Clinical characteristics, management modality, and in-hospital complications were recorded from admission to discharge. Data were analyzed by using SPSS v.23 and the p-value < 0.05 was considered statistically significant.
Results: Among a total of 150 pediatric patients enrolled in this study, osteomyelitis was diagnosed in 111 (74%), while the rest 39 (26%) had septic arthritis. The majority 105 (70%) of the study participants were male with a mean age of 8.79 ± 4.2 years. The culture was performed for only 3.6% of the patients. Almost all (98.7%) of the patients received intravenous (IV) antibiotics, and ceftriaxone was the most common IV antibiotic used as a monotherapy 66 (44.6%) or in combination with metronidazole 47 (31.8%) or gentamicin (12.8%). Almost half (45.3%) of the patients had poor treatment outcomes. Factors associated with poor treatment outcome were comorbidity [AOR=3.3, 95% CI (1.08– 10.16)] and use of combination antibiotics [AOR=2.9, 95% CI (1.16– 7.3)]. Rural residence [AOR=0.39, 95% CI (0.168– 0.92)] and surgical interventions [AOR=0.29, 95% CI (0.006– 0.144)] were associated with good treatment outcomes.
Conclusion: Almost half of pediatric patients with osteoarticular infections had poor treatment outcome. Health providers should increase the accessibility of microbiological tests and diagnostic imaging, which can guide treatment decisions and improve outcomes of patients with osteoarticular infections.
Keywords: management, infections, osteomyelitis, septic arthritis, children, sub-Saharan Africa
Introduction
Osteoarticular infections are clinically manifest as osteomyelitis, Septic arthritis, and both combined.1 Osteomyelitis is defined as the presence of clinical features (tenderness, pain, swelling, redness restriction of movement) and had at least one or more of the following: fever higher than 37.5 °C, leukocytosis [white cell count (WCC)] >13,000/mL, raised erythrocyte sedimentation rate (ESR) > 20 mm or a positive blood culture, while septic arthritis is defined as all clinical features of lab results similar to osteomyelitis but mostly restricted to the joints.2
Annually, approximately 7.3 million children die globally, and out of these 50% of children’s deaths occur in just five countries – Nigeria, Democratic Republic Congo, Ethiopia, Tanzania, and Uganda. The common cause of death for these children is an infection, of which osteoarticular infection is one.3 It is estimated that 0.1–30% of pediatric populations are affected by osteoarticular infections worldwide and the estimated cost for treatment of these infections is $17,000 to $150,000 per patient.4
Even though the incidence of these infections has declined in Europe starting in the late 1970, still it is among the leading reasons why patients visit healthcare facility, take time off work, become disabled, and a common cause of severe long-term pain in developing countries.4,5 In low and middle-income countries (LMIC), osteoarticular infections are still a common cause of suffering.6 A study from Nigeria reported that the majority (83%) of osteoarticular infections cases occurred among under-5 children, and they suffer from significant complications secondary to these infections.7,8 Similarly, other studies from South Africa and Malawi showed that about 66% of the patients with osteoarticular infections develop poor treatment outcomes and 6.7% of a total procedure done annually was due to unsuccessful management of these infections.9,10
Despite the increasing burden of osteoarticular infection in sub-Saharan African countries including Ethiopia, limited studies are available regarding the burden of the disease, management, and clinical outcomes from the region.11 Therefore, the present study aimed to assess, management approach, in-hospital clinical outcomes, and its determinants among pediatrics admitted to JUMC, Ethiopia.
Methods and Materials
Study Design and Clinical Setting
A prospective observational study was conducted at Jimma University Medical Center (JUMC) in Ethiopia. JUMC serves as a referral center for the southwestern part of the country (over 15 million catchment population). It is located 352 kilometers from the capital city Addis Ababa. The hospital has a general pediatric ward, pediatric intensive care unit (ICU), and pediatric surgical ward on top of other specialty services for adults. The orthopedic surgical ward has 6 rooms with 36 beds. This study was conducted from April 30-October 30, 2019.
Study Population
All consecutive pediatric patients admitted to JUMC with a diagnosis of osteoarticular infections were included regardless of the duration of the disease. Pediatric patients (age ≤ 15 years) diagnosed with osteoarticular infections clinically or with objective findings (culture and imaging) were included based on the willingness of the guardians. In the present study, osteomyelitis was indicated by the presence of clinical features, such as tenderness, pain, swelling, redness, and restriction of movements; with at least one or more of the following: fever higher than 37.5 °C, leukocytosis >13,000/mL, raised erythroid sedimentation rate (ESR) > 20 mm or a positive blood culture. Septic arthritis was considered in patients who presented with one or more of the above features that were restricted to the joints areas.2 Patients whose initial diagnosis changed to other medical conditions and patients whose their guardian declined consent was excluded.
Data Collection
The data collection tool was developed by reviewing prior literature conducted at a different settings. The data collection tool contains sociodemographic characteristics, clinical characteristics (including laboratory test and key diagnostics investigations), in-hospital management (medications and surgical interventions), and in-hospital outcomes (complications, length of hospital stay, and discharge clinical status). A pre-test was conducted on 5% of the study population before commencing data collection and appropriate modifications were made to the data collection tool. Three healthcare professionals (one B. Pharm, one BSc nurse, and one medical intern) working at the pediatric ward of JUMC collected the data after receiving two days of training on study purpose and data collection tool. Data collectors abstracted clinical data from active patient’s medical records and interviewed the guardians when necessary.
Study Outcomes and Validating Tools
The primary outcome of the present study was in-hospital treatment outcomes, which were assessed by following the patient’s clinical signs and symptoms and occurrence of complications until discharge. In-hospital complications were confirmed by the treating physician-based clinical findings and objective findings, such as culture, ultrasound, and X-ray imaging. Treating physicians’ discharge summary notes were reviewed in addition to following patients on a daily basis from admission to discharge.
Operational Definition and Definitions of Terms
- Good treatment outcome was considered in pediatric patients discharged with improved signs and symptoms without in-hospital complications or significant disability.
- Poor treatment outcome was indicated by the occurrence of significant in-hospital complications (such as deep vein thrombosis, amputation, systemic infection, pathologic dislocations, bone abscess, etc.) with lack of clinical improvement or considered in patients discharged against medical advice or dead in-hospital.
- Co-morbid conditions were considered in patients presented with chronic illness on top of osteoarticular infections.
- We used the following laboratory cut points in the present study: Hyperthermia: temperature higher than 37.5 °C;12 Leukocytosis: [white blood cell counts (WBC)] >13,000/mL;12 Leucopenia: WBC less than <5000 cell/mm;3,12 Low red blood cell (RBC) count: RBC count <3500x 103 cell/mm;3,13 Thrombocytopenia: platelet count less than 150×103 cell/mm;3,14 Thrombocytosis: platelet count greater than 450×103 cell/mm;3,14 High erythrocyte sedimentation rate (ESR) >20 mm.13
Ethical Approval and Assent to Participate
The study protocol was approved by the Institutional Review Board (IRB) of Jimma University, Institute of Health with a reference number of IHRPGD/567/19. Permission was obtained from the responsible bodies of the hospital before interviewing the patients’ guardians and extracting data from patients’ case records. In addition, written and verbal informed consent was obtained from all study guardians before commencing data collection.
Statistical Analysis
The collected data were checked for completeness, clarity, and accuracy and entered into Epidata version 4.4.1. Statistical package for social science (SPSS) version 23 (IBM, Armonk, NY, USA) was used for data analysis. Categorical variables were reported by frequency and percentage (%) and continuous variables were summarized with mean ± standard deviation. A multivariate logistic regression model was used to identify independent predictors of poor treatment outcomes. Variables with p-value < 0.25 on univariate analysis were considered as a candidate for multivariate logistic regression. Variables with p-value < 0.05 was considered statistically significant.
Results
Among a total of 150 pediatric patients enrolled in this study, osteomyelitis was diagnosed in 111 (74%), while the rest 39 (26%) had septic arthritis.
Sociodemographic Characteristic
The majority 105 (70%) of the study participants were male with a mean age of 8.79 ± 4.2 years. About two-thirds, 114 (76%) of the patients’ families had no formal education and the majority 107 (71.3%) of the patients were from rural areas. Twenty-five (16.7%) patients had comorbid conditions (Table 1).
 |
Table 1 Socio-Demographic of Pediatrics Patients with OAI Admitted to JUMC, Ethiopia |
Laboratory Parameters
Complete blood count test was done for 121 (80.7%) patients on admission. Among those patients who had CBC results, 47 (38.2%) patients had leukocytosis (raised white blood cell count ≥13,000/mL). Erythrocyte sedimentation rate was done for 100 (66.6%) patients on admission 90 (60%) raised ESR (>20 mm). Ultrasound imaging was done for 65 (43.3%) patients, while culture test was performed for only five patients (Table 2).
 |
Table 2 Laboratory and Diagnostic Findings Among Pediatric Patients with OAI Admitted to JUMC |
Management of Osteoarticular Infections
Medical Management of Osteoarticular Infections
Almost all patients 148 (98.6%) received intravenous antibiotics on admission. Ceftriaxone was the most common antibiotic used as a monotherapy 66 (44.6%) or in combination with metronidazole 47 (31.8%) or gentamicin (12.8%). Regarding the duration of IV antibiotics, most patients received for less than 1 month (39% for ≤2 weeks and 30% received for 3–4 weeks). Only 11 patients received IV antibiotics for a prolonged duration (>2 months). De-escalation of oral antibiotics was provided for about 16% of the patients and the most common oral antibiotic used was Ciprofloxacin 14 (9.3%). Anti-pain medications were given for 86 (57.3%) of the patients, and Tramadol was prescribed for the majority 30 (46.9%) of the patients (Table 3).
 |
Table 3 Management of Osteoarticular Infections Among Pediatrics Patients Admitted to JUMC |
Surgical Management
In addition to antibiotic therapy, the majority of patients, 117 (78%), were received a different surgical intervention. Sequestrectomy was performed for 27 (18%) patients; while surgical debridement and drainage were done for 18 (12%) of the patients. Eight (5.3%) patients were amputated secondary to the infections (Figure 1).
 |
Figure 1 Type of surgical interventions done for pediatric patients with osteoarticular infections admitted to JUMC, Ethiopia. |
Incidence of in Hospital Complications
Sixty-eight (45.4%) patients developed at least one complication during their hospital stay. The most common complications detected were bone necrosis in (9.3%), deep vein thrombosis in (7.3%), and amputation of the affected site in (7.3%) patients (Figure 2).
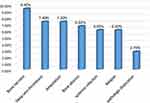 |
Figure 2 In-hospital complications of osteoarticular infections among pediatric patients admitted to JUMC, Ethiopia. |
Predictors of Treatment Outcomes
Of the 150 patients treated for osteoarticular infections, 65 (43.3) patients had poor treatment outcomes. In multivariable logistic regression analysis, patients who had comorbid medical conditions had about a three-fold increase in poor treatment outcome compared to those who had no comorbid condition (AOR=3.3, 95% CI: 1.08–10.16). Similarly, patients who took a combination of antibiotics (ceftriaxone + metronidazole) were three times more likely to have poor treatment outcomes compared to those who received ceftriaxone monotherapy (AOR=2.9, 95% CI: 1.16–7.3). On the other hand, patients from a rural area (AOR=0.39, 95% CI: 0.168–0.92) and those who received the surgical intervention (AOR=0.29, 95% CI: 0.006–0.144) had a good prognosis compared to those from an urban area and those who did not receive the surgical intervention (Table 4).
 |
Table 4 Predictors of Poor Treatment Outcome Among Pediatrics Patients with Osteoarticular Infections Admitted to JUMC |
Discussion
In this prospective observational study, we evaluated osteoarticular infections management, in-hospital complications and predictors of poor treatment outcomes among pediatric patients. Majority (74%) of pediatric patients with osteoarticular infections were presented with osteomyelitis and about 70% of the study participants were male. The most commonly used antibiotics were ceftriaxone monotherapy, and a combination of ceftriaxone with metronidazole or gentamicin. Almost half (45%) of the patients had poor treatment outcomes. Bone necrosis, deep vein thrombosis, and amputations were the three common in-hospital complications recorded among pediatric patients with osteoarticular infections.
In this study, about 45% of pediatric patients treated for osteoarticular infections had poor treatment outcomes. This result was comparable with the study conducted by Petruhina et al, which reported that 47% of patients with osteoarticular infections had poor treatment outcomes.15 On the other hand, studies conducted by Haeffs, et al, Arias, et al, Kuiper, et al, and Street et al were reported to have a low number of poor treatment outcomes (16.7%, 11.1%, 31%, and 14.3%, respectively) compared to the present study.6,16–18 This discrepancy in treatment outcomes might be due to geographical difference, duration of follow-up, the difference in management modality, and outcome assessment. Meanwhile, other studies from sub-Saharan Africa by Ebong et al and El Bushra were reported a higher prevalence of poor treatment outcomes (55–57%) compared to the present findings.19,20
All patients with poor treatment outcomes were developed at least one in-hospital complication. The in-hospital complications encountered among these patients were bone necrosis (9.3%), deep vein thrombosis (7.3%), amputation (7.3%), bone abscess (6.7%), systemic infections (6%), relapsed case (6%) and pathologic dislocation (2.6%) patients. Similar complications were reported in studies conducted in New Zealand and Sudan.18,19 Contrary to these findings, data from Cambodia, Latvia, and the USA reported decreased movement, residual pain, death, synovitis, and pneumonia as common complications of osteoarticular infections.5,15,21 This difference in complications was most likely due to differences in study design, diagnostic methods (culture, CT-scan), and duration of follow-up.
In the present study, factors associated with poor treatment outcomes were the use of combination antibiotics (ceftriaxone with metronidazole vs ceftriaxone monotherapy) and the presence of comorbidity. Patients treated with ceftriaxone and metronidazole had a three-fold increase in the risk of in-hospital complications compared to those treated with ceftriaxone monotherapy. This finding was supported by prior studies done by Omoke et al, and Park et al.7,22 However, studies conducted by Arias et al and Chiappini et al showed that patients treated with combination antibiotics had a good prognosis.16,23 The poor treatment outcome in patients who received combination antibiotics compared to single antibiotics might be due to a lack of microbiological tests and other diagnostic imaging, which would have helped the treatment decisions. In addition, other unmeasured confounders, such as disease severity, risk factors, multi-microbial and resistance pathogens, might contribute to the poor outcome observed among patients received combination antibiotics. Pediatric patients with comorbid medical conditions had poor treatment outcomes compared to those presented only with osteoarticular infections. This result is also reported by several studies.17,24,25
Factors associated with a good treatment outcome were surgical interventions and rural residency. Surgical interventions decreased the risk of complications and poor treatment outcomes by around 70%. Many studies have also reported significant associations between surgical interventions and good treatment outcomes.17,22,26 However, studies conducted by Haeffs et al and Li et al did not observe statistical significant between surgical management and treatment outcomes.6,27 Patients from the rural areas had a good treatment outcome compared to those who came from the urban areas. This might be due to the fact that patients from the urban areas had more comorbid conditions (diabetes and HIV/AIDS) compared to those from the rural areas and more patients from the rural areas received surgical interventions compared to those from the urban areas. In addition, patients from urban area had prolonged hospital stay compared to those from rural area. On top of these differences, other unmeasured confounders, such as difference in the causative pathogen, resistance pattern and antibiotics exposure between rural and urban residents, might contribute to the observed difference in outcomes.
This study prospectively illustrated clinical characteristics, management, and treatment outcomes of osteoarticular infections. However, this study has several limitations. First, this study was conducted on a small sample population due to resource constraints. Second, important objective findings, such as culture test, sensitivity analysis, c-reactive proteins, other inflammation markers, and diagnostic imaging, were not routinely performed in the present setting. As a result, it is challenging to judge the appropriateness of antibiotic treatment. Third, both acute and chronic osteoarticular infections were treated almost similarly due to a lack of detailed assessment of symptom onset and pathogenesis. Fourth, only in-hospital and short-term relapse was assessed in the present study due to resource constraint, lack of regular follow-up at the same hospital, and lack of technological access in the rural areas to at least follow-up them on phone. Lastly, other important outcomes, such as economic cost, quality of life, and quality of care, were not measured.
Conclusion
The majority of pediatric patients with osteoarticular infections were presented with osteomyelitis. Almost half of the pediatric patients admitted with osteoarticular infections had poor treatment outcomes. We recommend that health providers should increase the accessibility of microbiological tests and diagnostic imaging, which can guide treatment decisions and improve outcomes of patients with osteoarticular infections. In addition, a large registry should be conducted to assess the appropriateness of osteoarticular infection treatment based on local microbial sensitivity patterns.
Abbreviations
AOR, adjusted odds ratio; CBC, complete blood count; CI, confidence interval; COR, crude odds ratio; C-RP, C-reactive protein; ESR, erythrocyte sedimentation rate; IV, intra venous; JUMC, Jimma University Medical Center; OAI, osteoarticular; OM, osteomyelitis; PO, per oral; SA, septic arthritis; SF, Synovial Fluid; WBC, white blood.
Data Sharing Statement
The dataset that was used to support the finding of this study will be made available from the corresponding author upon reasonable request.
Ethics Approval and Consent to Participate
The study protocol was approved by the Institutional Review Board (IRB) of Jimma University, Institute of Health with a reference number of IHRPGD/567/19. Permission was obtained from responsible bodies of JUMC before interviewing the patients’ guardians and extracting data from active patient’s case records. Written and verbally informed assent was obtained from all the guardians of the study participants. All the study protocols were performed in accordance with the ethical principles of the Declaration of Helsinki.28 The confidentiality and privacy of participant’s data were maintained by using different codes throughout the data collection tools.
Acknowledgment
We would like to thank Jimma University for facilitating the study and covering stationery materials and data collection costs. We would also like to acknowledge all data collectors, supervisors, and respondents without whom this research would not have been realized.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The present research was funded by Jimma University Institute of Health; grant number [JUIH/567/11]. The funder had no contribution to this study regarding study design, data collection, and data analysis, interpretation of data, or writing the manuscript.
Disclosure
The authors declare that there are no conflicts of interest regarding this work.
References
1. Saavedra J, Falup-Pecurariu SO, Faust RS. Bone and joint infections. 2017.
2. Mahmoudi S, Pourakbari B, Borhani K, et al. Acute osteomyelitis and septic arthritis in children. Wien Med Wochenschr. 2017;167(11–12):259–263. doi:10.1007/s10354-017-0583-1
3. Afolabi BM. Sub-Sahara African neonates–ghosts to statistics. J Neonatal Biol. 2017;6(1):2167–0897.1000246. doi:10.4172/2167-0897.1000246
4. Schwarz EM, Parvizi J, Gehrke T, et al. 2018 international consensus meeting on musculoskeletal infection: research priorities from the general assembly questions. J Orthop Res. 2019;37(5):997–1006. doi:10.1002/jor.24293
5. Grammatico‐Guillon L, Maakaroun Vermesse Z, Baron S, Gettner S, Rusch E, Bernard L. Paediatric bone and joint infections are more common in boys and toddlers: a national epidemiology study. Acta Paediatr. 2013;102(3):e120–e125. doi:10.1111/apa.12115
6. Haeffs TH, Scott CA, Campbell TH, Chen Y, August M. Acute and chronic suppurative osteomyelitis of the jaws: a 10-year review and assessment of treatment outcome. J Oral Maxillofac Surg. 2018;76(12):2551–2558. doi:10.1016/j.joms.2018.05.040
7. Omoke NI, Obasi AA. Childhood pyogenic septic arthritis as seen in a teaching hospital South East Nigeria. Niger J Surg Res. 2017;23(1):26–32. doi:10.4103/1117-6806.199968
8. Akinyoola AL, Orimolade EA, Yusuf MB. Pathologic fractures of long bones in Nigerian children. J Child Orthop. 2008;2(6):475–479. doi:10.1007/s11832-008-0141-x
9. Nunn T, Cheung W, Rollinson P. A prospective study of pyogenic sepsis of the hip in childhood. J Bone Joint Surg Br. 2007;89(1):100–106. doi:10.1302/0301-620X.89B1.17940
10. Lavy C, Thyoka M, Pitani A. Clinical features and microbiology in 204 cases of septic arthritis in Malawian children. J Bone Joint Surg B. 2005;87-B(11):1545–1548. doi:10.1302/0301-620X.87B11.16735
11. Biruk W, Wubshet K. Chronic osteomyelitis at Tikur Anbessa Hospital, Addis Ababa University, Ethiopia. East Cent Afr J Surg. 2007;12(1):33–41.
12. Schmitt SK. Osteomyelitis. Infect Dis Clin. 2017;31(2):325–338.
13. Unkila-Kallio L, Kallio MJ, Peltola H, Eskola J. Serum C-reactive protein, erythrocyte sedimentation rate, and white blood cell count in acute hematogenous osteomyelitis of children. Pediatrics. 1994;93(1):59–62.
14. Prieto-Pérez L, Pérez-Tanoira R, Petkova-Saiz E, et al. Osteomyelitis: a descriptive study. Clin Orthop Surg. 2014;6(1):20–25. doi:10.4055/cios.2014.6.1.20
15. Petruhina J, Urbane UN, Petersons A, Pavare J. Epidemiology and antibacterial treatment of acute hematogenous osteomyelitis in patients hospitalized at children’s clinical University Hospital in Riga, Latvia. Acta Chir Latv. 2017;17(2):29–34. doi:10.1515/chilat-2017-0021
16. Arias CA, Betancur MCT, Pinzón MA, Arango DC, Taffur CAC, Prada EC. Differences in the clinical outcome of osteomyelitis by treating specialty: orthopedics or infectology. PLoS One. 2015;10(12):e0144736. doi:10.1371/journal.pone.0144736
17. Kuiper JW, Vos SJ, Saouti R, et al. Prosthetic joint-associated infections treated with DAIR (debridement, antibiotics, irrigation, and retention) analysis of risk factors and local antibiotic carriers in 91 patients. Acta Orthop. 2013;84(4):380–386. doi:10.3109/17453674.2013.823589
18. Street M, Puna R, Huang M, Crawford H. Pediatric acute hematogenous osteomyelitis. J Pediatr Orthop. 2015;35(6):634–639. doi:10.1097/BPO.0000000000000332
19. Ebong WW. Acute osteomyelitis in Nigerians with sickle cell disease. Ann Rheum Dis. 1986;45(11):911–915. doi:10.1136/ard.45.11.911
20. El Bushra A. Diabetic septic foot lesions in El Obeid, Western Sudan. Sudan J Med Sci. 2007;2(2):119–122.
21. Tice AD, Hoaglund PA, Shoultz DA. Outcomes of osteomyelitis among patients treated with outpatient parenteral antimicrobial therapy. Am J Med. 2003;114(9):723–728. doi:10.1016/S0002-9343(03)00231-6
22. Park K-H, Kim DY, Lee Y-M, et al. Selection of an appropriate empiric antibiotic regimen in hematogenous vertebral osteomyelitis. PLoS One. 2019;14(2):e0211888.
23. Chiappini E, Camposampiero C, Lazzeri S, Indolfi G, De Martino M, Galli L. Epidemiology and management of acute haematogenous osteomyelitis in a tertiary paediatric center. Int J Environ Res Public Health. 2017;14(5):477. doi:10.3390/ijerph14050477
24. Ali AM, Maya E, Lakhoo K. Challenges in managing paediatric osteomyelitis in the developing world: analysis of cases presenting to a tertiary referral centre in Tanzania. Afr J Paediatr Surg. 2014;11(4):308. doi:10.4103/0189-6725.143136
25. Li HK, Scarborough M, Zambellas R, et al. Oral versus intravenous antibiotic treatment for bone and joint infections (OVIVA): study protocol for a randomised controlled trial. Trials. 2015;16(1):583. doi:10.1186/s13063-015-1098-y
26. Gilbertie JM, Schnabel LV, Stefanovski D, Kelly DJ, Jacob ME, Schaer TP. Gram-negative multi-drug resistant bacteria influence survival to discharge for horses with septic synovial structures: 206 cases (2010–2015). Vet Microbiol. 2018;226:64–73. doi:10.1016/j.vetmic.2018.10.009
27. Li Y, Zhou Q, Liu Y, et al. Delayed treatment of septic arthritis in the neonate: a review of 52 cases. Medicine. 2016;95(51):e5682. doi:10.1097/MD.0000000000005682
28. Association WM. World Medical Association declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191–2194. doi:10.1001/jama.2013.281053
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
