Back to Journals » International Medical Case Reports Journal » Volume 13
Traumatic Mucormycosis of Auricular Cartilage in an Iranian Diabetic Patient
Authors Meidani M , Abtahi SH, Mohammadi R
Received 15 January 2020
Accepted for publication 11 March 2020
Published 18 March 2020 Volume 2020:13 Pages 95—99
DOI https://doi.org/10.2147/IMCRJ.S246072
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Ronald Prineas
Mohsen Meidani,1 Sayed Hamidreza Abtahi,2 Rasoul Mohammadi3
1Department of Infectious Diseases, Isfahan University of Medical Sciences, Isfahan, Iran; 2Department of Otolaryngology, Isfahan University of Medical Sciences, Isfahan, Iran; 3Department of Medical Parasitology and Mycology, School of Medicine, Infectious Diseases and Tropical Medicine Research Center, Isfahan University of Medical Sciences, Isfahan, Iran
Correspondence: Rasoul Mohammadi
Medical Mycology, Department of Medical Parasitology and Mycology, School of Medicine, Infectious Diseases and Tropical Medicine Research Center, Isfahan University of Medical Sciences, Isfahan, Iran
Tel + 983137929175
Fax + 983136688597
Email [email protected]
Abstract: Mucormycosis is an uncommon and acute fungal infection, with high morbidity and mortality. Traumatic mucormycosis mainly occurs in military conflicts, civilian trauma, and vehicle accidents. Hurricanes, tornadoes, floods, and tsunamis also play a major role in causing mucormycosis by inoculation. Herein, we presented a case of trauma-related mucormycosis in a 70-year-old diabetic male. He referred to a specialty clinic due to the auricular swelling after having fallen and having a major trauma in his ear. Pathologic examination of necrotic cartilage revealed broad ribbon like aseptate hyphae. Antifungal therapy with amphotericin B deoxycholate (1.5 mg/kg/day) was administered for 6 weeks as an initial therapy, and the patient was discharged with a regimen of posaconazole oral solution (400 mg PO bid with meals) for 8 weeks. He followed up for one year and there was no recurrence of the infection. In conclusion, traumatic mucormycosis is a rare but potentially life-threatening fungal infection. Early diagnosis and surgical excision are essential regarding the management of this critical condition. Knowing the underlying diseases is preferable to early diagnosis and timely initiation of antifungal therapy in order to improve survival rates.
Keywords: traumatic implantation, mucormycosis, auricular cartilage, diabetic patient, Iran
Introduction
Mucormycosis is a fatal fungal infection mainly occurring in patients with diabetes mellitus, hematological disorders, and solid organ transplant recipients. It might also involve immunocompetent patients after burn and trauma.1 The etiological agents belonging to the subphylum Mucoromycotina in the order Mucorales are ubiquitous environmental hyaline molds and produce airborne conidia which could often infect respiratory tract, sinuses, and wounds.2 Cutaneous mucormycosis is the third most prevailing type of this infection (10–19%) with the potential to spread haematogenously in patients with multiple predisposing factors, if it is left untreated.3 Traumatic mucormycosis predominantly occurs in military conflicts, civilian trauma, and vehicle accidents.4 Herein, we presented an auricular chondritis due to the order of Mucorales in a diabetic patient.
Case Presentation
A 70-year-old diabetic man with hypertension referred to Al-Zahra clinic, Isfahan, Iran, in account of the auricular swelling after having fallen and having an ear trauma. He had fallen down the staircase and his head had hit the fences. The patient was taking Atenolol, Aspirin, and Glibenclamide (10 mg/day). He had type 2 diabetes for about 18 years, and his disease was under control. There was a soft, red, and about 2×2 cm lesion on the left ear concha (Figure 1). Auricular hematoma was the first diagnosis; and surgical incision and drainage with bolster placement was performed with local anesthesia. After 3 times of incision and drainage, and improper responses to the treatment, the patient underwent general anesthesia; necrotic cartilage was removed then and sent for pathologic examination. Histopathologic evaluation revealed broad ribbon like aseptate hyphae (Figure 2). Due to unreachable AmBisome (amphotericin B liposomal), amphotericin B deoxycholate was prescribed for him following renal function test parameters, regarding its nephrotoxicity. The patient was treated successfully taking amphotericin B deoxycholate (1.5 mg/kg/day) for 6 weeks as the initial therapy and was discharged with a regimen of posaconazole oral solution (400 mg PO bid with meals) administrated for 8 weeks. He followed up for one year and healed up without any evidence of recurrence (Figure 3).
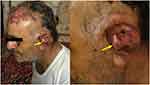 |
Figure 1 Auricular swelling of the left ear before surgical excision. |
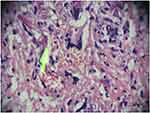 |
Figure 2 Broad aseptate hyphae (yellow arrow), typical for those species belonging to the Mucorales, Hematoxylin and Eosin (H&E) Stain, original magnification x 40. |
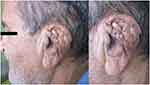 |
Figure 3 Left ear after surgical excision and antifungal therapy. |
Discussion
Mucormycosis is an uncommon, emerging fungal infection, with a high mortality rate among immunocompromised patients. In developed countries, even though the disease remains infrequent and is mainly seen in patients with hematological disorders, it is more prevalent in diabetic patients and in patients with a history of trauma.5 The prevalence of mucormycosis differs regionally from 0.01–0.2/100,000 population in the US and Europe6,7 to 14/100,000 population in India.5 The most common clinical manifestations of the infection are rhino-orbito-cerebral (27–34%), pulmonary (21–30%), cutaneous (20–26%), and disseminated (14–15%).8,9 Direct implantation of fungal conidia into the skin has been reported. Hurricanes, tornadoes, floods, and tsunamis play a major role in causing mucormycosis by inoculation of causative agents into the muscle, bones, and tendons.10–14 Corticosteroid therapy can increase the risk of cutaneous mucormycosis;15 however, the patient in the present case report had not taken any corticosteroid. Although trauma-related mucormycosis mainly occurs in adults, Kordy et al16 reported a case of traumatic mucormycosis due to the Apophysomyces elegans in a child from Saudi Arabia. The infection was caused by a car accident and tearing the deep soft tissue in the soil. The patient was treated with surgical excision and administration of liposomal amphotericin B. The principal strategies regarding the treatment of this infection is a reversal of underlying immune-impaired or metabolic conditions, glycemic control, resection of necrotic tissue, and timely empirical antifungal therapy with liposomal amphotericin B, posaconazole, or voriconazole.17,18 Even when fungal elements are seen in histopathologic examinations, cultures are only positive in 10–50% of cases.8 Coenocytic hyphae are fragile in nature, therefore, they might be damaged during tissue preparations.19 In the present case, we also could not isolate the etiological agents of infection on culture media. For a better recovery of zygomycetes on synthetic media, avoidance of excessive homogenization of tissues is recommended. Cunninghamella, Apophysomyces, and Rhizopus show high minimum inhibitory concentrations (MIC) against amphotericin B, and Mucor circinelloides reveals resistance against posaconazole,20,21 but the patient of the present case was treated successfully with conventional amphotericin B, and posaconazole as salvage therapy. Reduced susceptibility to antifungals is associated with necrosis of the affected tissues that prohibits penetration of antifungal drugs and immune cells into the infected areas.8 Optimal management of the disease depends on distinguishing the infection patterns, available distinctive symptoms, and curative options, which differ from region to region of the world. Daily doses of liposomal amphotericin B ranged from 1 mg/kg to 10 mg/kg;22 however, we treated our patient with a dose of 1.5 mg/kg/day of amphotericin B deoxycholate, because liposomal amphotericin B was unavailable due to the international sanctions. Amphotericin B deoxycholate has been the drug of choice for decades, yet its use is restricted by its remarkable nephrotoxicity, particularly in the doses and treatment periods needed for mucormycosis.22–24 Patients receiving 10 mg/kg/day, had significant serum creatinine increments that were mostly reversible.22,25 If fundamental kidney toxicity develops, the dose of antifungal can be decreased as necessary. However, doses below 5 mg/kg/day are suggested with marginal strength only.26 Kyvernitakis et al27 reported that combination therapy had no improved outcomes among 106 patients. Meanwhile, Reed et al28 represented a survival benefit of patients who were taking amphotericin B and caspofungin. Posaconazole oral suspension has been used successfully in first-line treatment, but, many important concerns due to its oral bioavailability led to the use of a delayed release pills with improved exposure and an intravenous infusion formulation.29 Posaconazole delayed release pills are suggested for salvage therapy, and if only they are available, they should be preferred over posaconazole oral suspension.22 In trauma-related mucormycosis, the trauma does not need to be immense. It could be caused by an insect bite, use of contaminated adhesive tape, and direct inoculation of a splinter to the cutaneous, subcutaneous or intramuscular tissues.30 Superficial cutaneous mucormycosis is usually caused in healthy individuals, and is characterized by pustules, vesicles, and less commonly, eschars. Patients usually recover with proper debridement and use of intravenous amphotericin B in less than one month.2,30
Conclusion
Traumatic mucormycosis is a rare but potentially life-threatening fungal infection. Early diagnosis and surgical excision are essential regarding the management of this critical condition. Knowing the underlying diseases is preferable to early diagnosis and timely initiation of antifungal therapy in order to improve survival rates.
Ethical Approval
All procedures performed in the studies involving human participants were in accordance with the ethical standards of the institutional Committee of Isfahan University of Medical Sciences.
Consent for Publication
Written informed consent was obtained from the patient for publication of data and images included in the present case report. Regarding the policies of Isfahan University of Medical Sciences, institutional approval was not required to publish this case report.
Acknowledgments
The authors appreciate Al-Zahra university hospital personnel for their cooperation.
Author Contributions
MM, and SHA clinically followed the patient and gave advice regarding the treatment of the patient. RM contributed to identifying the pathogen and drafted the manuscript. All authors contributed to the data analysis, and revising the article, they also gave the final approval of the version to be published, and agreed to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Roden MM, Zaoutis TE, Buchanan WL, et al. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin Infect Dis. 2005;41(5):634–653.
2. Garcia-Hermoso D, Criscuolo A, Lee SC, et al. Outbreak of invasive wound mucormycosis in a burn unit due to multiple strains of Mucor circinelloides f. circinelloides resolved by whole-genome sequencing. MBio. 2018;9(2):e00573–18. doi:10.1128/mBio.00573-18
3. Riley TT, Muzny CA, Swiatlo E, Legendre DP. Breaking the mold: a review of mucormycosis and current pharmacological treatment options. Ann Pharmacother. 2016;50(9):747–757. doi:10.1177/1060028016655425
4. Walsh TJ, Hospenthal DR, Petraitis V, Kontoyiannis DP. Necrotizing mucormycosis of wounds following combat injuries, natural disasters, burns, and other trauma. J Fungi. 2019;5(3):E57. doi:10.3390/jof5030057
5. Chakrabarti A, Singh R. Mucormycosis in India: unique features. Mycoses. 2014;57:85–90. doi:10.1111/myc.2014.57.issue-s3
6. Bitar D, Van Cauteren D, Lanternier F, et al. Increasing incidence of zygomycosis (mucormycosis), France, 1997–2006. Emerg Infect Dis. 2009;15(9):1395–1401. doi:10.3201/eid1509.090334
7. Rees JR, Pinner RW, Hajjeh RA, Brandt ME, Reingold AL. The epidemiological features of invasive mycotic infections in the San Francisco Bay area, 1992–1993: results of population-based laboratory active surveillance. Clin Infect Dis. 1998;27(5):1138–1147. doi:10.1093/clinids/27.5.1138
8. Skiada A, Lass-Floerl C, Klimko N, Ibrahim A, Roilides E, Petrikkos G. Challenges in the diagnosis and treatment of mucormycosis. Med Mycol. 2018;56(suppl_1):S93–S101. doi:10.1093/mmy/myx101
9. Skiada A, Pagano L, Groll A, et al. Zygomycosis in Europe: analysis of 230 cases accrued by the registry of the European Confederation of Medical Mycology (ECMM) Working Group on Zygomycosis between 2005 and 2007. Clin Microbiol Infect. 2011;17(12):1859–1867. doi:10.1111/j.1469-0691.2010.03456.x
10. Petrikkos G, Skiada A, Lortholary O, Roilides E, Walsh TJ, Kontoyiannis DP. Epidemiology and clinical manifestations of mucormycosis. Clin Infect Dis. 2012;54(suppl_1):S23–S34. doi:10.1093/cid/cir866
11. Neblett Fanfair R, Benedict K, Bos J, et al. Necrotizing cutaneous mucormycosis after a tornado in Joplin, Missouri, in 2011. N Engl J Med. 2012;367(23):
12. Maegele M, Gregor S, Yuecel N, et al. One year ago not business as usual: wound management, infection and psychoemotional control during tertiary medical care following the 2004 Tsunami disaster in southeast Asia. Crit Care. 2006;10(2):R50. doi:10.1186/cc4868
13. Andresen D, Donaldson A, Choo L, et al. Multifocal cutaneous mucormycosis complicating polymicrobial wound infections in a tsunami survivor from Sri Lanka. Lancet. 2005;365(9462):876–878. doi:10.1016/S0140-6736(05)71046-1
14. Patiño JF, Castro D, Valencia A, Morales P. Necrotizing soft tissue lesions after a volcanic cataclysm. World J Surg. 1991;15(2):240–247. doi:10.1007/BF01659059
15. Lionakis MS, Kontoyiannis DP. Glucocorticoids and invasive fungal infections. Lancet. 2003;362(9398):1828–1838. doi:10.1016/S0140-6736(03)14904-5
16. Kordy FN, Al-Mohsen IZ, Hashem F, Almodovar E, Al Hajjar S, Walsh TJ. Successful treatment of a child with posttraumatic necrotizing fasciitis caused by Apophysomyces elegans: case report and review of literature. Pediatr Infect Dis J. 2004;23(9):877–879. doi:10.1097/01.inf.0000136870.17071.fd
17. Rodriguez CJ, Tribble DR, Malone DL, et al. Treatment of suspected invasive fungal infection in war wounds. Mil Med. 2018;183(suppl_2):142–146. doi:10.1093/milmed/usy079
18. Epstein JB, Kupferman SB, Zabner R, et al. Early diagnosis and successful management of oral mucormycosis in a hematopoietic stem cell transplant recipient: case report and literature review. Support Care Cancer. 2016;24(8):
19. Walsh TJ, Gamaletsou MN, McGinnis MR, Hayden RT, Kontoyiannis DP. Early clinical and laboratory diagnosis of invasive pulmonary, extrapulmonary, and disseminated mucormycosis (zygomycosis). Clin Infect Dis. 2012;54(suppl_1):S55–S60. doi:10.1093/cid/cir868
20. Bonifaz A, Stchigel AM, Guarro J, et al. Primary cutaneous mucormycosis produced by the new species Apophysomyces mexicanus. J Clin Microbiol. 2014;52(12):
21. Vitale RG, de Hoog GS, Schwarz P, et al. Antifungal susceptibility and phylogeny of opportunistic members of the order mucorales. J Clin Microbiol. 2012;50(1):66–75. doi:10.1128/JCM.06133-11
22. Cornely OA, Alastruey-Izquierdo A, Arenz D, et al. Global guideline for the diagnosis and management of mucormycosis: an initiative of the European Confederation of Medical Mycology in cooperation with the Mycoses Study Group Education and Research Consortium. Lancet Infect Dis. 2019;19(12):e405–e421. doi:10.1016/S1473-3099(19)30312-3
23. Ullmann AJ, Sanz MA, Tramarin A, et al. Prospective study of amphotericin B formulations in immunocompromised patients in 4 European countries. Clin Infect Dis. 2006;43(4):e29–38. doi:10.1086/505969
24. Chakrabarti A, Chatterjee SS, Das A, et al. Invasive zygomycosis in India: experience in a tertiary care hospital. Postgrad Med J. 2009;85(1009):573–581. doi:10.1136/pgmj.2008.076463
25. Lanternier F, Poiree S, Elie C, et al. Prospective pilot study of high-dose (10 mg/kg/day) liposomal amphotericin B (L-AMB) for the initial treatment of mucormycosis. J Antimicrob Chemother. 2015;70(11):
26. Nosari A, Oreste P, Montillo M, et al. Mucormycosis in hematologic malignancies: an emerging fungal infection. Haematologica. 2000;85(10):1068–1071.
27. Kyvernitakis A, Torres HA, Jiang Y, Chamilos G, Lewis RE, Kontoyiannis DP. Initial use of combination treatment does not impact survival of 106 patients with haematologic malignancies and mucormycosis: a propensity score analysis. Clin Microbiol Infect. 2016;22(9):
28. Reed C, Bryant R, Ibrahim AS, et al. Combination polyene-caspofungin treatment of rhino-orbital-cerebral mucormycosis. Clin Infect Dis. 2008;47(3):364–371. doi:10.1086/591413
29. Duarte RF, Lopez-Jimenez J, Cornely OA, et al. Phase 1b study of new posaconazole tablet for prevention of invasive fungal infections in high-risk patients with neutropenia. Antimicrob Agents Chemother. 2014;58(10):
30. Losee JE, Selber J, Vega S, Hall C, Scott G, Serletti JM. Primary cutaneous mucormycosis: guide to surgical management. Ann Plast Surg. 2002;49(4):385–390. doi:10.1097/00000637-200210000-00009
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
