Back to Journals » Research and Reports in Urology » Volume 11
Transurethral resection of a large bladder hemangioma of mixed histological composition: a case report
Authors Martov AG, Pominalnaya VM, Kurkov AV , Baykov NA, Gomzikova EA, Fayzullin AL , Balykov IS , Ergakov DV
Received 20 December 2018
Accepted for publication 15 April 2019
Published 6 June 2019 Volume 2019:11 Pages 175—178
DOI https://doi.org/10.2147/RRU.S198854
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jan Colli
Video abstract presented by Alexey G Martov.
Views: 3798
Alexey G Martov,1,2 Viktoriya M Pominalnaya,3 Alexandr V Kurkov,3,4 Nikolay A Baykov,1,2 Ekaterina A Gomzikova,3 Alexey L Fayzullin,4 Ilya S Balykov,1,2 Dmitry V Ergakov1,2
1Department of Urology and Andrology, IPPE of A.I. Burnazyan SSC FMBC, Federal Biomedical Agency of Russia, Moscow, Russia; 2Department of Urology №2, D.D. Pletnev City Clinical Hospital, Moscow Health Department, Moscow, Russia; 3Department of Anatomic Pathology, D.D. Pletnev City Clinical Hospital, Moscow Health Department, Moscow, Russia; 4Institute for Regenerative Medicine, Sechenov University, Ministry of Health, Moscow, Russia
Abstract: Hemangioma is a rare benign vascular tumor of the bladder, which occurs mainly in children. It has no specific clinical symptoms but can result in severe and fatal complications as well as relapse. In the current clinical observation, a 35-year-old patient had a large solid tumor of the bladder spreading into the muscular layer. In histological and immunohistochemical analyses, verified hemangioma consisted of capillary, cavernous and arterio-venous components. The patient underwent transurethral resection of the bladder using computer chromoendoscopy. It is the first to the best of our knowledge complete transurethral removal of 3 cm in diameter bladder hemangioma.
Keywords: hemangioma, transurethral resection, urinary bladder, urinary bladder neoplasms
Introduction
Hemangioma is a benign vascular tumor that accounts for 0.6% of all bladder tumors.1,2 The tumor occurs more often in the first two decades of life.3,4 It can present as a single tumor or be combined with hemangiomas of other localizations (Klippel–Trenaunay and Sturge–Weber syndromes).1,5 Hemangioma manifests itself as asymptomatic macrohematuria; however, large tumors can be accompanied by life-threatening bleedings.1,6 This extremely rare tumor should be differentiated with various diseases of the bladder, including malignant neoplasms.5,7,8 The tumor requires a multidisciplinary approach and histological verification of the diagnosis. Dynamic observation of these cases is necessary because of the ability to relapse and cause complications in the postoperative period.
Case report
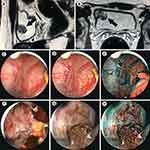
A 35-years-old man came to D.D. Pletnev City Clinical Hospital in 2017 complaining about episodic spontaneous blood admixtures in the urine, stopping independently, and cutting pain during urination. Two years before, he received a sports injury to the lower third of the anterior abdominal wall. Bladder tumor was suspected after a visit to a urologist at the place of residence. Cystoscopy revealed a solid tumor with a diameter of 3 cm. Unchanged blood was obtained with a puncture of the tumor, and a pinch biopsy of the tumor was also performed. Histological examination did not reveal tumor tissue, after which the patient was released under dynamic observation. Episodes of macrohematuria reoccurred in 2017. Following that, MRI revealed a large mass in the right lateral wall of the bladder, 2.6×2.1 cm in size (Figure 1A, B). The muscle layer in the tumor area was deformed and had a hyperintensive MR-signal in the DWI mode. The patient was suspected of having a muscle-invasive bladder tumor.
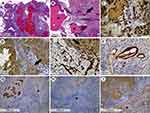
During videoendoscopic transurethral resection of the bladder using computer chromoendoscopy (SPIES, Storz Professional Imaging Enhancement System, resectoscope 26F), a solid 3 cm in diameter tumor was found on the right lateral wall of the bladder at a distance of 3 cm from the right ureteral orifice. The tumor had a wide base and ulcerative defects covered with blood clots (Figure 1C). The dilated vessels of different caliber were observed in submucosal layer (Figure 1D) in computer-enhanced contrast mode in conditions of more uniform illumination («Clara-Chroma»®, Karl Storz, Germany). Pathological vascularization common for urothelial carcinomas was not found in the tumor, its base or bladder mucosa (Figure 1E) by computer modulation of a light beam (SPECTRA B®, Karl Storz, Germany). A bladder transurethral resection of exophytic part of the tumor was performed (Figure 1F). Hemorrhage zones were located in the tumor bed by white light and by the mode of computer chromoendoscopy (Figure 1G, H). Moreover, there were visual signs of tumor pseudocapsule. Further, a separate removal of the tumor base located inside the bladder wall was performed. The final stage of the operation was hemostasis. In histological and immunohistochemical studies, the bladder tumor was shown to be a hemangioma of mixed histological structure (Figure 2).
The postoperative period proceeded without complications. No signs of relapse were observed at follow-up examinations (ultrasound, MRI, cystoscopy) at 3, 6 and 12 months after the operation.
Discussion
Bladder hemangiomas used to be diagnosed so rarely that we know of only several dozens of histologically verified cases in the late century.5 However, a timely diagnosis of hemangioma is important due to a positive prognosis comparing to muscle invasive bladder cancer.5 The conventional ways of surgical treatment of bladder hemangiomas include biopsy with or without fulguration, transurethral resection, partial cystectomy and embolization.5,6,9–11 The development of visualization techniques and physician awareness of hemangioma led to an increase in the number of case reports covering the application of new surgical methods.9,11 However, there are no clinical guidelines for large bladder hemangiomas.
In the presented case report, the size and localization of the tumor were too excessive for open or laparoscopic bladder resections. Moreover, there was a discordance between the hemangioma size determined by MRI and that observed by chromoendoscopy on account of heterogenous structure of the tumor. To our knowledge, this is the first reported application of computer-enhanced endoscopy for the removal of a large bladder hemangioma. The chromoendoscopic intraoperation control made possible a complete transurethral resection of the tumor without an extraperitoneal perforation of the bladder or an involvement of the right ureteral orifice in the section. Separate resections of the exophytic part of the tumor and its base were performed to confirm complete removal of the tumor through a high-resolution in situ visualization of blood vessels in the hemangioma. Immunohistochemistry revealed a unique simultaneous presence of capillary, cavernous and arterio-venous components in bladder hemangioma that has not been described previously in the literature (see Figure 2). We suspect that the sports injury in anamnesis caused gross hematuria and contributed to the tumor’s heterogenous histological structure.
In summary, we recommend performing transurethral resections with chromoendoscopy to treat large bladder hemangiomas with hard-to-reach localization. In order to study bladder hemangiomas, we make a proposition for urologists to collect separate biopsies of the exophytic part and the tumor base and examine by immunohistochemistry for alfa-SMA and at least one endothelial marker (eg, CD31, CD34). Future cases of mixed hemangiomas can help to establish clinical guidelines for diagnosis, treatment and follow-up of these patients.
Acknowledgments
This work was sponsored by Russian academic excellence project “5-100”. Patient provided a written informed consent for publication of the case details. The hospital’s approval was received to publish the case details.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Moch H, Humphrey P, Ulbright T. WHO Classification of Tumors of the Urinary and Male Genital Organs. Lyon, France: IARC Press; 2016.
2. Grignon DJ. Neoplasms of the urinary bladder. In: Bostwich DG, Eple NJ, editors. Urol Surg Pathol. St. Louis: Mosby; 1997:214–305.
3. Rosenberg J, Golimbu M, Suarez J, Morales P. Congenital arteriovenous malformation of the bladder. J Urol. 1973;109(4):605–608. doi:10.1016/S0022-5347(17)60492-0
4. Hendry W, Vinnicombe J. Haemangioma of bladder in children and young adults. Br J Urol. 1971;43(3):309–316. doi:10.1111/bju.1971.43.issue-3
5. Cheng L, Nascimento AG, Neumann RM, et al. Hemangioma of the urinary bladder. Cancer. 1999;86(3):498–504. doi:10.1002/(SICI)1097-0142(19990801)86:3<498::AID-CNCR19>3.0.CO;2-6
6. Abdullaev FK, Nikolaev VV, Kulaev VD, Cherkashina EN. [Urinary bladder hemangiomas in children: experience with endoscopic treatment]. Urologiia. 2011;1(6):46–49.
7. Caro DJ, Brown JS. Hemangioma of bladder. Urology. 1976;8(5):479–481. doi:10.1016/0090-4295(76)90281-8
8. Wong-You-Cheong JJ, Woodward PJ, Manning MA, Davis CJ. From the archives of the AFIP: inflammatory and nonneoplastic bladder masses: radiologic-pathologic correlation. Radiographics. 2006;26(6):1847–1868. doi:10.1148/rg.e24
9. Takemoto J, Yamazaki Y, Sakai K. A case of large bladder hemangioma successfully treated with endoscopic yttrium aluminium garnet laser irradiation. Int J Urol. 2011;18(12):854–856. doi:10.1111/j.1442-2042.2010.02670.x
10. Mor Y, Hitchcock RJ, Zaidi SZ, et al. Bladder hemangioma as a cause of massive hematuria in a child. A case report and literature review. Scand J Urol Nephrol. 1997;31(3):305–307. doi:10.3109/00365599709070355
11. Hu X, Deng K. Bladder cavernous hemangioma after pelvic radiotherapy in a female patient: A case report and literature review. Int J Surg Case Rep. 2018;53:479–482. doi:10.1016/j.ijscr.2018.11.044
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.