Back to Journals » International Journal of General Medicine » Volume 15
Transperitoneal Laparoscopic Unroofing versus Fenestration Under Seminal Vesiculoscopy for Seminal Vesicle Cyst, a Multi-Institutional Retrospective Cohort Study
Authors Ding K, Wang W , Kang Y, Zhang L, Tan S, Tang Z
Received 5 March 2022
Accepted for publication 23 May 2022
Published 7 June 2022 Volume 2022:15 Pages 5547—5556
DOI https://doi.org/10.2147/IJGM.S365210
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Ke Ding,1 Wei Wang,2 Ye Kang,3 Lei Zhang,2 Shuo Tan,1 Zhengyan Tang1
1The Department of Urology, Xiangya Hospital, Central South University, Changsha, Hunan, People’s Republic of China; 2The Department of Urology, the Second Xiangya Hospital, Central South University, Changsha, Hunan, People’s Republic of China; 3The Department of Urology, Hunan Provincial People’s Hospital, Hunan Normal University, Changsha, Hunan, People’s Republic of China
Correspondence: Shuo Tan; Zhengyan Tang, Department of Urology, Xiangya Hospital, Central South University, 87 Xiangya Street, Changsha, Hunan, 410008, People’s Republic of China, Tel +8615273133018 ; +8613507318268, Email [email protected]; [email protected]
Background: To illustrate the transperitoneal laparoscopic unroofing (TLU) and compare the efficacy and safety of TLU to fenestration under seminal vesiculoscopy (FUSV) in treating symptomatic seminal vesicle cyst (SVC).
Methods: We retrospectively reviewed all patients with symptomatic SVC who underwent TLU or FUSV between 2008 and 2020 at 3 institutions in Hunan. The two groups were evaluated with reference to radiological failure-free survival (R-FFS), fertility outcome, symptoms, and complications at a median 33.5-month follow-up.
Results: Of the 98 males, 58 (59.2%) received TLU, and 40 (40.8%) underwent FUSV. Baseline characteristics were comparable. Semen analysis, prostatitis-like symptoms, and the maximum diameter of cyst were partially improved after both surgeries at 12-month follow-up. The TLU groups suggested a higher incidence rate of fertility for SVC patients with comorbid infertility compared with the FUSV group (82.4% vs 70.3%, p = 0.041), as well as better R-FFS of cysts at five-year follow-up (Log rank test, p = 0.021). In addition, the number of patients with NIH-CPSI (National Institutes of Health Chronic Prostatitis Symptom Index) scores higher than 15 decreased more significantly after TLU (p = 0.004). Except for hematospermia within 3 months, no significant difference in adverse events was observed in the two groups during the long-term follow-up.
Conclusion: TLU was superior for patients with large and symptomatic SVC to FUSV, with more relieved symptoms, better R-FFS of cysts and fertility outcomes.
Registration Number of Clinical Trial: ChiCTR2100053850 in Chinese Clinical Trial Registry Platform (ChiCTR).
Keywords: seminal vesicle cyst, laparoscopic unroofing, seminal vesiculoscopy, radiological failure-free survival, male infertility
Introduction
Seminal vesicle cyst (SVC) is a rare disease in andrology, and the incidence of which is reported to be around 0.005% in males.1 The clinical symptoms mainly include pain and lower urinary tract symptoms (LUTS) as well as other prostatitis-like symptoms.2 In addition, diminished volume ejaculate, hematospermia, epididymitis and infertility appear frequently along with SVC due to obstruction caused by compression of cysts.2,3 Watchful waiting and conservative therapy are not considered appropriate for large and symptomatic SVC.3,4 Transurethral/transrectal aspiration were thought to just alleviate symptoms or be used for diagnosis only with a less than 30% successrate.5–8 These patients thus may benefit from open surgery and sporadic cases were reported with nearly 100% cure rates in short-term follow-up.2,7 Besides, invasive surgeries remain the optimal choice for SVC patients with ipsilateral renal agenesis, especially for some of those requiring nephrectomy.2 However, the extensive trauma and surgery-related complications warrant safer and more effective alternatives. Recently, some minimally invasive approaches including robot-assisted/laparoscopic unroofing or excision as well as fenestration under seminal vesiculoscopy have been reported effective for SVC or cysts from other mesonephric duct derivates.8–10 Nevertheless, limited studies described the outcomes of minimally invasive interventions due to its relatively low incidence.3,11 Here we provide a comparative analysis between TLU and FUSV regarding the functional and fertility outcomes as well as safety profiles.
Materials and Methods
Patients
Between 2008 to 2020, we retrospectively included 115 patients from 3 centers in China who underwent TLU and FUSV for SVC. Figure 1 shows the inclusion and exclusion criteria in the study. In total, 98 patients were identified for analysis eventually, of whom 58 underwent TLU while 40 received FUSV. Detailed medical history and physical examination were recorded in all patients, especially the family history and digital rectal examination (DRE). The fertility status was identified, and preoperative magnetic resonance imaging (MRI) was applied for evaluation to the urogenital system as well as computed tomography if indicated. All the semen analyses were under the guidance of WHO Laboratory Manual for the Examination and Processing of Human Semen (5th edn). Furthermore, prostatitis-like symptoms were quantitatively assessed using National Institutes of Health Chronic Prostatitis Symptom Index (NIH-CPSI) before and after surgery. Preoperative cystourethroscopy was conducted if hematuria/hematospermia induced by non-SVC disease was suspected. Besides, preoperative transurethral biopsy or ultrasound-guided transrectal needle biopsies were performed if radiologic findings indicated malignant cysts. Most patients presented no response to conservative therapy including anticholinergic medication, non-steroidal anti-inflammatory drugs, and pelvic floor therapy.
 |
Figure 1 Patient selection flow chart. *Patients may have had more than one reason for exclusion. |
Interventions
Three senior chief surgeons conducted the two procedures under general anesthesia as we previously reported.12 Before TLU, patients were placed in a lithotomy position for cystoscopy. A 5 Fr ureteral catheter was placed into ipsilateral ureter to avoid iatrogenic injury for patients without ureteral ectopia. After the patient was changed into supine position, pneumoperitoneum is achieved at 12 mmHg through Veress needle which was introduced via an infraumbilical incision. A 12-mm trocar for a 30-degree lens replaced the Veress needle. Then, the patient position was transferred to Trendelenburg position. A 5 mm and a 12 mm trocar were punctured in 1 cm below the camera port at the left or right mid clavicular line, and a 5 mm port used for assistance. Next, retrovesical peritoneal reflection was incised and the ipsilateral vas deferens duct was identified, which was used as a guidance to SV. The dissection of SVC was carried out as far as possible down to its base to ensure better exposure. The cyst was then punctured and cystic fluid was thoroughly aspirated. Finally, the cystic wall was excised close to base of SVC using an ultrasound knife and the resection margins was electrocoagulated to achieve more reliable hemostasis. Caution was taken to preserve the neurovascular bundle and avoid injury to rectum or ureter. A pelvic drainage tube was placed for 24–48 hours after surgery, and the Foley catheter was removed within 1 day.
Before FUSV, patients were placed in a lithotomy position for evaluation to lower urinary tract under 6.0 Fr pediatric ureteroscope. Having identified the verumontanum and utricular lumen, the ureteroscope was inserted through the opening under the guidance of a 3.0 Fr ureteral catheter. Generally, the opening of the ejaculatory duct was then easily identified but some patients required punctuation through a thin membrane using a ureteral catheter to establish the tunnel from utricle lumen to SV. If an obstruction of ejaculatory duct was observed, part of the verumontanum was resected for subsequent procedures. The cavity was examined for cyst, calculi, floccules, and blood clots. Then, fenestration was carried out with a holmium laser and the cavity was irrigated with antibiotic saline. After adequate hemostasis, a 22 Fr Foley catheter was indwelled and removed within 48–72 hours.
Follow-Up and Outcomes
All patients were re-checked for semen analyses as well as symptoms, and cysts were monitored using CT (or MRI if necessary) at 12-month follow-up. A radiographic evaluation was modified according to the criteria established by Yoder and Wolf. Postoperative radiographic success was defined as no recurrence of cyst after complete resection, and partial success as no more than 5 mm increase in maximum diameter of remnant cyst compared with the first postoperative examination to cyst within 30 days. Thus, all other cases were regarded as radiological failures. Besides, a maximum diameter of regrown cyst which was bigger than half of the preoperative diameter was still considered a failure. The primary outcomes were fertility outcomes and radiological failure-free survival (R-FFS) during a median 33.5-month follow-up.
Statistical Analysis
Statistical analyses were processed by SPSS, version 22.0 (SPSS Inc., IBM Corp, USA). Categorical characteristics were analyzed by Pearson’s Chi-squared or Fisher’s exact tests as appropriate. Continuous variables were compared with Mann–Whitney U-tests. Kaplan–Meier curves of RFS and cumulative incidence of fertility were compared using the standard Log rank test. A value of p <0.05 was considered statistically significant (two-tailed).
Results
Patient Characteristics
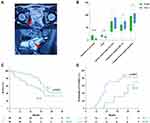
Among 98 male patients with symptomatic SVC (Figure 2A), the median age was 30.0 years (interquartile range [IQR]: 25.0 to 37.3), and 40 (40.8%) underwent FUSV while 58 (59.2%) received TLU (Table 1). 31 (31.6%) of all patients were still combined with unilateral renal agenesis although 8 patients were excluded due to surgical removal of the ipsilateral kidney without function. 68 (69.4%) and 39 (39.8%) patients failed conservative therapy and transrectal ultrasound-guided aspiration, respectively. There were no significant differences in preoperative semen analysis between the two groups. However, the FUSV group showed shorter operative time and hospital stay than the TLU group (p < 0.001).
 |
Table 1 Baseline Characteristics and Perioperative Outcomes for TLU versus FUSV |
Efficacy
Both groups demonstrated a significant decrease in the maximum diameter of cysts as well as the NIH-CPSI score at 12-month follow-up (Table 2, Figure 2B). 34 (58.6%) and 22 (37.9%) patients who underwent TLU respectively achieved radiographic success and partial success in comparison with 14 (35.0%) and 17 (42.5%) of patients after FUSV at month 12 (p=0.025, and p = 0.679, respectively) (Table 2). Alleviation in prostatitis-like symptoms was observed in 32, i.e. more than half of patients (55.2%) after TLU while only 10 (25%) patients remained responsive to FUSV 12 months after surgery. No significant improvements in sperm concentration and progressive motility were observed, whereas the seminal fructose showed a remarkable increase from baseline, especially in the TLU group (88.9 vs 65.0 μmol/ejaculate, p < 0.001, respectively). Besides, only 3 (5.2%) patients exhibited seminal fructose less than 13 μmol/ejaculate after TLU, compared with 10 (25.0%) patients in the FUSV group. Although no statistical significance in progressive motility of sperm was shown in the two groups, there were still significantly fewer patients who were identified as having asthenozoospermia after TLU than FUSV, considering the comparable preoperative asthenozoospermia (6.9% vs 25.0%, p = 0.026, respectively).
 |
Table 2 Comparison of Functional Outcomes at 12-Month Follow-Up Between TLU and FUSV |
During the median 33.5-month follow-up (IQR: 22.8–44.3), patients in the FLU group had better 5-year R-FFS than the FUSV group (Log rank test, p = 0.021) (Figure 2C). The 2-year and 5-year R-FFS rate was 89.3% and 61.5%, 42.0% and 33.1% for TLU and FUSV, respectively. Among 30 patients who were preoperatively diagnosed with infertility, patients after TLU showed a higher incidence rate of fertility against the FUSV group (Log rank test, p = 0.041) (Figure 2D).
Adverse Events
By the follow-up, 25 (25.5%) of all patients encountered adverse events which were classified by the Clavien-Dindo scale (Table 3). Except that recurrent/persistent hematospermia occurred in 8 (20.0%) patients within the first 3 months after FUSV compared with 1 (1.7%) patient after TLU, the incidence rates of all other complications showed no differences.
 |
Table 3 Postoperative Adverse Events Classified by Clavien-Dindo Scale |
Discussion
Seminal vesicle cyst can be congenital or acquired, and the former is usually accompanied by unilateral renal agenesis (Zinner syndrome) and heterotopic vas deferens.13 These urinary and reproductive malformations generally arise from an abnormality of the mesonephric duct.14 Symeonidis et al emphasized that physicians should consider Zinner syndrome when a solitary kidney was observed in patients with SVCs.15 Besides, a detailed radiologic evaluation of the genitourinary system, including CT and MRI, is warranted in the presence of kidney anomalies. They also stated that Zinner syndrome could be incidentally detected during a radiologic workup and warrants further awareness. Both urologists and radiologists should maintain a high index of suspicion even when incidentally detected, thus advocating the need for long-term follow-up and the potential subsequent therapy in case of clinical deterioration and infertility. Also, it can be acquired due to inflammation, ejaculation obstructions, stones in the male reproductive system, and prostate surgeries.16 Asymptomatic SVC generally achieved good clinical results by watchful waiting and monitoring under transrectal ultrasonography (TRUS).2,15 However, some patients exhibit prostatitis-like symptoms including pelvic pain as well as LUTS.2,8 As the firstly considered treatment, conservative therapy includes α1-blockers, muscarinic receptor antagonists, non-steroidal anti-inflammatory drugs, and pelvic floor therapy for SVC. For patients who have comorbid benign prostate hyperplasia and/or associated infection, 5α-reductase inhibitors and/or cultural-based antibiotics are needed.2,8,16 Although it presented an only partial response to symptomatic SVC as reported by Ouden, conservative therapy is still recommended before any invasive interventions, especially for patients with mild to moderate symptoms.2
Surgeries are recommended for patients who failed conservative therapy and aspiration under TRUS guidance in treating large and symptomatic SVCs. Various procedures have been reported.2,4,8,17 Although the high success rate of open surgery was observed, the associated and considerable complications including large abdominal incision, rectal and bladder injuries made minimally invasive alternatives imperative.17,18 After first performed in managing retrovesical cysts (RV) in the 1990s, transperitoneal laparoscopic unroofing (TLU) or excision was reported with the same acceptable outcomes as well as fewer associated adverse events in treating SVC.4,5,8,10,16 In addition, transurethral seminal vesiculoscopy was recently introduced to various seminal diseases such as hematospermia, seminal vesicle stones, or seminal vesiculitis and achieved reliable and effective results.19 Electrocautery or laser were later used under seminal vesiculoscopy for fenestration of SVC and also obtained satisfactory efficacy in limited series.11,20,21 Wang et al showed a symptom relief, reduced size or absence of cyst, and no recurrence within 6-month follow-up in 7 cases after FUSV.11 Our series showed similar outcomes due to a significant NIH-CPSI decrease as well as a relief of obstructive symptoms, and satisfactory cysts control as the relatively high rate of R-FFS in the TLU and FUSV group.
However, the comparison of these two minimally invasive interventions was rarely reported. It is worth noting that both the 2-year or 5-year R-FFS in the TLU group was better than the FUSV group, as well as the radiographic evaluation. The incidence of complete resection after TLU was significantly more than FUSV, according to radiographic success rate at 12-month follow-up (58.6% vs 35.0%, p = 0.025, respectively) for SVC, and it is speculated that the residual cysts wall might increase the risk of postoperative radiographic failure. Ouden et al also showed a 25% failure rate of patients undergoing transurethral fenestration in SVC patients, requiring further open surgeries.2 And yet most of the literatures demonstrated a nearly 100% success rate within a short-term follow-up after TLU in treating SVC.3,4,16 In our series, none of the 2 patients who failed after TLU required further interventions for the persistent relieved symptoms, while 5 of 10 cases who failed within 6 months after FUSV received further therapy including medication, aspiration, and even invasive surgery. The TLU procedure may presumably provide excellent operation visualization, flexible surgical instruments, and various surgical routes to guarantee complete resection of the cyst wall, despite the deeply hidden cysts in pelvic cavity. In contrast, it is difficult for the laser fiber to reach the deep cyst wall under the guided seminal vesiculoscopy, particularly with an acute angle to the cyst opening during the FUSV procedure. Thus, the remnant cyst wall and its secretory activity may be the reason for recurrence similar to simple renal cysts.22
Our results indicated the possible superiority of TLU over FUSV in relieving prostatitis-like symptoms were mainly due to better cysts control. However, the symptoms persisted in more than half of patients at the 12th month after surgeries. Rare studies evaluated and described the prostatitis-like symptoms of SVC patients, and we speculate that the cyst-induced pelvic pain and LUTS may not be eliminated due to persistence of the chronic inflammation, pelvic floor dysfunction, infections, and comorbidities in lower urinary tract. Furthermore, our results revealed more improved seminal fructose and relieved postoperative asthenozoospermia, and a higher cumulative incidence rate of fertility after TLU (p = 0.003, p = 0.014, p = 0.041, respectively) despite the comparable sperm concentration in both groups (p = 0.638). Similarly, Benyó et al have claimed a remarkable improvement of total motile sperm count at 18-month follow-up after TLU, and Valla et al have also reported an infant who had preserved fertility after laparoscopic excision of a congenital seminal vesicle cyst. These positive outcomes may be attributed to the disappeared obstruction and compression to SV, just as acceptable fertile outcomes after eliminating obstruction of the seminal pathways at the level of the prostate gland in treating RV, which were previously reported by Schroeder et al23
In terms of adverse events, no higher risk rate was observed in the TLU group despite the larger incision, longer operation time as well as hospital stay. Besides, the increased operation time and hospital stay were still acceptable due to the adequate exposure of pelvic seminal cyst under laparoscopy and indwelled pelvic drainage, which increased the risk of perioperative infection. The estimated blood loss was too little to be evaluated during FUSV, and the TLU procedure also reported a relatively low blood loss (ranging from 5–20 mL) because of the minimal invasion of the two surgeries.2,4 No grade IV or V complications were shown in our study. Of note is that ureteral and rectal injuries appeared in both groups, thus requiring more careful resection and prevention of deep incision and thermal damage even with the guidance of seminal vesiculoscopy. Besides, complete or adequate exposure of SVC under TLU may be helpful to avoid iatrogenic injury.
The retrospective nature and relatively small sample size may be the main limitations of the present study. Potential bias may exist in the semen analysis for the measurement variability despite the consecutive 2 analyses being conducted for a more precise assessment. A more convincing elucidation of the comparison of TLU and FUSV thus warrants a larger pool of cases, prospective, and randomized controlled design. However, we provided a relatively larger series and longer follow-up than recent literature, and the strict definition of parameters as well as systematic analysis in outcomes also reduced these potential biases.
Conclusions
TLU is superior to FUSV for the higher success rate of cyst control, more relieved prostatitis-like symptoms, and better infertility outcomes in treating symptomatic SVC after failed conservative therapy. Meanwhile, TLU can be a safe procedure with acceptable incidence rates of adverse events. In addition, careful patient selection and avoidance of iatrogenic injury are still required during treatment.
Data Sharing Statement
The authors intend to share personally unidentified participant data, including data related to characteristics and functional parameters. All the data and further inquiries can be directed to Shuo Tan and Zhengyan Tang from the day the article is published.
Ethical Approval and Informed Consent
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Ethics committee approval was obtained at Xiangya hospital, Second Xiangya hospital, Hunan Provincial People’s Hospital (reference number: 2021101094, 2021k045, and 2021k76) and individual consent for this retrospective analysis was waived. The ethic committees approved the request to waive the informed consent. The research presented no risk of harm to subjects and involved no procedures for which written consent is normally required for this retrospective study. All data were anonymized or maintained with confidentiality.
Acknowledgments
The authors acknowledge the clinical staff for their help to investigate the present study.
Disclosure
The authors have no conflicts of interest to disclose.
References
1. Sheih C, Hung C, Wei C, Lin C. Cystic dilatations within the pelvis in patients with ipsilateral renal agenesis or dysplasia. J Urol. 1990;144:324–327. doi:10.1016/s0022-5347(17)
2. van den Ouden D, Blom J, Bangma C, de Spiegeleer A. Diagnosis and management of seminal vesicle cysts associated with ipsilateral renal agenesis: a pooled analysis of 52 cases. Eur Urol. 1998;33(5):433–440. doi:10.1159/000019632
3. Kord E, Zisman A, Darawsha A, et al. Minimally Invasive Approach for Treatment of Seminal Vesicle Cyst Associated with Ipsilateral Renal Agenesis. Urol Int. 2017;99(3):338–342. doi:10.1159/000464298
4. Zhang D, Li X, Gao Y, et al. Transperitoneal laparoscopic excision of seminal vesicle cyst: a single-center experience. J Endourol. 2012;26(9):1153–1158. doi:10.1089/end.2012.0071
5. Coşkun B, Dalkılıç A, Sönmez N, et al. A case of seminal vesicle cyst associated with ipsilateral renal agenesis diagnosed during an investigation of urinary incontinence. Turkish j Urol. 2013;39(1):53–55. doi:10.5152/tud.2013.011
6. Fujinaga S, Hirano D, Hara S, et al. Seminal vesicle abscesses associated with ipsilateral multicystic dysplastic kidney in an infant. Pediatric Nephrology. 2008;23(9):1551–1554. doi:10.1007/s00467-008-0839-5
7. Fuselier JHA, Peters DH. Cyst of seminal vesicle with ipsilateral renal agenesis and ectopic ureter: case report. J Urol. 1976;116(6):833–835.
8. Hong Y, Onal B, Diamond D, et al. Robot-assisted laparoscopic excision of symptomatic retrovesical cysts in boys and young adults. J Urol. 2011;186(6):2372–2378. doi:10.1016/j.juro.2011.07.113
9. Haddock P, Wagner J. Seminal vesicle cyst with ipsilateral renal agenesis and ectopic ureter (Zinner syndrome). Urology. 2015;85(5):e41–e42. doi:10.1016/j.urology.2015.02.015
10. Basillote J, Shanberg A, Woo D, et al. Laparoscopic excision of a seminal vesicle cyst in a child. J Urol. 2004;171(1):369–371. doi:10.1097/01.ju.0000102300.07368.5c
11. Wang M, Li B, Huang Z, et al. Transurethral endoscopic treatment of seminal vesicle cysts (report of seven cases). Int Urol Nephrol. 2015;47(5):717–721. doi:10.1007/s11255-015-0944-x
12. Xue R, Tang Z, Chen Z, Huang L. Clinical outcomes of transperitoneal laparoscopic unroofing and fenestration under seminal vesiculoscopy for seminal vesicle cysts. Asian J Androl. 2018;20(6):621–625. doi:10.4103/aja.aja_62_18
13. Tan Z, Li B, Zhang L, et al. Classifying seminal vesicle cysts in the diagnosis and treatment of Zinner syndrome: a report of six cases and review of available literature. Andrologia. 2020;52(1):e13397. doi:10.1111/and.13397
14. Meiraz D, Fischelovitch J, Lazebnik J. Agenesis of the kidney associated with congenital malformation of the seminal vesicle. Br J Urol. 1973;45(5):541–544. doi:10.1111/j.1464-410x.1973.tb06818.x
15. Symeonidis EN, Gkekas C, Tsifountoudis I, et al. Incidental finding of Zinner syndrome in a Greek military recruit: a case report of a rare clinical entity. Mil Med Res. 2019;6(1):4. doi:10.1186/s40779-019-0194-9
16. Hou Y, Hu X, Duan Y, Tan W, Guo X. Laparoscopic treatment of a giant seminal vesicle cyst with hemorrhage: a case report. Medicine. 2021;100(21):e26142. doi:10.1097/md.0000000000026142
17. Kreager J, Jordan W. Transcoccygeal approach to the seminal vesicles. Am Surg. 1965;31:126–127.
18. Okur H, Gough D. Management of müllerian duct remnants. Urology. 2003;61(3):634–637. doi:10.1016/s0090-4295(02
19. Han C, Liang Q, Dong B, et al. The transurethral seminal vesiculoscopy in the diagnosis and treatment of the seminal vesicle disease. Cell Biochem Biophys. 2013;66(3):851–853. doi:10.1007/s12013-013-9527-6
20. Okoye B, Jones D, Lancashire M, Brown E, Ritchie A. Transvesical endoscopic drainage of a seminal vesicle cyst. Br J Urol. 1995;76(6):810. doi:10.1111/j.1464-410x.1995.tb00787.x
21. Gözen A, Alagöl B. Endoscopic management of seminal-vesical cyst with right renal agenesis causing acute urinary retention: case report. J Endourol. 2006;20(11):919–922. doi:10.1089/end.2006.20.919
22. Shiraishi K, Eguchi S, Mohri J, Kamiryo Y. Laparoscopic decortication of symptomatic simple renal cysts: 10-year experience from one institution. BJU Int. 2006;98(2):405–408. doi:10.1111/j.1464-410X.2006.06249.x
23. Schroeder-Printzen I, Ludwig M, Köhn F, Weidner W. Surgical therapy in infertile men with ejaculatory duct obstruction: technique and outcome of a standardized surgical approach. Human Reproduction. 2000;15(6):1364–1368. doi:10.1093/humrep/15.6.1364
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.