Back to Journals » Veterinary Medicine: Research and Reports » Volume 10
Transient methemoglobinemia suspected secondary to ingestion of Brassica species in a dog
Authors Hendricks J, Gates K
Received 21 November 2018
Accepted for publication 14 February 2019
Published 29 April 2019 Volume 2019:10 Pages 37—42
DOI https://doi.org/10.2147/VMRR.S195458
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Young Lyoo
Jeanette Hendricks, Kathryn Gates
Department of Emergency and Critical Care, Advanced Critical Care Emergency and Specialty Services, Culver City, CA, USA
Abstract: This case report describes methemoglobinemia in a dog suspected to be the result of consumption of a large volume of fermented bok choy. The patient presented with clinical signs and co-oximetry consistent with methemoglobinemia without ingestion of a known toxin. A large volume of fermented bok choy had been ingested earlier that day and decontamination procedures were performed as a result. Supportive care led to resolution of clinical signs and appropriate clearance of methemoglobin. While erythrocyte oxidant damage is a consequence of ingestion of plants in the genus Brassica (such as bok choy) in ruminant species due to rumen microbiota producing sulfur-containing compounds, specifically dimethyl disulfide, there are potential pathways that can lead to similar effects in monogastric animals. The methemoglobin formation in this patient may have resulted from the large volume consumed with the natural fermentation releasing dimethyl disulfide and leading to oxidant damage analogous with that in ruminants. This case report provides additional mechanisms for methemoglobin formation in dogs and to direct the clinician toward methemoglobinemia in patients with compatible clinical signs with ingestion of specific plant species.
Keywords: methemoglobin, canine, bok choy, Brassica
Introduction
Methemoglobinemia in small animals has been documented to result from a variety of causes including congenital deficiencies such as methemoglobin reductase deficiency (cytochrome b5R deficiency),1,2 acetaminophen ingestion,3 topical benzocaine products,4 skunk musk,5 hydroxycarbamide,6 phenazopyridine,7 and nitrates/nitrites.8 Hemoglobin, as an oxygen-binding molecule, requires iron in the ferrous state for oxygen binding to occur. Methemoglobin formation results from iron oxidation from the ferrous (2+) to the ferric (3+) form in the hemoglobin of the red blood cell. This creates an inactive form of hemoglobin that decreases the release of oxygen to the tissues. In healthy states, methemoglobin comprises <3% of total hemoglobin.9
There have been several studies citing acetaminophen ingestion as causing methemoglobinemia, particularly in cats.3,10 In emergency cases of methemoglobinemia, underlying acetaminophen ingestion is often suspected as this is a common household pharmaceutical drug and the other potential causes are rarely reported. Plants in the genus Brassica contain compounds known to cause erythrocyte oxidant damage, including methemoglobin formation, in ruminant species. This occurs as a result of sulfur-containing compounds in plant tissues and their conversion to toxic products by rumen microbiota.11 In humans, vegetables in this genus have been found to be goitrogenic.12 There is a single case report of a woman that developed myxedema coma from chronic, large volume bok choy ingestion.13
This case report details acute clinical signs attributed to spontaneous methemoglobinemia without any known toxin exposure in an elderly dog with large volume bok choy ingestion as the proposed mechanism for methemoglobin generation.
Case report
History
A 15-year-old male castrated Cardigan Welsh Corgi was referred for cyanosis and tachypnea. The owner had noted an acute onset of tachypnea and ptyalism. The patient became acutely lethargic, slow to ambulate, and eventually collapsed prompting his presentation to a local emergency clinic. There was no known toxin exposure, but he had been observed ingesting a large volume of fermented bok choy that day. The bok choy had been prepared by the owners with water and a small amount of salt to form a brine for fermentation. No other food ingredients, additives, or potential toxic substances had been added.
On presentation to the local emergency clinic, he was reported to be non-ambulatory and cyanotic. He was mildly tachycardic and tachypneic at presentation and was reported to appear distressed. He had initial bloodwork completed at that time including a complete blood count (IDEXX Procyte Dx) and an in-house chemistry panel (IDEXX Catalyst One) (Table 1). His CBC revealed a mild normocytic, normochromic anemia without a reticulocyte count reported (Hematocrit 36.66%, reference interval 37–55%). An in-house chemistry panel revealed a mild hypophosphatemia (2.5 mg/dL, reference interval 2.9–6.6 mg/dL) that was repeated and found to be normal at 5.9. He had both abdominal and thoracic radiographs performed showing an ingesta-filled stomach without radiographic evidence of a mechanical obstruction. His thoracic radiographs revealed an unremarkable thorax without a clear cause of cyanosis. Given the reported ingestion of a large quantity of bok choy, emesis was induced with 0.54 mg of apomorphine (Apomorphine, Compound Central Pharmacy) IV, which resulted in the patient vomiting a large volume of green vegetable material. He was then referred for further diagnostics and treatment.
 | Table 1 Chemistry and complete blood count results at presentation |
Clinical findings
At presentation, he was tachypneic and mildly tachycardic with cyanotic mucous membranes. Systolic blood pressure measured with a Doppler was 106 mmHg. A venous blood gas panel including co-oximetry was obtained (NOVA, Nova Biomedical) that revealed a methemoglobin of 34.5% (reference interval <3%). The packed cell volume (PCV) and total solids (TS) was 36%/6.6 (reference interval PCV 37–55%, TS 5.4–7.1 g/dL measured by handheld refractometer-VetLab Supplies). The gross appearance of his blood was a chocolate brown color (Figure 1). The patient history was reviewed with the owner to rule out potential toxin exposure leading to methemoglobinemia. There was no known acetaminophen in the household or exposure to any medications or compounds previously known to lead to methemoglobin formation. Given the age and lack of historic clinical signs, a congenital cause for the methemoglobin in this patient was considered less likely. The owner confirmed that the patient consumed a large volume of fermented bok choy that was being stored at their house prior to disposal, consistent with the plant material produced upon induction of emesis. Acetaminophen serum levels were submitted to an external laboratory (NMS Laboratories) and measured by high-performance liquid chromatography to rule out acetaminophen exposure.
The patient was hospitalized with oxygen supplementation, N-Acetylcysteine (N-Acetylcysteine, Hospira) intravenously for 7 doses total (initial dose of 140 mg/kg IV then subsequent doses were 70 mg/kg IV), Vitamin C (Vitamin C, Vet One injectable solution) 30 mg/kg subcutaneously every 6 hrs and Denamarin (SAMe/Silybin, Nutramaxx) supplementation (225 mg) daily (in the event that the patient had acetaminophen exposure) as part of decontamination and treatment for methemoglobinemia with treatments consistent with those administered with acetaminophen toxicity. He made improvements throughout this hospitalization, and his venous blood gas was rechecked 6 hrs after initiating therapy with a decrease in his methemoglobin to 3.6%. He was transitioned out of oxygen given his clinical improvement as the tachypnea and cyanosis resolved. The venous blood gas was rechecked after another 6 hrs (12 hrs after initiation of treatment) revealing clearance of methemoglobin with the level at 0.1% (Table 2). A chemistry panel was repeated 12 hrs after initiation of therapy and was within normal limits (Table 3). A recheck PCV was performed at this time revealing a decrease to 28%/6.0 (Reference interval PCV 37–55%, TS 5.4–7.1 g/dL) likely as a result of oxidative erythrocyte damage. The patient was discharged 24 hrs after admission with resolution of his clinical signs and low methemoglobin levels. He was discharged from the hospital with Denamarin 225 mg per os for a 7-day course for hepatoprotective and antioxidant effects as well as decreasing osmotic fragility of erythrocytes in the event he had accidental ingestion of acetaminophen. The acetaminophen serum level results were available 10 days following discharge, and the results showed no acetaminophen detected (therapeutic range provided 5–20 mcg/mL; results 0 mcg/mL). Future hospital visits months after discharge revealed no recrudescence of clinical signs and low methemoglobin levels (<3% methemoglobin).
 | Table 2 Table recording methemoglobin levels and venous blood gas variables performed during hospitalization |
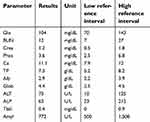 | Table 3 Repeat chemistry panel 12 hrs following admission |
Discussion
This report details a case of transient methemoglobinemia from an undetermined cause with the only known potential toxin exposure or ingestion being a large volume of fermented bok choy. Acetaminophen levels were measured, as exposure is a common cause of methemoglobin formation in small animal species. Although acetaminophen levels have not been validated in the canine species, the results in this case do not detect any acetaminophen ruling out accidental exposure as a cause. A congenital cytochrome B5 reductase deficiency can lead to episodic clinical signs and cannot be excluded as the underlying or a contributing cause of the methemoglobinemia in this case.14 While a congenital methemoglobinemia cannot be completely excluded, given the patient age, lack of similar events, and documented normal blood gas co-oximetry at other visits, it is considered less likely. An assay to evaluate b5R activity would have strengthened this report and allowed for exclusion of congenital causes. This case report proposes that the ingestion of a large quantity of fermented bok choy may have been associated with a transient methemoglobinemia in this patient, which warrants future investigations.
Bok choy is a plant in the genus Brassica, which is a group of plants known to lead to oxidant damage and hemolytic/Heinz body anemias in ruminant species when fed as forage.15,16 Plants in the genus Brassica are known to contain a large amount of sulfur products in their tissues, including a sulfur-based amino acid compound S-methyl-L-cysteine sulfoxide (SMCO).17,18 The concentration of SMCO has been studied in plant species and is known to be variable based on type of species, year of cultivation, age of the plant, season, and geographic region.19,20 While these influences make SMCO levels difficult to predict, bok choy has been documented to contain high amounts of this substance.19,21 Studies with animals fed these organosulfur compounds show numerous kinds of toxicological effects. In ruminants, SMCO is converted to dimethyl disulfide by microorganisms in the rumen, which is absorbed and leads to oxidative damage to red blood cells causing clinical signs of toxicity in these species.16,22 While the mechanism of this oxidative damage is not well characterized, one precipitating factor appears to be reduced glutathione levels in the erythrocyte allowing oxidative damage to occur.16 The enzyme responsible for production of dimethyl disulfide has been documented outside of ruminant species and in microorganisms in the human gastrointestinal system.23 Heinz body anemia has also occurred in adult fowls fed dimethyl disulfide and rats fed SMCO.24,25 Pharmacokinetics of SMCO in rats and dogs was characterized by high bioavailability.25 While there are documented cases of dimethyl disulfide produced outside of the rumen microbiota, the fermented state of the bok choy in this case may have had a contributing factor in the bioavailability of these toxic substances. Natural fermentation leads to lactic acid bacterial growth thus creating probiotic microorganisms more commonly thought to provide health benefits to the host.26 A causative link between the natural fermentation in this case and the production of toxic sulfur compounds requires further investigation. A known mechanism for methemoglobinemia with Brassica consumption in dogs has not been documented and this would be the first report.
There are several techniques used to diagnose the presence of methemoglobin in blood. On gross examination, blood appears chocolate brown in color. A peripheral blood smear can be completed to evaluate for oxidative damage (such as the presence of Heinz bodies). Direct quantification can be made through co-oximetry readings, which were utilized in this case. An indirect method of evaluating for methemoglobinemia is determining the saturation gap, which is calculated from comparing a pulse oximetry reading to arterial blood gas saturation. In this instance, methemoglobinemia should be suspected if the saturation gap exceeds 5%.27 Clinical suspicion for methemoglobinemia is often based on clinical signs and exposure history. The clinical signs of methemoglobinemia in small animal patients are related to the decrease in oxygen offloading by hemoglobin leading to hypoxia. These signs arise when methemoglobin levels reach greater than 20%, and they include tachycardia, tachypnea, lethargy, weakness, ptyalism, cyanosis/chocolate brown mucous membranes, and mentation changes.27 Methemoglobinemia has been reported to be fatal if levels reach 80%.1,9,10 These signs are consistent with the signs observed in this patient at presentation. The resolution of these clinical signs paralleled the co-oximetry readings of decreasing methemoglobin percentages to negligible levels. The patient was discharged less than 24 hrs after hospitalization without recurrent signs consistent with toxicity.
Treatment of methemoglobinemia is focused around decontamination in the case of toxicities and stabilization. Oxygen therapy can be utilized to increase the amount of dissolved oxygen, but it holds little benefit to hemoglobin saturation. Decontamination therapies involving emesis and activated charcoal can be considered. In this case, both emesis and supportive care with anti-oxidant therapy were pursued given concern that there may have been accidental acetaminophen exposure. This involved the administration of vitamin C as well as N-Acetylcysteine. N-Acetylcysteine is used in cases of acetaminophen toxicity as it directly binds and inactivates the metabolites as well as serving as a glutathione precursor.9,10,27 Vitamin C can help reduce methemoglobin back to hemoglobin.10 It is unclear if in this case these compounds resulted in any clinical benefit for the patient or if the patient's spontaneous resolution occurred as a result of decontamination and clearance of the toxin.
This case provides evidence of methemoglobinemia in a dog without exposure to any previously published toxins and with confirmed absence of acetaminophen ingestion. Co-oximetry is not widely available limiting direct methemoglobin measurements in many veterinary practices. Expanding our understanding of the potential causes of methemoglobinemia could aid in recognition and diagnosis. Ingestion of a large volume of bok choy potentially provides an additional cause of acquired methemoglobinemia in dogs. While a disulfide assay is not available, a definitive causality cannot be established in this case. Future laboratory studies exposing dogs to dimethyl disulfide to serve as a confirmatory toxic cause of erythrocyte oxidant damage should be completed. Genetic testing to explore congenital causes of methemoglobinemia was not pursued in this case and cannot be excluded as a confounding factor. A congenital cause is considered less likely given the age of the patient, the lack of clinical signs outside of this episode, and normal methemoglobin levels on blood gas panels from other hospital visits. The methemoglobinemia in this patient is likely associated with the bok choy ingested, and future investigations to support these findings could support the findings in this case.
In conclusion, we provide a case report of a dog with acquired methemoglobinemia secondary to ingestion of fermented bok choy that resolved with decontamination and supportive care. This provides an additional mechanism of transient methemoglobinemia in dogs with compatible clinical signs.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Fine DM, Eyster GE, Anderson LK, Smitley A. Cyanosis and congenital methemoglobinemia in a puppy. J Am Vet Med Assoc. 1999;35(1):33–35.
2. Shino H, Otsuka-Yamasaki Y, Ooi K, Inanami O, Sato R, Yamasaki M. Familial congenital methemoglobinemia in Pomeranian dogs caused by a missense variant in the NADH-Cytochrome B5 Reductase gene. J Vet Intern Med. 2018;32(1):165–171. doi:10.1111/jvim.15031
3. Richardson JA. Management of Acetaminophen and Ibuprofen toxicosis in dogs and cats. J Vet Emerg Crit Care. 2006;10(4):285–291. doi:10.1111/j.1476-4431.2000.tb00013.x
4. Davis JA, Greenfield RA, Brewer TG. Benzocaine-induced methemoglobinemia attributed to topical application of the anesthetic in several laboratory species. Am J Vet Res. 1993;54:1322.
5. Fierro BR, Agnew DW, Duncan AE, Lehner AF, Scott MA. Skunk musk causes methemoglobin and Heinz body formation in vitro. Vet Clin Pathology. 2013;42(3):291–300. doi:10.1111/vcp.12074
6. Wray JH. Methemoglobinemia caused by hydrxycarbamide (hydroxyurea) ingestion in a dog. J Small Animal Pract. 2008;49:216. doi:10.1111/j.1748-5827.2007.00449.x
7. Harvey JW, Kornick HP. Phenazopyridine toxicosis in the cat. J Am Vet Med Assoc. 1976;169(3):327–331.
8. Zhang P, Ohara A, Mashimo T. Cardiovascular effects of an ultrashort-acting nitric oxide-releasing compound, zwitterionicdiamine/NO adduct in dogs. Circulation. 1996;94:2235. doi:10.1161/01.CIR.94.9.2235
9. Rahilly LJ, Mandell DC. Methemoglobinemia. In: Silverstein DC, Hopper K, editors. Small Animal Critical Care Medicine.
10. Aronson LR, Drobatz K. Acetaminophen toxicosis in 17 cats. J Vet Emerg Crit Care. 1996;6(2):65–69. doi:10.1111/j.1476-4431.1996.tb00034.x
11. Smith RH. S-methylcysteine sulphoxide, the Brassica anaemia factor (a valuable dietary factor for man?). Vet Sci Communi. 1978;2(1):47–61. doi:10.1007/BF02291432
12. Felker P, Bunch R, Leung AM. Concentrations of thiocyanate and goitrin in human plasma, their precursor concentrations in Brassica vegetables, and associated potential risk for hypothyroidism. Nutr Rev. 2016;74(4):248–258. doi:10.1093/nutrit/nuv110
13. Chu M, Seltzer TF. Myxedema coma induced by ingestion of raw bok choy. NEJM. 2010;362(20):1945–1946. doi:10.1056/NEJMc0911005
14. Jaffey JA, Harmon MR, Villani NA, et al. Long-term treatment with methylene blue in a dog with hereditary methemoglobinemia caused by cytochrome b5 reductase deficiency. J Vet Intern Med. 2017;31(6):1860–1865. doi:10.1111/jvim.14843
15. Dawes ME. Brassica Spp. Toxicity. In: Chase C, Lutz K, McKenzie E, Tibary A, editors. Blackwell’s Five-Minute Veterinary Consult.
16. Prache S. Haemolytic anaemia in ruminants fed forage brassicas: a review. Vet Res. 1994;25(6):497–520.
17. Stoewsand GS. Bioactive organosulfur phytochemicals in Brassica oleracea vegetables – a review. Food Chem Toxic. 1995;33(6):537–543. doi:10.1016/0278-6915(95)00017-V
18. Wiley N, Wilkins J. An analysis of intertaxa differences in sulfur concentration in angiosperms. J Plant Nutrition Soil Sci. 2006;169:717–727. doi:10.1002/jpln.200520590
19. Mae T, Ohira K, Fujiwara A. Fate of (+) S-methyl-L-cysteine sulfoxide in Chinese cabbage, Brassica pekinensis RUPR. Plant Cell Physiol. 1971;12:1–11. doi:10.1093/oxfordjournals.pcp.a074591
20. McKenzie RA, Carmichael AM, Duigan SA, Gibson JA, Taylor JD. Sulfur-associated polioencephalomalacia in cattle grazing plants in the family Brassicaceae. Aust Vet J. 2009;87(1–2):27–32. doi:10.1111/j.1751-0813.2008.00387.x
21. Morris CJ, Thompson JF. The identification of (+) S-methyl-L-cysteine sulfoxide in plants. Chem Ind. 1955;951.
22. Benevenga NG, Case GL, Steele RD. Occurrence and metabolism of S-methyl-L-cysteine and S-methyl sulfoxide in plants and their toxicity and metabolites in animals. In: Cheeke PR, editor. Toxicants of Plant Origin, Volume III Proteins and Amino Acids. Florida: CRC Press; 1989:203–228.
23. Shamat MA. The role of the gastrointestinal microflora in the metabolism of drugs. Int J Pharm. 1993;97(1–3):1–13. doi:10.1016/0378-5173(93)90121-U
24. Maxwell MH. Production of Heinz body anaemia in the domestic fowl after ingestion of dimethyl disulphide: a haematological and ultrastructural study. Res Vet Sci. 1981;30(2):233–238.
25. Amano H, Kazamori D, Itoh K. Pharmacokientics and N-acetylation metabolism of S-mehtyl-L-cysteine and trans-S-1-propenyl-L-cysteine in rats and dogs. Xenobiotica. 2016. doi:10.3109/00498254.2016.1144229
26. Sung-Mee L, Im DS. Screening and characterization of probiotic lactic acid bacteria isolated from Korean fermented foods. J Microbiol Biotechnol. 2009;19(2):178–186.
27. Skold A, Cosco DL, Klein R. Methemoglobinemia: pathogenesis, diagnosis, and management. South Med J. 2011;104(11):757–761. doi:10.1097/SMJ.0b013e318232139f
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

