Back to Journals » Journal of Pain Research » Volume 11
Transforaminal epidural steroid injection combined with pulsed radio frequency on spinal nerve root for the treatment of lumbar disc herniation
Authors Ding YY , Li H, Zhu Y, Yao P, Zhao G
Received 16 May 2018
Accepted for publication 10 July 2018
Published 14 August 2018 Volume 2018:11 Pages 1531—1539
DOI https://doi.org/10.2147/JPR.S174318
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Michael A Ueberall
Yuanyuan Ding,1 Hongxi Li,1 Yongqiang Zhu,1 Peng Yao,1 Guangyi Zhao2
1Department of Pain Management, Shengjing Hospital of China Medical University, Shenyang, China; 2Department of Anesthesiology, Shengjing Hospital of China Medical University, Shenyang, China
Background: Lumbar disc herniation (LDH) is a common disease in clinical practice. The symptoms recur and are aggravated by time; severe pain and long-term movement disorder cause physiological and psychological problems that affect the quality of life of patients. Therefore, relieving the pain symptoms and promoting functional recovery are the primary goals that have gained increased attention.
Objective: To assess the efficacy of CT-guided transforaminal epidural steroid injection (TFESI) combined with pulsed radio frequency (PRF) on spinal nerve root for the treatment of LDH.
Study design: Retrospective comparative study.
Setting: Shengjing Hospital of China Medical University.
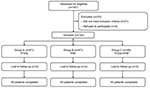
Methods: A total of 135 patients with LDH were selected from the Department of Pain Management in the Shengjing Hospital of China Medical University between January 2014 and December 2016. All patients were divided into three groups according to the order of entry (n=45): TFESI (group A); PRF on spinal nerve root (group B); and TFESI combined with PRF on spinal nerve root (group C). The visual analog scale (VAS), Oswestry disability index (ODI), and global perceived effect (GPE) before treatment and at different time points after treatment were observed, and patients’ satisfaction was assessed.
Results: At every point of observation, the VAS and ODI decreased significantly as compared to that before treatment in all groups (P<0.05). The VAS and ODI in group A at 3 and 6 months after treatment were significantly higher than that in the other two groups (P<0.05). At day 1, day 14, and 1 month after treatment, the VAS and ODI in group C were significantly lower than that in group B (P<0.05). The GPE in group C was high in the early days, while that at day 14 and 1 month after treatment was significantly higher than that in the other two groups (P<0.05); no significant difference was observed in GPE at 3 and 6 months after treatment between groups B and C (P>0.05).
Conclusion: TFESI combined with PRF for the treatment of LDH could effectively and rapidly relieve lumbago and radicular pain and achieve long-term remission. Although the method is widely applicable, the precise selection of patients is imperative.
Keywords: transforaminal epidural steroid injection, pulsed radio frequency, spinal nerve root, lumbar disc herniation, low back pain, radicular pain
Introduction
Lumbar disc herniation (LDH) is a common disease in clinical practice, characterized by recurrent low back pain, with or without lower limb radicular pain, numbness, and intermittent claudication. In a case of severe LDH, muscle strength is decreased and bowel and bladder dysfunction occurs. The symptoms recur and are aggrevated by time; severe pain and long-term movement disorder cause physiological and psychological problems that affect the quality of life of patients.1 Therefore, relieving the pain symptoms and promoting functional recovery of lumbar vertebrae are the primary goals that have gained increased attention.
Lumbago and leg pain is common; approximately 70% of individuals experience low back pain with different severities throughout their lives.2 LDH has a high prevalence of 1.6%–13.4% and a lifetime prevalence of 12.2%–43%.3 In clinical practice, some patients present with residual lumbago and leg pain after lumbar disc surgery. Consequently, the nerve compression has been relieved by surgery, and therefore, pain is not caused simply by mechanical compression, and the primary reason might be tissue trauma and local inflammatory response. A study found that the long-term efficacy in LDH surgery group was not superior to that of conventional non-operative treatment group after 2 years of follow-up.4 Therefore, minimally invasive treatment is the main development direction for the treatment of LDH via various methods. The characteristics of the method include less trauma, quick recovery, and cost-efficiency, and hence, easily accepted by most patients; moreover, comprehensive therapy could also be adopted.
Transforaminal epidural steroid injection (TFESI) is a classical, minimally invasive treatment for radicular pain with a definite short-term efficacy, and the pain relief or functional recovery outcome is more robust at 2 weeks than 2 months;5,6 however, the medium- and long-term efficacy is controversial due to drug metabolism. Unlike conventional continuous radio frequency (CRF), the radio frequency (RF) current of pulsed radio frequency (PRF) is temporarily discontinuous and the heat dissipation in 480 ms intermittent period makes the tip temperature <42°C, which does not destroy the structure of nerve fibers and maintains the integrity of function.7 Also, it does not cause hypesthesia, skin numbness, or dyskinesia. Presently, PRF is widely used in the treatment of neuralgia, stubborn low back pain, and joint pain;8–10 however, some investigators also pursue a wait-and-see attitude toward the efficacy of PRF, and hence, further observation is recommended.
In addition, most of the PRF treatment points for lumbago and leg pain caused by LDH have been dorsal root ganglion (DRG) or lumbar disc;11 however, PRF does not injure the nerve and is very safe, and thus, spinal nerve root can be chosen as a therapeutic target. Therefore, in the present study, the efficacy and safety of CT-guided TFESI combined with PRF on spinal nerve root were assessed for the treatment of LDH.
Methods
Patients
A total of 135 patients with LDH, treated at the Department of Pain Management, Shengjing Hospital of China Medical University between January 2014 and December 2016, were selected. The study was approved by the Ethics Committee of Shengjing Hospital Affiliated to China Medical University. All patients were divided into three groups according to the order of entry (n=45): group A (TFESI), group B (PRF on spinal nerve root), and group C (TFESI combined with PRF on spinal nerve root) (Figure 1). All the patients received regular therapy before treatment, and all the responsible segments were treated.
The inclusion criteria for the patients were as follows: 1) radicular pain in lower extremities, with or without lower back pain; 2) CT or MRI showed herniated disk; however, the herniated nucleus pulposus was still wrapped by annulus fibrosus or a posterior longitudinal ligament without free fragment shedding; 3) the remission of the symptoms was not obvious after conservative treatments such as inactivity, drug, and physical therapy; and 4) patients rejected or were intolerant to surgery and required minimally invasive treatment.
Exclusion criteria: 1) puncture site was infected; 2) vertebral canal and intervertebral foramen were severely narrow, nerve root compression, ≥ grade 2 spondylolisthesis, lumbar inflammation, tuberculosis, tumor, and definite other intraspinal lesions; 3) severe neurological deficit, such as bowel and bladder dysfunction and progressive dyskinesia; 4) pregnancy; 5) abnormal coagulation index, severe hematopathy, abnormal liver and kidney function, or cardiopulmonary disease; and 6) poor blood-glucose control in diabetic patients or those with mental illness who were unable to co-operate with the treatment. All patients provided written informed consent before treatment regarding the associated risks and complications.
Methods of treatment
The responsible segments were determined according to the symptoms, physical examinations, and imaging examinations, and multisegmental treatment was performed. Patients were placed in the prone position for the CT-guided segment scanning. Consecutively, puncture point, depth, and angle were marked after the RF cannula needle was slowly inserted under the guidance of CT, the tip of the needle was located in the intervertebral foramen, the RF electrode was inserted, and the RF test (Baylis Medical Inc., Montreal, Canada) was conducted. The RF was set at 50 Hz and 0.1–0.3 V to stimulate the sensory nerve; numbness and radicular pain were consistent with the painful area of the primary complaint. The RF was set at 2 Hz and 0.4–1.0V to test the motor nerve, and mild exercise was effectuated in the waist and gluteal muscles; also, muscle tremor and pulsation of the lower limbs occurred. The patients in group A were treated with TFESI, and 2.5 mL analgesic solution (2% lidocaine 5 mL + VitB12 1 mg + compound betamethasone 5 mg + normal saline 2 mL) was injected. The patients in group B were treated with PRF at 42°C for 300 seconds (pulse width 20 ms), and 2.5 mL solution (2% lidocaine 5 mL + VitB12 1 mg + normal saline 3 mL) was injected. The patients in group C were treated with PRF with the same parameters as group B and TFESI as group A. Finally, the needle was pulled out and the puncture point pressed. If patients did not present any adverse reactions, they could return to their wards.
Efficacy assessment and follow-up
Patients were assessed before treatment as well as on day 1, day 14 and 1, 3, and 6 months after treatment. Patients were followed-up by nonsurgical group medical staff using the double-blind method.
- Visual analog scale (VAS): judged the degree of pain: no pain (0) and unbearable pain (10 points).
- Oswestry disability index (ODI): assessed the degree of disability and pain. ODI consisted of ten items including pain intensity, self-care, lifting, walking, sitting, standing, interfering with sleep, sex life, social life, and tourism. Each item had six options and was scored as 0–5 points. The sum of scores was expressed as a percentage: 0% represented no pain or disability, while 100% represented the most severe pain and disability.
- Global perceived effect (GPE): assessed the satisfaction: a) unsatisfied: GPE ≤4, symptoms were not changed or worse; b) satisfied: 4< GPE ≤5, symptoms were improved; and c) very satisfied: GPE ≥6, symptoms were improved distinctly or patients recovered (Table 1). Success was defined as score ≥6 (6 or 7 on a 7-point Likert scale).
  | Table 1 Likert scale 7-point scoring system: global perceived effect |
Statistical analysis
SPSS version 18.0 software was used for statistical analysis. Chi-squared test was used for the analysis of the enumeration data. When the sample size was small, Fisher’s exact test was used. Kolmogorov–Smirnov test was used for the normal measurement data. The variables of normal distribution were expressed as mean ± SD, while the variables that did not conform to normal distribution were expressed as median ± interquartile range. For comparison of the variables of normal distribution, single factor analysis of variance was used, while least significant difference was used for pairwise comparison. On the other hand, the comparison of the variables that did not conform to normal distribution was conducted by Kruskal–Wallis test, and Mann–Whitney U test was used for pairwise comparison. P<0.05 was considered a significant difference.
Results
Preoperative general condition of patients
A total of 135 patients were enrolled. No statistical difference was observed in the general preoperative conditions including gender, age, preoperative pain duration, pain position, and preoperative VAS among the three groups (P>0.05). The severity of pain in the three groups before treatment was categorized into different degrees based on the analgesic drugs used by patients with different pain scale, and no statistical difference was observed in the scale classification (P>0.05) (Table 2).
Intraoperative condition of patients
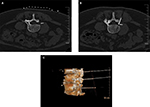
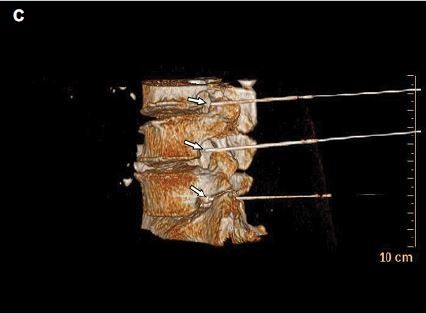
The treatments were performed successfully in all patients. CT was used for positioning at a definite angle and depth. The tip of the needle was observed at the spinal nerve root in plain CT scan images. Three-dimensional CT showed that multiple RF needles were localized at the spinal nerve root of the lumbar intervertebral foramen, and sensory and motor tests could induce symptoms that were consistent with the preoperative range. No severe complications, such as spinal injury and paraplegia, occurred in the patients in the three groups due to CT guidance (Figure 2). The complications were mild and reversible and resolved within 3 months.
VAS score
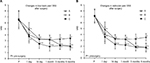
At every point of observation, the postoperative low back pain and lower limb radicular pain (VAS score) decreased in the three groups and differed significantly compared to before treatment (P<0.05). Groups A and C showed a rapid analgesic effect and VAS score decreased 1 day post-treatment, which was statistically different from that in group B (P<0.05); however, the maintenance time of analgesia effect in group A was short, and the onset of effect in group B was slow. The VAS scores in group A at 3 and 6 months after treatment were significantly higher than that in the other two groups (P<0.05), while in groups B and C, the pain relief could be maintained for a prolonged period. The VAS scores in group C at 14 days and 1 month after treatment were significantly lower than that in the other two groups (P<0.05). However, at 3 and 6 months after treatment, no statistical difference was observed in the VAS scores between groups B and C (P>0.05) (Figure 3).
Degree of disability (ODI)
At every time point of observation, the quality of life in the three groups was improved after treatment, and the ODI score decreased, which differed significantly from that before treatment (P<0.05). Groups A and C showed a rapid improvement in the quality of life and ODI score decreased 1 day after treatment, which was statistically different from that in group B (P<0.05). However, the maintenance time of the improved quality of life in group A was short, and the onset of effect in group B was slow. On the other hand, the ODI scores in group A at 3 and 6 months after treatment were significantly higher than that in the other two groups (P<0.05), while in groups B and C, the quality of life could be improved for a prolonged period. The ODI scores in group C at 14 days and 1 month after the treatment were significantly lower than that in the other two groups (P<0.05). However, at 3 and 6 months post-treatment, no significant difference was observed in the ODI scores between groups B and C (P>0.05) (Table 3).
Satisfaction (GPE)
The percentage of patients with GPE ≥6 was shown in Table 4. The GPE in group A decreased with time; it was significantly lower at 3 and 6 months after treatment than that in the other two groups (P<0.05). The GPE in group B at 1 day after treatment was significantly lower than that in groups A and C (P<0.05). Subsequently, the GPE increased gradually and reached a peak at 3 months after treatment, although statistical difference was not observed at 3 and 6 months after treatment between groups B and C (P>0.05). The GPE in group C was higher in the early days, while that at 14 days and 1 month after treatment was significantly higher than that in the other two groups (P<0.05); no significant difference was observed in the GPE at 3 and 6 months after treatment between groups B and C (P>0.05) (Table 4).
Discussion
LDH is one of the most common causes of low back pain and radicular pain, which is easily overlooked; it is stubborn and affects the daily life of patients. Therefore, there is an urgent need to alleviate the pain and improve the quality of life of these patients.
Presently, several methods are available for LDH treatment that primarily encompass surgery and non-surgical treatment. Although the non-surgical treatment is of great significance, it requires a prolonged duration, has a poor curative effect, and relapse occurs frequently. On the other had, with surgery, trauma is significant, recovery is slow, and it might cause severe complications.12 Minimally invasive interventional therapy has been widely used in the treatment of chronic pain, and it has gradually become the developmental direction for the treatment of chronic lumbago and leg pain. Each minimally invasive interventional therapy has limitations, and the success rate varies greatly. Single therapy often has poor efficacy, and hence, it is necessary to select the patients accurately; comprehensive treatment could achieve the best results. Accurate CT scanning in therapy can be used to locate and determine the position of the needle tip. The present study accurately selected the inclusion and exclusion criteria. Physical compression caused by the free lumbar nucleus pulposus, severe stenosis of the spinal canal and intervertebral foramen, and definite nerve root bone compression should be resolved as a priority, otherwise, minimally invasive procedures would be ineffective.
The mechanisms underlying low back pain and radicular pain caused by LDH are yet to be elucidated. Mechanical pressure could lead to nerve injury and functional alteration. Although, after oppression caused by LDH was relieved by surgery, some patients did not show any improvement in symptoms. Thus, in addition to mechanical oppression, nerve root inflammatory response also played a critical role.13–15 Steroids exert anti-inflammatory and immunosuppressive effects, and intervertebral foramen injection was administered directly to the nerve root, which relieved the pain markedly;16,17 however, the maintenance time was short. The present study found that all three treatments could effectively alleviate lower back pain and lower extremity radicular pain, which could improve the quality of life and increase the degree of satisfaction in patients. However, at 3 and 6 months after TFESI (Group A), VAS and ODI scores were significantly higher than those of the other two groups (P<0.05), while GPE was lower than those of the other two groups (P<0.05). At day 1, day 14, and 1 month after treatment, VAS and ODI scores in the combination group (Group C) were significantly lower than that in the PRF group (Group B) (P<0.05). This early analgesic effect was associated with the anti-inflammatory effect of steroid injection, which could improve function in the patient at an early stage as well as the compliance of the patient. In groups B and C, pain relief could be maintained for a prolonged period. PRF affected neuromodulation in the spinal nerve root, which achieved a long-term analgesic effect. TFESI combined with PRF displayed the characteristics of rapid onset and prolonged maintenance; the overall efficacy was superior to that of the single method treatment.
The pulse current of PRF stimulated the nerve for regulation and relieved the pain after nerve injury.18 The present study found that the pain relief, improvement of quality of life, and increased satisfaction in PRF and combination groups could be maintained for up to 6 months. In group C, the patients returned to activity or work in 2–3 weeks. Due to few side effects of treatment, the patient could be active 1 day after treatment. Some studies reported the DRG PRF treatment with maximal safety at 42°C as compared to that of the conventional CRF therapy.19 Moreover, it relieved acute nerve root pain20 for a prolonged period.21 L-2 DRG PRF treatment was used for chronic lower back pain with or without pain in the lower extremities – pain relief in patients without pain in the lower extremities was maintained up to 3 months. On the other hand, pain relief could be maintained for 1 month in patients with pain in the lower extremities; a medium-term remission was achieved.22,23 VAS, Neck Disability Index, and ODI scores were significantly decreased, suggesting that L-2 DRG PRF treatment had the same effect as TFESI;24 however, for lumbar nerve root pain due to different causes, PRF was more effective in herniated disc and spinal stenosis than failed back surgery syndrome.25 PRF treatment of the intervertebral disc could reduce the numeric rating scale score, improve the Roland-Morris disability questionnaire score, and alleviate chronic lumbar discogenic low back pain.11 One year after the PRF treatment of lumbar degenerative disc pain, the number of patients with >50% pain relief reached 56%.26 As compared to intradiscal electrothermal therapy (IDET), patients were followed-up for 6 months, and no significant difference was observed between the PRF and IDET groups.27 Also, VAS and ODI scores were decreased, sitting tolerance time was increased, and the treatment was effective; however, the efficacy was limited.28 In the present study, the therapeutic sites of PRF were mostly intervertebral discs or DRG, and only a few studies are available on the effect on the spinal nerve root.29–31 When PRF exerted an effect on DRG, the morphology was observed. As a result, the cell morphology was found to be normal under the light microscope, while the ultrastructural changes could be observed only under an electron microscope. These changes were subclinical and reversible. The accumulation of endoplasmic reticulum and the increase in the number of vacuoles in the cytoplasm could be observed in ganglion cells, which could selectively act on the neuronal axons of the small-diameter C, and Aδ nociceptive fibers could be affected selectively,32 unmyelinated axons were normal, and the Schwann cell membranes were completed.19,33 Therefore, the regulation of PRF on the nerve did not cause damage, and the biological effect was not related to the thermal injury. Consequently, a normal neural structure was maintained, and the PRF treatment could be carried out for the spinal nerve root.
Nevertheless, the underlying mechanism of alleviating pain with PRF was yet unclear. A recent study found that PRF enhanced the expression of anti-inflammatory factor GABAB-R1, Na/K-ATPase, and 5-HT3r in DRG, decreased the expression of proinflammatory factors TNF-α and IL-6, increased the expression of Na/K-ATPase in the spinal cord, and decreased the neurogenic inflammatory response. This phenomenon reduced the peripheral and central sensitization,34 inhibited the activation of MAPK in the spinal cord, reduced the release of cytokines, reduced the release of excitatory amino acid,35 inhibited the JNK activation in the spinal cord dorsal horn,36 and inhibited the sensitization of the spinal cord, thereby alleviating acute pain and improving neuropathic pain.37 In the rat LDH model, PRF DRG reduced the mechanical pain, the activity of microglia, and the expression of pERK.38 Moreover, PRF was carried out in the sacral epidural, which downregulated the activity of microglia at the level of intervertebral disc herniation and adjacent lumbar vertebrae, as well as, decreased the expression of CGRP.39 Therefore, the long-term analgesic effect of PRF might be associated with neuromodulation.40
Conclusion
TFESI combined with PRF for the treatment of LDH could effectively and rapidly relieve lumbago and radicular pain, reduce VAS and ODI scores, relieve pain symptoms, improve the quality of life, cure rate, and satisfaction of patients, as well as, achieve long-term remission. This treatment could be applied widely; however, the precise selection of patients is imperative.
Acknowledgment
The Natural Science Foundation of Liaoning Province (no 20170541032) supported the research of this article, granted to Professor Peng Yao.
Disclosure
The authors report no conflicts of interest in this work.
References
Froud R, Patterson S, Eldridge S, et al. A systematic review and meta-synthesis of the impact of low back pain on people’s lives. BMC Musculoskelet Disord. 2014;15:50. | ||
Hoy D, Brooks P, Blyth F, Buchbinder R. The epidemiology of low back pain. Best Pract Res Clin Rheumatol. 2010;24(6):769–781. | ||
Konstantinou K, Dunn KM. Sciatica: review of epidemiological studies and prevalence estimates. Spine. 2008;33(22):2464–2472. | ||
Zentner J, Schneider B, Schramm J. Efficacy of conservative treatment of lumbar disc herniation. J Neurosurg Sci. 1997;41(3):263–268. | ||
El-Yahchouchi C, Wald J, Brault J, et al. Lumbar transforaminal epidural steroid injections: does immediate post-procedure pain response predict longer term effectiveness? Pain Med. 2014;15(6):921–928. | ||
Rivera CE. Lumbar epidural steroid injections. Phys Med Rehabil Clin N Am. 2018;29(1):73–92. | ||
Tun K, Cemil B, Gurcay AG, et al. Ultrastructural evaluation of pulsed radiofrequency and conventional radiofrequency lesions in rat sciatic nerve. Surg Neurol. 2009;72(5):496–500. | ||
Huang RY, Liao CC, Tsai SY, et al. Rapid and delayed effects of pulsed radiofrequency on neuropathic pain: electrophysiological, molecular, and behavioral evidence supporting long-term depression. Pain Physician. 2017;20(2):E269–E283. | ||
Kim DH, Han SR, Choi CY, Sohn MJ, Lee CH. Efficacy of pulsed radiofrequency medial branch treatment in low back pain patients. J Back Musculoskelet Rehabil. 2016;29(2):361–366. | ||
Gulec E, Ozbek H, Pektas S, Isik G. Bipolar versus unipolar intraarticular pulsed radiofrequency thermocoagulation in chronic knee pain treatment: a prospective randomized trial. Pain Physician. 2017;20(3):197–206. | ||
Fukui S, Nitta K, Iwashita N, Tomie H, Nosaka S, Rohof O. Intradiscal pulsed radiofrequency for chronic lumbar discogenic low back pain: a one year prospective outcome study using discoblock for diagnosis. Pain Physician. 2013;16(4):E435–E442. | ||
Bouche K, Daenens AS, Caemaert J, Vanderstraeten G, Danneels L. Long term outcome of lumbar discectomy: results from a biopsychosocial perspective. Acta Neurol Belg. 2011;111(4):287–295. | ||
Inoue N, Espinoza Orías AA. Biomechanics of intervertebral disk degeneration. Orthop Clin North Am. 2011;42(4):487–499. | ||
Takebayashi T, Cavanaugh JM, Cüneyt Ozaktay A, Kallakuri S, Chen C. Effect of nucleus pulposus on the neural activity of dorsal root ganglion. Spine. 2001;26(8):940–944. | ||
Mclain RF, Kapural L, Mekhail NA. Epidural steroid therapy for back and leg pain: mechanisms of action and efficacy. Spine J. 2005;5(2):191–201. | ||
Benny B, Azari P. The efficacy of lumbosacral transforaminal epidural steroid injections: a comprehensive literature review. J Back Musculoskelet Rehabil. 2011;24(2):67–76. | ||
Burgher AH, Hoelzer BC, Schroeder DR, Wilson GA, Huntoon MA. Transforaminal epidural clonidine versus corticosteroid for acute lumbosacral radiculopathy due to intervertebral disc herniation. Spine. 2011;36(5):E293–E300. | ||
Perret DM, Kim DS, Li KW, et al. Application of pulsed radiofrequency currents to rat dorsal root ganglia modulates nerve injury-induced tactile allodynia. Anesth Analg. 2011;113(3):610–616. | ||
Erdine S, Yucel A, Cimen A, Aydin S, Sav A, Bilir A. Effects of pulsed versus conventional radiofrequency current on rabbit dorsal root ganglion morphology. Eur J Pain. 2005;9(3):251–256. | ||
Teixeira A, Grandinson M, Sluijter ME. Pulsed radiofrequency for radicular pain due to a herniated intervertebral disc – an initial report. Pain Pract. 2005;5(2):111–115. | ||
Sluijter ME, Cosman ER, Rittman WB, van Kleef M. The effects of pulsed radiofrequency fields applied to the dorsal root ganglion: a preliminary report. Pain Clin. 1998;11(2):109–117. | ||
Chao SC, Lee HT, Kao TH, et al. Percutaneous pulsed radiofrequency in the treatment of cervical and lumbar radicular pain. Surg Neurol. 2008;70(1):59–65. | ||
Tsou HK, Chao SC, Wang CJ, et al. Percutaneous pulsed radiofrequency applied to the L-2 dorsal root ganglion for treatment of chronic low-back pain: 3-year experience. J Neurosurg Spine. 2010;12(2):190–196. | ||
Lee DG, Ahn SH, Lee J. Comparative effectivenesses of pulsed radiofrequency and transforaminal steroid injection for radicular pain due to disc herniation: a prospective randomized trial. J Korean Med Sci. 2016;31(8):1324–1330. | ||
Abejón D, Garcia-del-Valle S, Fuentes ML, Gómez-Arnau JI, Reig E, van Zundert J. Pulsed radiofrequency in lumbar radicular pain: clinical effects in various etiological groups. Pain Pract. 2007;7(1):21–26. | ||
Rohof O. Intradiscal pulsed radiofrequency application following provocative discography for the management of degenerative disc disease and concordant pain: a pilot study. Pain Pract. 2012;12(5):342–349. | ||
Fukui S, Nitta K, Iwashita N, Tomie H, Nosaka S, Rohof O. Results of intradiscal pulsed radiofrequency for lumbar discogenic pain: comparison with intradiscal electrothermal therapy. Korean J Pain. 2012;25(3):155–160. | ||
Jung YJ, Lee DG, Cho YW, Ahn SH. Effect of intradiscal monopolar pulsed radiofrequency on chronic discogenic back pain diagnosed by pressure-controlled provocative discography: a one year prospective study. Ann Rehabil Med. 2012;36(5):648–656. | ||
Xiao L, Li J, Li D, et al. A posterior approach to cervical nerve root block and pulsed radiofrequency treatment for cervical radicular pain: a retrospective study. J Clin Anesth. 2015;27(6):486–491. | ||
Fujii H, Kosogabe Y, Kajiki H. Long-term effects of pulsed radiofrequency on the dorsal root ganglion and segmental nerve roots for lumbosacral radicular pain: a prospective controlled randomized trial with nerve root block. Masui. 2012;61(8):790–793. | ||
Wang F, Zhou Q, Xiao L, et al. A randomized comparative study of pulsed radiofrequency treatment with or without selective nerve root block for chronic cervical radicular pain. Pain Pract. 2017;17(5):589–595. | ||
Hamann W, Abou-Sherif S, Thompson S, Hall S. Pulsed radiofrequency applied to dorsal root ganglia causes a selective increase in ATF3 in small neurons. Eur J Pain. 2006;10(2):171–176. | ||
Protasoni M, Reguzzoni M, Sangiorgi S, et al. Pulsed radiofrequency effects on the lumbar ganglion of the rat dorsal root: a morphological light and transmission electron microscopy study at acute stage. Eur Spine J. 2009;18(4):473–478. | ||
Vallejo R, Tilley DM, Williams J, Labak S, Aliaga L, Benyamin RM. Pulsed radiofrequency modulates pain regulatory gene expression along the nociceptive pathway. Pain Physician. 2013;16(5):E601–E613. | ||
Yang CH, Chen KH, Huang HW, Sheen-Chen SM, Lin CR. Pulsed radiofrequency treatment attenuates increases in spinal excitatory amino acid release in rats with adjuvant-induced mechanical allodynia. Neuroreport. 2013;24(8):431–436. | ||
Chen KH, Yang CH, Juang SE, et al. Pulsed radiofrequency reduced complete Freund’s adjuvant-induced mechanical hyperalgesia via the spinal c-Jun N-terminal kinase pathway. Cell Mol Neurobiol. 2014;34(2):195–203. | ||
Lin ML, Lin WT, Huang RY, et al. Pulsed radiofrequency inhibited activation of spinal mitogen-activated protein kinases and ameliorated early neuropathic pain in rats. Eur J Pain. 2014;18(5):659–670. | ||
Cho HK, Cho YW, Kim EH, Sluijter ME, Hwang SJ, Ahn SH. Changes in pain behavior and glial activation in the spinal dorsal horn after pulsed radiofrequency current administration to the dorsal root ganglion in a rat model of lumbar disc herniation: laboratory investigation. J Neurosurg Spine. 2013;19(2):256–263. | ||
Cho HK, Kang JH, Kim SY, et al. Changes in neuroglial activity in multiple spinal segments after caudal epidural pulsed radiofrequency in a rat model of lumbar disc herniation. Pain Physician. 2016;19(8):E1197–E1209. | ||
Lee JJ, Sohn JH, Choi HJ, et al. Clinical efficacy of pulsed radiofrequency neuromodulation for intractable meralgia paresthetica. Pain Physician. 2016;19(3):173–179. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.