Back to Journals » Drug Design, Development and Therapy » Volume 15
Transferrin-Decorated Protein-Lipid Hybrid Nanoparticle Efficiently Delivers Cisplatin and Docetaxel for Targeted Lung Cancer Treatment
Authors Mao K, Zhang W, Yu L, Yu Y, Liu H, Zhang X
Received 16 December 2020
Accepted for publication 25 February 2021
Published 10 August 2021 Volume 2021:15 Pages 3475—3486
DOI https://doi.org/10.2147/DDDT.S296253
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Tuo Deng
Kaiping Mao,1 Weina Zhang,2 Lan Yu,3 Yi Yu,1 Haixia Liu,1 Xiaotao Zhang3
1Department of Thoracic surgery, The Affiliated Hospital of Qingdao University, Qingdao, 266000, People’s Republic of China; 2Department of Plastic surgery, The Affiliated Hospital of Qingdao University, Qingdao, 266000, People’s Republic of China; 3Department of Cancer Stereotactic Radiotherapy, Affiliated Qingdao Central Hospital of Qingdao University, Qingdao, 266042, People’s Republic of China
Correspondence: Xiaotao Zhang Tel +86 532-68667866
Email [email protected]
Purpose: Non-small cell lung cancer (NSCLC) therapy faces the barriers including drug resistance. A transferrin-functionalized protein-lipid hybrid nanoparticle (PLHN) was designed loading both cisplatin (CIS) and docetaxel (DTX) for the lung cancer treatment.
Methods: CIS and DTX were loaded into the hybrid nanoparticle and then decorated with transferrin (Tf). The Tf-functionalized protein-lipid hybrid nanoparticle (Tf-CIS/DTX-PLHN) was investigated by determining the release behavior, cytotoxicity in vitro, and anticancer efficiency in vivo.
Results: Tf-CIS/DTX-PLHN showed a nano-size of 189.5 ± 5.9 nm, and a surface tested to be − 16.9 ± 2.1 mV. Tf-CIS/DTX-PLHN exhibited obviously better antitumor ability in vitro and in vivo compared with the non Tf contained CIS and DTX co-loaded lipid nanoparticles (CIS/DTX-LN), single drug loaded nanoparticles, and free drugs.
Conclusion: Since remarkable enhanced efficiency of Tf and synergistic effect of the drugs, it could inhibit the lung tumor growth and help with the lung cancer treatment.
Keywords: lung cancer, hybrid nanoparticles, nanostructured lipid nanoparticles, protein nanoparticles, transferrin
Introduction
Lung cancer causes the most amount of cancer death among people worldwide.1 Non-small cell lung cancer (NSCLC) accounts for over 80% of lung cancer, and the majority of patients (about 80%) are diagnosed with advanced or metastatic NSCLC (stage IV disease) and are not suitable for surgery.2,3 Among various chemotherapies, platinum-based treatments were the gold standard for the first-line therapy of patients with stage IV disease.4,5 Phase 3 randomized trials have proven that many of the platinum-contained combinations (cisplatin and docetaxel, cisplatin and paclitaxel, cisplatin and gemcitabine, carboplatin and paclitaxel, etc) exhibit no remarkable difference in objective response rates, survival and toxicity.6,7 Based on phase 3 trials, nanoparticle albumin-bound paclitaxel (Abraxane®) with less neurotoxicity and improved response rate has been approved by the US FDA for patients with advanced NSCLC.8 Therefore, the combined application of nanotechnology and two or more therapeutic agents has been the research hotspot recently.
Nano-sized drug delivery systems (Nano-DDS) have been explored for cancer therapy because of its advantages: lower toxicity, high efficacy from their capacity to accumulate at tumor zone, sustained release and extended bioavailability.9 Recently, continuous efforts have been devoted to exploit functional lipid-based nanoparticles to deliver two or more anti-tumor compounds, that include liposomes, solid lipid nanoparticles, and nanostructured lipid carriers.10–12 Despite the success of their co-loading hydrophilic and hydrophobic drugs, all lipid-based nanoparticles suffer from some disadvantages, such as lower drug loading, instability in serum, increased reticuloendothelial system (RES) clearance, decreased blood circulation times and efficacy.13,14 Therefore, protein-lipid hybrid nanoparticles (PLHN), a new generation of lipid-based nanoparticles have arisen to bring fruitful outcomes, reducing RES clearance, enhancing cellular uptake, and facilitating conjugation of drug or targeting ligand.15–18 In our study, we designed a multifunctional PLHN, consist of protein decorated nanostructured lipid carriers to deliver both cisplatin (CIS) and docetaxel (DTX).
Transferrin (Tf) is a polypeptide glycoprotein.19 Due to overexpression of transferrin receptor (TfR) in various malignant tumor cells such as lung cancer, brain cancer, breast cancer and colorectal cancer, it has been used as a tumor targeting motif in various delivery systems. So Tf was used as part of the lung cancer targeted delivery systems in some studies.20–22 In the present study, a multifunctional PLHN, modified by Tf and carried CIS and DTX (Tf-CIS/DTX-PLHN) was developed and evaluated.
Materials and Methods
Materials
Soya lecithin (SL, injectable) was provided by Lipoid GmbH (Ludwigshafen, Germany). Compritol® 888 ATO (ATO) was a gift from Gattefossé (Saint-Priest, Lyon, France). DSPE-PEG2000-COOH was produced by Seebio Biotech (Shanghai) Co., Ltd. (Shanghai, China). Transferrin, 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC), N-Hydroxysuccinimide (NHS), and 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide (MTT) were bought from Sigma Aldrich (St. Louis, MO). The CIS resistant NSCLC cell line (A549/CIS cells), another NSCLC cell line (H1975 cells), and human normal lung epithelial cells (BEAS-2B cells) were obtained from American Type Culture Collection (ATCC, Manassas, VA, USA) and cultured in RPMI Medium 1640 supplemented with 10% (v/v) FBS and 1% (v/v) penicillin–streptomycin at 37°C in an atmosphere of 5% CO2.
Preparation of CIS and DTX Contained Lipid Nanoparticles
CIS and DTX contained lipid nanoparticles (CIS/DTX-LN) was prepared by the lipid emulsify and solvent injection method.23 CIS (30 mg), DTX (50 mg), 100 mg of SL and 100 mg of ATO were mixed and warmed to 70–75°C (1), 100 mg of DSPE-PEG2000-COOH and 1% of polysorbate 80 (v/v) were dissolved in 50 mL of distilled water and heated to 70–75°C (2). (1) was injected to (2) rapidly under stirring (400 rpm, 30 min) to get CIS/DTX-LN. CIS/DTX-LN was washed and filtered by microporous membrane (0.45 μm pore size), then stored at 4°C.
CIS or DTX loaded lipid nanoparticles (CIS-LN or DTX-LN) were prepared using CIS (100 mg) or DTX (100 mg), respectively. Blank lipid nanoparticles (Blank LN) was prepared using no drug.
Preparation of Tf-CIS/DTX-PLHN
The preparation of Tf modified nanoparticles was conducted by conjugating the amido group of Tf with the DSPE-PEG2000-COOH of lipid nanoparticles.24 Briefly, CIS/DTX-LN suspension (200 mg of CIS/DTX-LN) was incubated with EDC (50 mg) and NHS (30 mg) for 10 min, then Tf (100 mg) were added and stirred (4°C, 12 h) to form an amide linkage between Tf and DSPE-PEG2000-COOH. The density of Tf on the particles was measured using a BCA assay kit by recording the absorbance at 595 nm and determining the protein concentration by comparison to a standard curve.
Characterization of Tf-CIS/DTX-PLHN
The size and size distribution of particles, as well as zeta potential were tested by a Zetasizer (Malvern Instruments Ltd., Malvern, UK).25 The morphology of Tf-CIS/DTX-PLHN and CIS/DTX-LN was observed under a TEM (JEOL, Tokyo, Japan). Encapsulation efficiency (EE) and drug loading (DL): The concentration of CIS was measured using ICP-MS.26 High performance liquid chromatography (HPLC) method was used to determine the amount of DTX using a UV detector at 230 nm.27 The stability of nanoparticles was evaluated cell culture medium (as stated in Materials section) and 50% FBS at 37 °C.
In vitro Drug Release
The drug release patterns of nanoparticles were analyzed using dialysis method.28 Drugs loaded nanoparticles (3 mL) were sealed in dialysis bags (MWCO 8–14 kDa) and incubated in PBS (100 mL, pH 7.4) containing 0.1% (w/v) Tween 80 at 37°C. At different time points, 300 μL of release medium was replaced by 300 μL of fresh PBS. The CIS and DTX content were determined by the method described in the above section.
Cytotoxicity Assay
MTT assay was applied to evaluate the cell viability of drugs loaded nanoparticles and free drugs.29 96-well plates were used to culture A549/CIS, H1975 or BEAS-2B cells at a density of 1×104 cells per well. DMEM medium (100 μL) was added to the wells, incubating for 24 h. 200 μL of fresh medium was utilized to replace the culture medium containing Tf-CIS/DTX-PLHN, CIS/DTX-LN, CIS-LN, DTX-LN, blank LN, free CIS/DTX, free CIS, and free DTX. MTT assay was carried out after incubating the cells for another 24 h. The cell viability was calculated compared with the untreated cells at the absorbance at 490 nm.
Synergistic Effect
Combination index (CI) was calculated to evaluate the synergistic effects of drugs loaded in CIS/DTX-LN system when administrated to A549/CIS cells.30 CI when the drug concentration causing 50% inhibition (CI50) was achieved by the equation: CI50 = (D)CIS/(D50)CIS + (D)DTX/(D50)DTX. (D)CIS and (D)DTX means CIS and DTX in combination exhibited 50% of cytotoxicity; (D50)CIS and (D50)DTX means one drug (CIS or DTX) exhibited 50% of cytotoxicity, respectively. CI50 smaller and larger than show synergism and antagonism, respectively.
Cellular Uptake
Cellular uptake of were analyzed using coumarin-6 (C6) loaded nanoparticles, which were prepared by adding C6 (20 mg) along with drugs to get mixture (1) in the “Preparation of CIS and DTX contained lipid nanoparticles” section.30 C6 loaded nanoparticles were added to A549/CIS cells that were seeded at 24-well black plates and incubated for 4 h. Then, the cells were washed three times with D-Hank’s solution, collected and centrifuged and the cell uptake efficiency was quantified using a BD FACSCalibur flow cytometer.
Apoptosis Assay
The levels of apoptosis induced by Tf-CIS/DTX-PLHN, CIS/DTX-LN, CIS-LN, DTX-LN and free CIS/DTX were determined by Annexin V-FITC binding assay was performed using the Annexin V-FITC detection kit I according to the manufacturer’s instructions.31,32 The cells were cultured and treated with Tf-CIS/DTX-PLHN, CIS/DTX-LN or free CIS/DTX or 48 h, the cells were trypsinized and collected by centrifugation at 1500 rpm for 5 min. The cell pellet was resuspended with 100 µM binding buffer and incubated with 5 µL of FITC-conjugated Annexin V and 5 µL of propidium iodide (PI) for 15 min at room temperature in the dark. The samples were immediately analyzed by a flow cytometer (FACSCalibur, BD Biosciences, San Diego, CA).
Xenograft Tumor Model and Biodistribution
All animal experiments were approved by the Institutional Animal Care and Use Committee of the Second Affiliated Hospital of Qingdao University and operated following the Guide of the National Institutes of Health for the care and use of experimental animals. Tumor-bearing BALB/C nude mice (5 weeks old, weighing 18–20 g) were established by subcutaneous injecting A549/CIS cells (3×106) into the right flanks of the mice and waited for the tumor grew to around 100 mm3.33 The mice were then injected with Tf-CIS/DTX-PLHN, CIS/DTX-LN, and free CIS/DTX via tail vein (10 mice each group). At 2, 24 and 96 h, tumor, heart, kidney, lung, liver, and spleen samples were excised and homogenized in lysis buffer, mixed with methanol, and then centrifuged for 30 min. The CIS and DTX content were determined the same as “Characterization of nanoparticles” part.
In vivo Anti-Cancer Efficacy
Tumor-bearing BALB/C mice were administered with physiological saline, Tf-CIS/DTX-PLHN, CIS/DTX-LN, CIS-LN, DTX-LN, blank LN, free CIS/DTX, free CIS, and free DTX via tail vein injection every 2 days for 16 consecutive days (10 mice each group).34 Mice were killed on day 18, the sizes of the tumor were measured using calipers before every injection. Tumor volumes (V) were c achieved by the equation: V = the longest axis × the perpendicular shorter tumor axis2 × 0.5 and tumor growth curves were presented after treatment. The tumor images (before and after treatment) and changes of body weight of mice were also recorded.
TUNEL Assay of Tumor Tissue
Tumor tissue was embedded in paraffin, sliced to 5 micron and detected by TUNEL assay using in situ cell death assay kit to evaluate the apoptotic cells in tumor tissue.35 Apoptotic cells were photographed under fluorescence microscope and TUNEL-positive cells were measured with Image J Software.
Statistical Analysis
Student’s t-test and one-way analysis of variance (ANOVA) was used for the statistical analysis. The parametric variables were expressed as means ±standard deviation (SD). Differences were considered statistically significant at P < 0.05 (*).
Results
Characterization of Nanoparticles
The characterization of nanoparticles in terms of particle size, polydispersity index (PDI), zeta potential, EE, DL and Tf coupling density were summarized in Table 1. Differences were found between Tf-CIS/DTX-PLHN and CIS/DTX-LN in terms of size and surface charge. In particular, the size of Tf-CIS/DTX-PLHN was 189.5 ± 5.9 nm while CIS/DTX-LN exhibit a diameter of 156.3 ± 4.9 nm. The zeta potential of Tf-CIS/DTX-PLHN and CIS/DTX-LN was −16.9 ± 2.1 and −25.3 ± 2.6 mV, respectively. The PDIs of the nanoparticles were below 0.2. The Tf density was found to be 56.3± 3.2%. As can be seen from the TEM images, CIS/DTX-LN appeared as uniform nanosized particles, while Tf-CIS/DTX-PLHN showed double layered particles (Figure 1). The size of the nanoparticles tested showed no significant changes during 72 h of study in both cell culture medium and 50% FBS, illustrated the fine stability of the systems.
 |
Table 1 Characterization of Nanoparticles |
 |
Figure 1 The schematic illustration and TEM images of Tf-CIS/DTX-PLHN and CIS/DTX-LN (scale bar showed 200 nm). |
In vitro Drug Release
Figure 2 shows the accumulated drug release behaviors of nanoparticles wherein. The result revealed no burst release for all formulations, suggested the stability of these particles at the physiological condition. All the drugs contained nanoparticles showed sustained release patterns. Notably, Tf-CIS/DTX-PLHN showed a slower drugs release kinetics compared with CIS/DTX-LN.
 |
Figure 2 In vitro CIS (A) and DTX (B) release profiles of Tf-CIS/DTX-PLHN, CIS/DTX-LN, CIS-LN, and DTX-LN. Data presented as mean ±SD. |
Cytotoxicity and Synergistic Effect
Blank nanoparticles showed no obvious cytotoxicity, which may prove its biocompatibility (Figure 3A). At the equivalent drug concentrations, drugs loaded nanoparticles showed higher cell inhibition efficiency than the drugs without nanoparticle loading (P < 0.05). The combination treatment using CIS and DTX together (CIS/DTX-LN) synergistically decreases the viability of cells than the single drug loaded DTX-LN and CIS-LN. When evaluated on H1975 cells, Tf-CIS/DTX-PLHN exhibited higher cell inhibition ability compared with CIS/DTX-LN (Figure 3B). However, drugs loaded LN showed no obvious influence on human normal lung epithelial cells (BEAS-2B cells) compared with free drugs (Figure 3C). Whether the dual drugs showed synergistic effect when loaded in one system was further evaluated by CI50 values (Figure 3D). The effect of drug combinations of different ratios (from 1:5 to 5:1, CIS: DTX) in CIS/DTX-LN was compared with the effect of treatment with either agent alone. Result showed that the drug ratio of 3:5 had the best synergistic effect with CI values less than 1 at the Fa values from 0.2 to 0.8, so this ratio was used for the nanoparticles preparation.
Cellular Uptake
The cellular uptake efficiency of Tf-CIS/DTX-PLHN showed higher uptake efficiency compared to CIS/DTX-LN and other nanoparticles that not contained Tf (Figure 4, P < 0.05), which may be explained by the targeting effect of transferrin to the cancer cells that could improve the uptake of the nanoparticles.
 |
Figure 4 Cellular uptake efficiency quantified by flow cytometry on A549/CIS cells. Data presented as mean ±SD. *P < 0.05. |
Apoptosis Assay
The level of apoptosis induced by CIS and DTX combined CIS/DTX-LN was higher than CIS-LN and DTX-LN (Figure 5), indicating that the combination treatment induced cell death more effectively than the individual drug treatments. Tf-CIS/DTX-PLHN exhibited a considerable increase in the apoptotic cell population than that of CIS/DTX-LN, which may be concluded as the targeted efficiency of Tf.
 |
Figure 5 Apoptosis rates analyzed by flow cytometer. Data presented as mean ±SD. |
In vivo Biodistribution
Tf-CIS/DTX-PLHN showed higher drug distribution in tumor than non Tf involved CIS/DTX-LN and free drugs at 24 h and 96 h post injection (P < 0.05) (Figure 6). At both 2 and 24 h, Tf-CIS/DTX-PLHN and CIS/DTX-LN distributed less in the heart and kidney (P < 0.05). At 96 h, drug distribution in tumor decreased obviously, which may suggest another injection of formulations.
In vivo Antitumor Efficacy and Systemic Toxicity
Figure 7A reveals that Tf-CIS/DTX-PLHN had the most significant in vivo tumor inhibition ability, which was better than that of other drugs loaded nanoparticles, free drugs and saline control groups (P < 0.05). CIS/DTX-LN presented better tumor suppression ability compared to CIS-LN and DTX-LN (P < 0.05). More remarkable antitumor efficacy was observed by drugs loaded nanoparticles groups compared with free drugs groups (P < 0.05). The body weight changes were summarized in Figure 7B. Obvious body weight lost were found on free drugs administrated groups, in the contrast, nanoparticles groups exhibited no remarkable body weight change. Tumor images (before and after treatment) were applied in Supplementary Figure 1. The effect of nanoparticles on apoptosis of cells in transplanted tumors was examined by the TUNEL assay. TUNEL-positive cells ratio of Tf-CIS/DTX-PLHN group was higher than other groups (Figure 8, P < 0.05), which suggested the better efficiency of Tf modified Tf-CIS/DTX-PLHN on the proliferation and apoptosis of cells in vivo. This result was in accordance with the in vitro experiments.
 |
Figure 7 In vivo tumor inhibition effect evaluated by using mice bearing lung carcinoma model: Tumor volume (A); and body weight (B). Data presented as mean ±SD. *P < 0.05. |
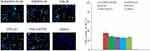 |
Figure 8 TUNEL assay of tumor tissue: TUNEL-positive cells were measured with Image J Software. Data presented as mean ±SD. *P < 0.05. |
Discussion
Transferrin-decorated protein-lipid hybrid nanoparticle was prepared in this study. CIS/DTX-LN was firstly prepared by the lipid emulsify and solvent injection method. Then Tf was conjugated to nanoparticles surface to obtain Tf-CIS/DTX-PLHN. Tf-CIS/DTX-PLHN exhibited larger particle size (189.5 ± 5.9 nm) compared with CIS/DTX-LN (156.3 ± 4.9 nm), which is also reported by others that the adding of Tf slightly enlarged the particle sizes.36 Increased surface charge of Tf functionalized nanoparticles may attribute to the positive charged functional groups of Tf. PDIs less than 0.2 indicated the uniform distribution of the nanoparticles.
Scientific rigorous in vitro experiments could help with the evaluating of the in vivo characters of the system. The in vitro release study is a kind of test in the environment of simulated temperature, medium condition and motion state.37 The drug release results showed that the drugs were sustained released from the nanoparticles. Slower drugs release kinetics of Tf-CIS/DTX-PLHN compared with CIS/DTX-LN may attribute to the Tf layer that hindered the drug release.
Drug delivery systems that induce significant cytotoxic effects will provide a valuable pathway for the delivery of antitumor drugs.38 At the equivalent drug concentrations, drugs loaded nanoparticles showed higher cell inhibition efficiency compared with the free drugs (P < 0.05). This is because free drugs diffuse through cell membranes but nanoparticles are internalized through the endocytic pathway, resulting in larger amount uptake and higher cytotoxicity than free drugs.39 It is unlikely that such a high concentration of the free drug would persist for such a long duration of treatment for in vivo applications; however, drug-loaded nanoparticles may contribute to their passive accumulation in tumor tissue by enhancing the permeability and retention (EPR) effect. Combination index (CI) was raised by Chou and Talalay to evaluate the synergistic effects among drugs.40 When the CIS: DTX ratio was 3:5, the most prominent synergistic effect was achieved. Higher cellular uptake efficiency and increased apoptotic cell population of Tf-CIS/DTX-PLHN than that of CIS/DTX-LN could prove the targeting effect of transferrin to the receptor contained cancer cells. Cell apoptosis results illustrated that CIS and DTX combined CIS/DTX-LN induced cell death more effectively than the individual CIS-LN and DTX-LN.
In vivo tissue biodistribution of Tf-CIS/DTX-PLHN in tumor was higher than non Tf involved CIS/DTX-LN and free drugs at 24 h post injection, and less in the heart and kidney, these behaviors may reduce the toxicity of in vivo injection. These results were in line with the research carried by other researchers.41,42 They found that the preferential accumulation of nanoparticles in tumor due to the EPR effect.41 The existence of PEG chain on particle surface plays a stealth role on the system, which is responsible for the long circulating of LPNs. Also coating of Tf could increase the accumulation in the tumor.42 The application of this particle might be limited because the biodistribution may be different in a primary or lung metastasis model. We would like to evaluate the anticancer effect in an orthotopic tumor model in the future research.
In vivo anti-tumor nanoparticles have more prominent potential than free drugs, which may be due to enhanced nanoparticle accumulation in tumor sites and effective encapsulation of drugs against leakage in the bloodstream that can promote intracellular release of drugs and contribute to enhanced anti-cancer efficacy.43 CIS/DTX-LN presented more profound anti-tumor capacity than CIS-LN and DTX-LN, which is in line with the synergistic effect evaluation results that the drugs combination could achieve additive effect than the drugs used alone. Tf contained Tf-CIS/DTX-PLHN showed higher tumor suppression ability than that of CIS/DTX-LN, which could prove that the protein-lipid hybrid nanoparticle had a remarkable benefit. No significant change of body weight further proved the low systemic toxicity of the nanoparticles, which could be safe when administrated in vivo.
Conclusion
In this study, Tf-functionalized protein-lipid hybrid nanoparticle (Tf-CIS/DTX-PLHN) was developed with a nano-size of 189.5 ± 5.9 nm, and a surface tested to be −16.9 ± 2.1 mV. Tf-CIS/DTX-PLHN exhibited a remarkable tumor cell inhibition ability, outstanding tumor suppression capacity compared with other systems. The results indicated that Tf-CIS/DTX-PLHN it could inhibit the lung tumor growth and help with the lung cancer treatment. No significant change of body weight further proved the low systemic toxicity of the nanoparticles, which could be safe when administrated in vivo.
Disclosure
The authors do not have any conflict of interest for this work to declare.
References
1. Torre LA, Siegel RL, Jemal A. Lung cancer statistics. Adv Exp Med Biol. 2016;893:1–19.
2. Scheff RJ, Schneider BJ. Non-small-cell lung cancer: treatment of late stage disease: chemotherapeutics and new frontiers. Semin Intervent Radiol. 2013;30(2):191–198. doi:10.1055/s-0033-1342961
3. Chen L, Kim JS, San Antonio B, Zhu YE, Mitchell L, John W. Safety outcomes in advanced non-small-cell lung cancer patients treated with first-line platinum-based regimens in the United States. J Thorac Dis. 2019;11(11):4474–4483. doi:10.21037/jtd.2019.11.11
4. Azzoli CG, Baker S
5. NSCLC Meta-Analyses Collaborative Group. Chemotherapy in addition to supportive care improves survival in advanced non-small-cell lung cancer: a systematic review and meta-analysis of individual patient data from 16 randomized controlled trials. J Clin Oncol. 2008;26(28):4617–4625. doi:10.1200/JCO.2008.17.7162
6. Schiller JH, Harrington D, Belani CP, et al.; Eastern Cooperative Oncology Group. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002;346(2):92–98.
7. Kelly K, Crowley J, Bunn PA
8. Socinski MA, Bondarenko I, Karaseva NA, et al. Weekly nab-paclitaxel in combination with carboplatin versus solvent-based paclitaxel plus carboplatin as first-line therapy in patients with advanced non-small-cell lung cancer: final results of a phase III trial. J Clin Oncol. 2012;30(17):2055–2062. doi:10.1200/JCO.2011.39.5848
9. Al-Attar T, Madihally SV. Recent advances in the combination delivery of drug for leukemia and other cancers. Expert Opin Drug Deliv. 2020;22:1–11.
10. Liu TI, Lu TY, Chang SH, Shen MY, Chiu HC. Dual stimuli-guided lipid-based delivery system of cancer combination therapy. J Control Release. 2020;318:16–24. doi:10.1016/j.jconrel.2019.12.002
11. Li S, Wang L, Li N, Liu Y, Su H. Combination lung cancer chemotherapy: design of a pH-sensitive transferrin-PEG-Hz-lipid conjugate for the co-delivery of docetaxel and baicalin. Biomed Pharmacother. 2017;95:548–555. doi:10.1016/j.biopha.2017.08.090
12. Liu B, Han L, Liu J, Han S, Chen Z, Jiang L. Co-delivery of paclitaxel and TOS-cisplatin via TAT-targeted solid lipid nanoparticles with synergistic antitumor activity against cervical cancer. Int J Nanomedicine. 2017;31(12):955–968. doi:10.2147/IJN.S115136
13. Gaber M, Medhat W, Hany M, Saher N, Fang JY, Elzoghby A. Protein-lipid nanohybrids as emerging platforms for drug and gene delivery: challenges and outcomes. J Control Release. 2017;28(254):75–91. doi:10.1016/j.jconrel.2017.03.392
14. Müller RH, Mäder K, Gohla S. Solid lipid nanoparticles (SLN) for controlled drug delivery - a review of the state of the art. Eur J Pharm Biopharm. 2000;50(1):161–177. doi:10.1016/S0939-6411(00)00087-4
15. Jung SH, Kim SK, Jung SH, et al. Increased stability in plasma and enhanced cellular uptake of thermally denatured albumin-coated liposomes. Colloids Surf B Biointerfaces. 2010;76(2):434–440. doi:10.1016/j.colsurfb.2009.12.002
16. Xu Y, Jin X, Ping Q, et al. A novel lipoprotein-mimic nanocarrier composed of the modified protein and lipid for tumor cell targeting delivery. J Control Release. 2010;146(3):299–308. doi:10.1016/j.jconrel.2010.05.022
17. Pandey V, Gajbhiye KR, Soni V. Lactoferrin-appended solid lipid nanoparticles of paclitaxel for effective management of bronchogenic carcinoma. Drug Deliv. 2015;22(2):199–205. doi:10.3109/10717544.2013.877100
18. Kuo YC, Cheng SJ. Brain targeted delivery of carmustine using solid lipid nanoparticles modified with tamoxifen and lactoferrin for antitumor proliferation. Int J Pharm. 2016;499(1–2):10–19. doi:10.1016/j.ijpharm.2015.12.054
19. Xu G, Chen Y, Shan R, Wu X, Chen L. Transferrin and tocopheryl-polyethylene glycol-succinate dual ligands decorated, cisplatin loaded nano-sized system for the treatment of lung cancer. Biomed Pharmacother. 2018;99:354–362. doi:10.1016/j.biopha.2018.01.062
20. Tan S, Wang G. Lung cancer targeted therapy: folate and transferrin dual targeted, glutathione responsive nanocarriers for the delivery of cisplatin. Biomed Pharmacother. 2018;102:55–63. doi:10.1016/j.biopha.2018.03.046
21. Zhang B, Zhang Y, Yu D. Lung cancer gene therapy: transferrin and hyaluronic acid dual ligand-decorated novel lipid carriers for targeted gene delivery. Oncol Rep. 2017;37(2):937–944. doi:10.3892/or.2016.5298
22. Zhang Q, Tian X, Cao X. Transferrin-functionalised microemulsion co-delivery of β-elemene and celastrol for enhanced anti-lung cancer treatment and reduced systemic toxicity. Drug Deliv Transl Res. 2019;9(3):667–678. doi:10.1007/s13346-019-00623-4
23. Yue Y, Zhao D, Yin Q. Hyaluronic acid modified nanostructured lipid carriers for transdermal bupivacaine delivery: in vitro and in vivo anesthesia evaluation. Biomed Pharmacother. 2018;98:813–820. doi:10.1016/j.biopha.2017.12.103
24. Lopalco A, Cutrignelli A, Denora N, Lopedota A, Franco M, Laquintana V. Transferrin functionalized liposomes loading dopamine HCL: development and permeability studies across an in vitro model of human blood-brain barrier. Nanomaterials (Basel). 2018;8(3):178. doi:10.3390/nano8030178
25. Juang V, Chang CH, Wang CS, Wang HE, Lo YL. pH-responsive PEG-shedding and targeting peptide-modified nanoparticles for dual-delivery of irinotecan and microRNA to enhance tumor-specific therapy. Small. 2019;15(49):e1903296. doi:10.1002/smll.201903296
26. Guo S, Wang Y, Miao L, et al. Lipid-coated cisplatin nanoparticles induce neighboring effect and exhibit enhanced anticancer efficacy. ACS Nano. 2013;7(11):9896–9904. doi:10.1021/nn403606m
27. Guan Q, Sun D, Zhang G, et al. Docetaxel-loaded self-assembly stearic acid-modified bletilla striata polysaccharide micelles and their anticancer effect: preparation, characterization, cellular uptake and in vitro evaluation. Molecules. 2016;21(12):1641. doi:10.3390/molecules21121641
28. Han S, Sun R, Su H, et al. Delivery of docetaxel using pH-sensitive liposomes based on D-α-tocopheryl poly(2-ethyl-2-oxazoline) succinate: comparison with PEGylated liposomes. Asian J Pharm Sci. 2019;14(4):391–404. doi:10.1016/j.ajps.2018.07.005
29. Li M, Tang Z, Lv S, et al. Cisplatin crosslinked pH-sensitive nanoparticles for efficient delivery of doxorubicin. Biomaterials. 2014;35(12):3851–3864. doi:10.1016/j.biomaterials.2014.01.018
30. Guo S, Zhang Y, Wu Z, et al. Synergistic combination therapy of lung cancer: cetuximab functionalized nanostructured lipid carriers for the co-delivery of paclitaxel and 5-demethylnobiletin. Biomed Pharmacother. 2019;118:109225. doi:10.1016/j.biopha.2019.109225
31. He YC, He L, Khoshaba R, et al. Curcumin nicotinate selectively induces cancer cell apoptosis and cycle arrest through a P53-mediated mechanism. Molecules. 2019;24(22):4179. doi:10.3390/molecules24224179
32. Tan KT, Li S, Li YR, Cheng SL, Lin SH, Tung YT. Synergistic anticancer effect of a combination of paclitaxel and 5-demethylnobiletin against lung cancer cell line in vitro and in vivo. Appl Biochem Biotechnol. 2019;187(4):1328–1343. doi:10.1007/s12010-018-2869-1
33. Wang H, Zhang F, Wen H, et al. Tumor- and mitochondria-targeted nanoparticles eradicate drug resistant lung cancer through mitochondrial pathway of apoptosis. J Nanobiotechnology. 2020;18(1):8. doi:10.1186/s12951-019-0562-3
34. Xie F, Ding RL, Wf H, et al. In vivo antitumor effect of endostatin-loaded chitosan nanoparticles combined with paclitaxel on Lewis lung carcinoma. Drug Deliv. 2017;24(1):1410–1418. doi:10.1080/10717544.2017.1378938
35. Liu H, Shen M, Zhao D, et al. The effect of triptolide-loaded exosomes on the proliferation and apoptosis of human ovarian cancer SKOV3 cells. Biomed Res Int. 2019;2019:2595801.
36. Zhou J, Li M, Lim WQ, et al. A transferrin-conjugated hollow nanoplatform for redox-controlled and targeted chemotherapy of tumor with reduced inflammatory reactions. Theranostics. 2018;8(2):518–532. doi:10.7150/thno.21194
37. Dong X, Shu X, Wang Y, et al. Synthesis, characterization and in vitro release performance of the pegylated valnemulin prodrug. J Vet Med Sci. 2018;80(1):173–180. doi:10.1292/jvms.17-0434
38. Pang J, Xing H, Sun Y, Feng S, Wang S. Non-small cell lung cancer combination therapy: hyaluronic acid modified, epidermal growth factor receptor targeted, pH sensitive lipid-polymer hybrid nanoparticles for the delivery of erlotinib plus bevacizumab. Biomed Pharmacother. 2020;125:109861. doi:10.1016/j.biopha.2020.109861
39. Zhong Y, Zhang J, Cheng R, et al. Reversibly crosslinked hyaluronic acid nanoparticles for active targeting and intelligent delivery of doxorubicin to drug resistant CD44+ human breast tumor xenografts. J Control Release. 2015;205:144–154. doi:10.1016/j.jconrel.2015.01.012
40. Chou TC, Talalay P. Analysis of combined drug effects: a new look at a very old problem. Trends Pharmacol Sci. 1983;4:450–454. doi:10.1016/0165-6147(83)90490-X
41. Nan Y. Lung carcinoma therapy using epidermal growth factor receptor-targeted lipid polymeric nanoparticles co-loaded with cisplatin and doxorubicin. Oncol Rep. 2019;42(5):2087–2096.
42. Wang J, Su G, Yin X, et al. Non-small cell lung cancer-targeted, redox-sensitive lipid-polymer hybrid nanoparticles for the delivery of a second-generation irreversible epidermal growth factor inhibitor-afatinib: in vitro and in vivo evaluation. Biomed Pharmacother. 2019;120:109493. doi:10.1016/j.biopha.2019.109493
43. Zheng G, Zheng M, Yang B, Fu H, Li Y. Improving breast cancer therapy using doxorubicin loaded solid lipid nanoparticles: synthesis of a novel arginine-glycine-aspartic tripeptide conjugated, pH sensitive lipid and evaluation of the nanomedicine in vitro and in vivo. Biomed Pharmacother. 2019;116:109006. doi:10.1016/j.biopha.2019.109006
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


