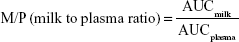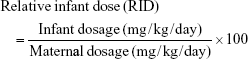Back to Journals » Drug Design, Development and Therapy » Volume 12
Transfer of rosuvastatin into breast milk: liquid chromatography–mass spectrometry methodology and clinical recommendations
Authors Lwin EMP , Leggett C, Ritchie U, Gerber C, Song Y , Hague W , Turner S, Upton R , Garg S
Received 15 August 2018
Accepted for publication 21 September 2018
Published 29 October 2018 Volume 2018:12 Pages 3645—3651
DOI https://doi.org/10.2147/DDDT.S184053
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Georgios Panos
Ei Mon Phyo Lwin,1 Catherine Leggett,2 Usha Ritchie,2 Cobus Gerber,1 Yunmei Song,1 William Hague,3,4 Sean Turner,2 Richard Upton,1 Sanjay Garg1
1School of Pharmacy and Medical Sciences, University of South Australia, Adelaide, SA 5000, Australia; 2SA Pharmacy, Women’s and Children’s Hospital, North Adelaide, SA 5006, Australia; 3Robinson Research Institute, University of Adelaide, SA 5006, Australia; 4Obstetric Medicine, Women’s and Children’s Hospital, North Adelaide, SA 5006, Australia
Introduction: Rosuvastatin reduces concentrations of total cholesterol (TC) and is used for the management of hypercholesterolemia and prevention of acute coronary syndromes. There are no published reports estimating infant exposure to rosuvastatin through breast milk.
Purpose: The aims of this study were to quantify concentrations of rosuvastatin in human milk and plasma from a lactating woman taking rosuvastatin and to investigate potential infant exposure.
Materials and methods: A 38-year-old breastfeeding mother was commenced on rosuvastatin 20 mg daily for secondary prevention of an acute coronary syndrome. Eight maternal breast milk samples and a single plasma sample were collected over a 24-hour period. The samples were quantified using a sensitive liquid chromatography–mass spectrometry (LC-MS/MS) method.
Results: The average concentration of rosuvastatin in breast milk was 30.84 ng/mL, and a peak concentration of 58.59 ng/mL occurred at 17 hours after oral administration. Although the milk-to-plasma (M/P) ratio was 16.49 at 14 hours, the theoretical infant dosage (TID) and relative infant dose (RID) were 0.005 mg/kg/day and 1.50%, respectively.
Conclusion: The findings suggest that only small amounts of rosuvastatin pass into breast milk. Should the maternal condition necessitate treatment, consideration could be given to the use of rosuvastatin during breastfeeding provided the infant is monitored.
Keywords: rosuvastatin, LC-MS/MS, human plasma, human milk, lactation
Introduction
Rosuvastatin is a 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitor indicated for the management of hypercholesterolemia and dyslipidemia and prevention of acute coronary syndrome. The health benefits of statin use are protection from stroke or heart attack and possible cardiovascular protection in diabetic individuals.1 Rosuvastatin reduces total cholesterol (TC), low-density lipoprotein (LDL) cholesterol and triglycerides (TGs) and increases high-density lipoprotein (HDL).2 Rosuvastatin is administered at a usual daily dose of 5–20 mg, as its half-life is approximately 19 hours.3 Theoretically, rosuvastatin may be more suitable for use in breastfeeding than other statins as it has a relatively large molecular weight (1,001.14 g/mol), a higher degree of protein binding (90%) and a higher volume of distribution compared with other statins.3,4 In addition, rosuvastatin has a pKa of 4.6 and is considered to be a hydrophilic statin. The use of cholesterol-lowering medications during breastfeeding is currently not recommended due to potential adverse effects on the breastfed infant. Cholesterol and other products of cholesterol synthesis are vital to infant development, and concerns have been raised that the use of statins may disrupt infant lipid metabolism.3,4
There is limited published evidence of statin transfer into rat and human milk. Henck et al5 reported that atorvastatin concentrations in rat milk are three times higher than those in plasma, but this may not accurately reflect human milk concentrations.6 Schutte et al6 confirmed the presence of rosuvastatin in human milk after oral administration. This study, however, was not conducted according to Food and Drug Administration (FDA) guidelines and did not report either a 24-hour concentration–time curve or provide an estimation of infant exposure.7
Given this paucity of information, we therefore aimed to measure the amount of rosuvastatin present in human breast milk and plasma from a lactating woman taking rosuvastatin to estimate the exposure of the breastfed infant to the drug.
Materials and methods
The pure forms of rosuvastatin powder and rosuvastatin-d3 powder (Toronto Research Chemicals Inc., Toronto, ON, Canada) were used as a reference standard and an internal standard (IS), respectively. Control and standard samples were prepared using human plasma (Red Cross Australia) and human milk. Acetonitrile (EMD Millipore, Billerica, MA, USA) was used for the protein precipitation and mobile phase. Formic acid (BDH Chemicals Ltd., Poole, England) and MilliQ water (Sartorius Stedim Biotech, Goettingen, Germany) were used to prepare the mobile phase.
Methods
Assay (liquid chromatography–mass spectrometry [LC-MS/MS] method)
An assay method developed and validated for the detection of rosuvastatin in human plasma by Kumar et al8 was adopted. The method was optimized to quantify nanogram levels of rosuvastatin in human plasma and milk. Partial validation was conducted by checking sensitivity, specificity and linearity. The analytical assay for rosuvastatin was performed using a Gemini C-18 (50 × 4.6 mm i.d., 3 μm particle size) column supplied by Phenomenex® (Torrance, CA, USA) and LC-MS/MS 8060 (Shimadzu, Kyoto, Japan). Positive ion mode was used for both rosuvastatin and the IS. The ion transitions for rosuvastatin and the IS were m/z 482.25/258.20 and m/z 485.05/261.30, respectively. An isocratic elution at a flow rate of 0.4 mL/min was employed, including mobile phase (70% of acetonitrile and 30% of 0.1% formic acid [v/v]) with a run time of 5 minutes. The column oven was set at 25°C, and the injection volume was 5 μL. Other LC-MS/MS instrument parameters including dwell time (ms), Q1 pre-bias (V), Collision energy (CE) and Q3 pre-bias (V) were 100.0, −47.0, −33.8, and −25.0, respectively, for rosuvastatin, and 100.0, −23.0, −38.3, and −26.0, respectively, for the IS.
Preparation of the stock solutions
An initial stock solution of rosuvastatin (1,000 μg/mL) was prepared in 75% acetonitrile. The solution was serially diluted with 75% acetonitrile to make concentrations of 100 μg/mL, 10 μg/mL, 1 μg/mL, 100 ng/mL and 50 ng/mL. An IS stock solution (10 μg/mL) was also prepared in 75% acetonitrile.
Preparation of standard curve, quality control and test samples
Final concentration range of rosuvastatin (0.1–100 ng/mL, eight concentrations in total) standard solutions was prepared. Blank whole milk and plasma were removed from the −80°C freezer and allowed to thaw at room temperature before sample preparation. Stock solutions of rosuvastatin and IS were spiked into drug-free human milk and human plasma, obtaining a total volume of 500 μL. The spiked samples were mixed thoroughly by vortex mixing for approximately 1 minute. Our previously developed protein precipitation and ultracentrifugation methods3,9,10 were used for the separation of rosuvastatin from biological samples. Drug-free human plasma and milk samples were used as negative controls.
Study design and participants
This study was approved by the Women’s and Children’s Health Network (WCHN) Human Research Ethics Committee (HREC14/WCHN/115) and the University of South Australia Research Ethics Committee (0000033979).
Our participant was a 38-year-old breastfeeding woman (weight 65 kg, height 154 cm, nonsmoker) recently diagnosed with a non-ST-elevated myocardial infarction and commenced on rosuvastatin 20 mg at night for secondary prevention. Her infant was 13 months old, and the decision was made to cease breastfeeding upon commencing rosuvastatin. Written informed consent was obtained from the woman for the collection and analysis of milk and plasma samples.
Collection and preparation of patient samples
Sample collection was conducted once rosuvastatin reached steady state (≥5 days). Eight expressed milk samples (up to 10 mL each) were collected over a 24-hour period (ie, at least one dosing interval). The woman was asked to attend the Women’s and Children’s Hospital (WCH), Adelaide, SA, Australia, to provide the first milk sample and one maternal blood sample. Subsequent milk samples were collected in the woman’s home using pre-labeled containers. According to the FDA recommendation, the patient was advised to express milk from each breast and mix. Milk samples were stored in a domestic refrigerator and returned within 24 hours where they were stored at −80°C until analysis.
Statistical analyses
Pharmacokinetic (PK) parameters of rosuvastatin in breast milk were calculated in accordance with FDA guidelines,7 including the area under the milk concentration–time curve from time zero to time of last measurable concentration (AUClast), the dose-normalized area under the milk concentration–time curve over 24 hours (AUC_D; AUC0–24), the dose-normalized milk peak concentration (Cmax_D), the time to peak plasma concentration (Tmax), the average steady-state milk drug concentration during multiple-dose administration (Cavg) and the terminal half-life (t1/2). The dose-normalized area under the curve (AUC) (AUC_D) was calculated by dividing the AUClast by the dose. The AUC and Cmax values were normalized to maternal dose, and average milk concentration was calculated. The parameters were normalized to the dose of 20 mg. Noncompartmental PK analysis with Phoenix™ Winnonlin® 7.0 software was used to calculate the PK parameters of the drug.
Theoretical infant dose (TID), milk-to-plasma (M/P) ratio and relative infant dose (RID) of rosuvastatin were calculated (Equations 1–3).3,11
|
|
|
|
|
|
According to Equation 1, as stated by Begg et al,12 Bennett,13 Hägg and Spigset,14 and Kristensen et al,15 150 mL/kg/day is the standardized milk consumption for a 2-month-old infant.7
Results
Assay optimization
The existing assay method8 was optimized for high sensitivity of rosuvastatin detection in both human plasma and milk. The optimized method provided the lower limit of quantification (LLOQ) of 0.1 ng/mL (Figures 1 and 2) and a linear range of 0.1–100 ng/mL (0.1, 0.3, 0.5, 1, 5, 10, 50 and 100 ng/mL) with a correlation coefficient (r2) of 0.998 or better in both human plasma and milk. Chromatograms of rosuvastatin in human plasma and milk are shown in Figure 3. The retention time of both rosuvastatin and the IS was 1.7 minutes, with no matrix interference from plasma or milk. Each point was collected at every 0.412 seconds; hence, 8.03 points were captured across a peak. The optimized analytical method was selective and specific to differentiate and quantify the analyte and IS in the presence of other components in the plasma and milk samples.
  | Figure 1 Chromatograms of rosuvastatin (0.1 ng/mL) (A) and IS (50 ng/mL) (B) in human milk. |
Analysis of patient samples
Rosuvastatin milk concentrations ranged from 16.58 to 58.59 ng/mL over the 24-hour study period (Figure 4). The milk concentration of rosuvastatin remained relatively constant (20 ng/mL) for the first 10 hours after oral administration before reaching a peak (Cmax 58.59 ng/mL) at 17 hours (plasma Tmax) and subsequently declining.
  | Figure 4 Concentration–time curve of rosuvastatin in breast milk. |
A maternal plasma concentration of 2.47 ng/mL was observed at 14.42 hours after taking the rosuvastatin dose. The drug concentration in breast milk was higher compared with plasma, and the M/P ratio was calculated as 16.49 at 14 hours after the dose.
PK studies
The participant did not have any medical conditions or concomitant medication use known to affect the PK of rosuvastatin. PK parameters were predicted using analyzed data from milk samples. Figure 5 shows the calculated and predicted rosuvastatin concentration–time profile in breast milk.
  | Figure 5 Observed and predicted concentration–time profiles of rosuvastatin in breast milk. |
The estimated PK parameters of rosuvastatin in breast milk and prediction of infant exposure are summarized in Table 1. The estimated half-life and Tmax were 5.66 and 16.83 hours, respectively. The estimated infant dose was calculated as 4.63 μg/kg/day. Although the M/P ratio was relatively high (16.49), the RID (percentage of mother’s weight-adjusted dose) was only 1.50.
Discussion
Although this is the second report of rosuvastatin use in a breastfeeding woman, our study is novel and we have provided an estimate of infant exposure.
Despite PK parameters that would suggest low extent of transfer into human milk (large molecular weight, high protein binding and hydrophilic properties), our findings show that rosuvastatin does transfer preferentially into milk, as demonstrated by the high M/P ratio (M/P ratio 16.49). This finding is consistent with the case study by Schutte et al,6 who reported higher milk concentrations compared with those in plasma at 21–23 hours after the maternal dose was taken. Although confirming that rosuvastatin transfers into milk, the M/P ratio should be interpreted with care, as it is not indicative of overall infant exposure to a medicine; if the plasma and milk values are overall low, the ratio on its own is not clinically relevant. In addition, in our situation, milk and plasma concentrations were only correlated at a single time point and therefore may not be reflective of changing patterns of extent of transfer over a dosing interval.
More importantly, we determined a RID for rosuvastatin of 1.5% based on the mother’s weight-adjusted dose. A medicine with a RID of <10% is generally considered as compatible with breastfeeding.3 Our calculated RID is consistent with that reported by Hale and Rowe,3 who calculated a RID of 0.6%–0.77% for rosuvastatin based on the study by Schutte et al.6 The TID for rosuvastatin based on the average rosuvastatin milk concentration and a standard infant milk consumption volume of 150 mg/kg/day was found to be 4.63 μg/kg/day. Although there are no defined infant dosing limits for rosuvastatin, it should be noted that this exposure is significantly lower than an adult treatment dose; a 50 kg patient taking a usual maximum dose of 20 mg/day would receive 400 μg/kg/day. This finding is reassuring that infant exposure to rosuvastatin via breast milk would have been low in our participant.
The use of lipid-lowering medications during breastfeeding is often discouraged. Cholesterol biosynthesis is known to be vital for infant development,3 and in most clinical situations a temporary discontinuation in treatment for the duration of pregnancy and breastfeeding would rarely give cause for concern. However, there are some clinical scenarios where treatment is considered more critical, for example, as in our case where the participant required treatment for secondary prophylaxis of a myocardial infarction. In such situations, there may historically have been a clinical recommendation to discontinue breastfeeding. However, our study found that overall infant exposure to rosuvastatin via breast milk appears low. With the known benefits of breastfeeding (eg, enhancing infant development and providing protection against infection),16 the results of our study provide reassurance that rosuvastatin use during breastfeeding may be considered. As this is only the second case reported in the literature, further investigation is required before stronger recommendations can be made regarding the safety of rosuvastatin during breastfeeding. Until further information is available, the authors advise that rosuvastatin use during breastfeeding should occur with careful monitoring of the infant for growth and normal developmental milestones.
There are some limitations to our study. The M/P ratio calculated from a single time point will be different from the results calculated from AUCs. Hence, other important parameters including RID and TID were justified. Second, as breast milk samples were collected in the home environment, we were reliant on self-reporting and documentation of collection times by the participant. Detailed instructions and data collection labels were provided to reduce variability and improve reliability. Finally, the infant was not breastfed while the mother was taking rosuvastatin, and therefore it was not possible to quantify rosuvastatin in infant plasma, which is considered the best way of determining infant exposure via breast milk.
Conclusion
Our study confirms that rosuvastatin appears to pass preferentially into breast milk; however, the estimated infant exposure is low. The decision to treat a breastfeeding woman with rosuvastatin should be made with careful consideration of the potential benefit of maternal treatment vs the risks to the infant (ie, not receiving breast milk or theoretical harm from the medication). If there is clear clinical benefit for the mother and given the known benefits of breastfeeding for infants, our study provides reassurance that the rosuvastatin dose delivered to the infant through breast milk is likely to be small. Given the paucity of information regarding the effects of exposure to low doses of rosuvastatin through breast milk, careful monitoring of the infant for growth, weight gain and developmental milestones is recommended.
Acknowledgments
We would like to acknowledge the other untreated women at the WCH, Adelaide, SA, Australia, who supplied milk samples, the clinicians at the hospital for allowing us access to their patients and the Australian Red Cross for supplying human plasma from untreated women. We are also grateful for the provision of an Australian Government (Research Training Program) Scholarship. This study did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
Disclosure
The authors report no conflicts of interest in this work.
References
Eldor R, Raz I. American Diabetes Association indications for statins in diabetes: is there evidence? Diabetes Care. 2009;32(Suppl 2):S384–S391. | ||
Singh SS, Sharma K, Patel H, et al. Estimation of rosuvastatin in human plasma by HLPC tandem mass spectroscopic method and its application to bioequivalence study. J Braz Chem Soc. 2005;16(5):944–950. | ||
Hale TW, Rowe HE. Medications & Mothers’ Milk. 17th ed. New York, USA: Springer Publishing Company, LLC; 2017. | ||
Holmsen ST, Bakkebø T, Seferowicz M, Retterstøl K. Statins and breastfeeding in familial hypercholesterolaemia. Tidsskr Nor Laegeforen. 2017;137(10):686–687. | ||
Henck JW, Craft WR, Black A, Colgin J, Anderson JA. Pre- and postnatal toxicity of the HMG-CoA reductase inhibitor atorvastatin in rats. Toxicol Sci. 1998;41(1):88–99. | ||
Schutte AE, Symington EA, du Preez JL. Rosuvastatin is transferred into human breast milk: a case report. Am J Med. 2013;126(9):e7–e8. | ||
U. S. Department of Health and Human Services (U.S. DHHS); Food and Drug Administration (FaDAF); Center for Drug Evaluation and Research (CDER); Center for Biologics Evaluation and Research (CBER). Guidance for Industry: Clinical Lactation Studies – Study Design, Data Analysis, and Recommendations for Labeling. Maryland, USA: Food and Drug Administration; 2005. | ||
Kumar PP, Murthy TEGK, Basaveswara Rao MV. Development, validation of liquid chromatography-tandem mass spectrometry method for simultaneous determination of rosuvastatin and metofrmin in human plasma and its application to a pharmacokinetic study. J Adv Pharm Technol Res. 2015; 6(3):118–124. | ||
Phyo Lwin EM, Gerber C, Song Y, et al. A new LC-MS/MS bioanalytical method for atenolol in human plasma and milk. Bioanalysis. 2017;9(7):517–530. | ||
Lwin EMP, Gerber C, Song Y, et al. A new LC-MS/MS bioanalytical method for perindopril and perindoprilat in human plasma and milk. Anal Bioanal Chem. 2017;409(26):6141–6148. | ||
Anderson PO, Sauberan JB. Modeling drug passage into human milk. Clin Pharmacol Ther. 2016;100(1):42–52. | ||
Begg EJ, Duffull SB, Saunders DA, et al. Paroxetine in human milk. Br J Clin Pharmacol. 1999;48(2):142–147. | ||
Bennett PN. Drugs and Human Lactation. 2nd ed. Amsterdam: Elsevier; 1988. | ||
Hägg S, Spigset O. Anticonvulsant use during lactation. Drug Saf. 2000;22(6):425–440. | ||
Kristensen JH, Ilett KF, Hackett LP, Yapp P, Paech M, Begg EJ. Distribution and excretion of fluoxetine and norfluoxetine in human milk. Br J Clin Pharmacol. 1999;48(4):521–527. | ||
Uhl K. Activities at FDA: Drug use in pregnancy and lactation. Jpn J Clin Pharmacol Therap. 2006;37(6):324–330. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.