Back to Journals » Drug Design, Development and Therapy » Volume 8
Transdermal fentanyl for pain due to chemoradiotherapy-induced oral mucositis in nasopharyngeal cancer patients: evaluating efficacy, safety, and improvement in quality of life
Authors Guo S, Wu S, Zhou J, Feng H, Li F, Wu J, Sun J, He Z
Received 7 January 2014
Accepted for publication 28 March 2014
Published 12 May 2014 Volume 2014:8 Pages 497—503
DOI https://doi.org/10.2147/DDDT.S60187
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Su-Ping Guo,1,* San-Gang Wu,2,* Juan Zhou,3,* Hui-Xia Feng,1 Feng-Yan Li,1 Ying-Jia Wu,1 Jia-Yuan Sun,1 Zhen-Yu He1
1Department of Radiation Oncology, Sun Yat-sen University Cancer Center, State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Guangzhou, People's Republic of China; 2Department of Radiation Oncology, Xiamen Cancer Center, the First Affiliated Hospital of Xiamen University, Xiamen, People's Republic of China; 3Department of Obstetrics and Gynecology, Xiamen Cancer Center, the First Affiliated Hospital of Xiamen University, Xiamen, People's Republic of China
*These authors contributed equally to this work
Abstract: This study evaluated the efficacy, safety, and quality of life (QoL) measure of transdermal fentanyl (TDF) for moderate-to-severe pain due to oral mucositis caused by chemoradiotherapy in patients with advanced nasopharyngeal carcinoma (NPC). Patients with NPC who experienced moderate-to-severe oral mucosal pain during chemoradiotherapy (n=78) received TDF for pain relief. Pain relief and QoL were compared before and after treatment. The mean numeric rating scale score was reduced from 7.41±0.96 before treatment to 5.54±0.86, 3.27±0.73, 2.88±0.62, and 2.82±0.68 on days 1, 4, 7, and 10, respectively, after treatment (P<0.001). Karnofsky performance status and SPAASMS (Score for pain, Physical activity levels, Additional pain medication, Additional physician/emergency room visits, Sleep, Mood, and Side effects) scores showed significant improvement after treatment, indicating an improved QoL of patients (both P<0.001). The most common adverse reactions were nausea and vomiting (10.26%). No serious life-threatening adverse events and no symptoms of drug withdrawal were observed. TDF is effective, safe, and improves QoL in treating pain due to oral mucositis caused by chemoradiotherapy in NPC patients.
Keywords: nasopharyngeal cancer, transdermal fentanyl, noncancerous pain, quality of life, mucositis
Introduction
Although nasopharyngeal carcinoma (NPC) is seen in many countries and regions worldwide, it is common in the People’s Republic of China, especially in the south.1 It is reported that approximately 70% of cases are stage III and IV at diagnosis, due to the anatomical structure of the nasopharynx and biological behavior of NPC.2,3 Over the past 20 years, clinical trials have proven the benefit of concurrent chemoradiotherapy for improving disease-free survival and overall survival in patients with locally advanced NPC, and concurrent chemoradiotherapy has become the standard treatment for locally advanced NPC.4–7 However, concurrent chemoradiotherapy is associated with increased toxicity, and oral mucositis (OM) is common. Wee et al7 reported that concurrent chemoradiotherapy resulted in significantly more cases of grade 3–5 OM compared with radiotherapy alone (48.1% versus 31.8%, respectively, P=0.0149). OM is accompanied by pain, which not only affects the quality of life (QoL) of patients, but also leads to difficulty eating and possibly an increase in treatment cost.
There are currently few strategies for preventing and reducing OM pain caused by concurrent chemoradiotherapy, and the primary treatments are oral cleaning, promotion of local mucosal recovery, nutritional supplements, antibiotics, and analgesics.8–11 Commonly used analgesics are local anesthetic drugs, such as lidocaine mouthwash. Although local anesthetics reduce oral mucosal pain, their duration of action is short and application is not convenient. The use of oral opioid analgesics has been reported, but their effects are affected by ingestion, so they cannot be widely used clinically.12,13 Additionally, the European Society for Medical Oncology and the National Comprehensive Cancer Network guidelines recommend patient-controlled analgesia (PCA) for the treatment of OM pain in hematopoietic stem cell-transplant and bone marrow-transplant patients.14,15 PCA administers analgesics intravenously at a constant rate, the effect is long-lasting and stable, the analgesic dose can be adjusted, and it has been shown that the method can control pain effectively.15 However, it is an invasive treatment and the cost is high, and thus its clinical use is limited.
Transdermal administration of the opioid fentanyl, a synthetic opioid-receptor agonist, is used in the treatment of pain. Via a transdermal patch, fentanyl is delivered into a subcutaneous reservoir, from which it is taken up into the systemic circulation. Because of continuous release, transdermal fentanyl (TDF) can achieve clinically meaningful analgesia for 72 hours per patch. For this reason, TDF has been recommended for the management of chronic cancer pain and general pain, including noncancerous pain.16,17 Reports on the efficacy of TDF for the treatment of OM pain caused by concurrent chemoradiotherapy are limited.18
The purpose of this open-label, prospective, single-center study was to evaluate the efficacy and safety of TDF for the treatment of mucositis pain caused by chemoradiotherapy in patients with advanced NPC.
Materials and methods
The study was conducted in an open-label fashion in the Department of Radiation Oncology, Sun Yat-sen University Cancer Center, from January 2011 to June 2013. The study was approved by the ethics committee of the Sun Yat-sen University Cancer Center.
Inclusion, exclusion, and pain criteria
Pain was evaluated according to the numeric rating scale (NRS), with 0 indicating no pain, 10 indicating the worst pain, 1–3 indicating mild pain that did not interfere with sleep, 4–6 indicating moderate pain that interfered with sleep, and scores ≥7 indicating severe pain with severe sleep interference.
Patients with NPC who developed chemoradiotherapy-induced OM and had an NRS pain score >5 and OM grade >1 according to the National Cancer Institute Common Toxicity Criteria (NCI CTC) 3.019 were included in this study. Patients with a history of opioid abuse, known allergy or hypersensitivity to fentanyl, or dysfunction of major organs, such as renal failure (creatinine >2.5 mg/dL), hepatic insufficiency (aspartate aminotransferase or alanine aminotransferase >80 units/L), heart or respiratory failure, and severe mental illness (including schizophrenia, major depression, panic disorder, schizoaffective disorder, obsessive–compulsive disorder, bipolar disorder, and autism), were excluded from this study.
Radiotherapy and concurrent chemotherapy regimens
Radical radiotherapy was implemented. The nasopharynx and neck were subjected to intensity-modulated radiation therapy. Target volumes were delineated according to our institutional treatment protocol, in agreement with the International Commission on Radiation Units and Measurements, reports 50 and 62.20,21 The prescribed doses were 70 Gy to the planning target volume (PTV) including the primary gross tumor volume, 60–62 Gy to the PTV enclosing clinical target volume 1 (ie, high-risk regions), 54 Gy to the PTV enclosing clinical target volume 2 (ie, low-risk regions and neck nodal regions), and 60–64 Gy to the nodal primary gross tumor volume in 33 fractions. Treatment was delivered once daily with five fractions per week, and all targets were treated at the same time using the simultaneous integrated boost technique.
Concurrent chemotherapy regimens consisted of cisplatin (80 mg/m2, day 1) with 5-fluorouracil (600 mg/m2, days 1–5) or cisplatin (80 mg/m2, day 1) with taxol (120 mg/m2, day 1) on day 1, with radiotherapy every 3 weeks for two or three cycles.
Examinations and TDF administration
Oral and dental examinations were carried out in all patients, and severe dental problems, such as inflammatory periapical abnormalities, periodontal status, and other dental disease, if any, were treated by a dentist before treatment. TDF (Duragesic®; Janssen Pharmaceutica, Beerse, Belgium) was administered at a rate of 25 μg/hour for patients with an NRS pain score >5 during concurrent chemoradiotherapy. The application site for the patch was examined to confirm that is was free of any skin irritation. The dose of TDF was increased by 25 μg/hour increments to maintain the NRS score ≤3 after the first 24 hours according to no change in pain control. All subjects were routinely treated with oral hygiene, and antiviral, antibacterial, or antifungal agents were prescribed as needed.
All patients were evaluated using the NRS and SPAASMS (Score for pain, Physical activity levels, Additional pain medication, Additional physician/ER Visits, Sleep, Mood, and Side effects) score (Table 1).22 SPAASMS values were evaluated by a physician and recorded before treatment with the TDF patch, then 4, 7, and 10 days later. NRS pain scores were recorded by the patients once a day (1 day before and during treatment until the score was ≤3).
Evaluation of efficacy, safety, and QoL
All patients had a physical examination and routine laboratory tests before treatment was initiated. They also underwent oral examinations by the same clinician to assess the degree of OM using the NCI CTC from the first day of chemoradiotherapy.
Analgesic efficacy was evaluated by comparing the NRS pain scores from before and after treatment with TDF. Safety assessments included evaluation of adverse events, laboratory tests, and vital signs. Adverse events were recorded during treatment, and the skin was examined for local reactions during treatment and after removal of TDF. QoL was assessed using Karnofsky performance status (KPS) standards of the Union for International Cancer Control and SPAASMS before and after 3 days of treatment with TDF.
Statistical analysis
All data were analyzed using the SPSS 18.0 statistical software package for Windows (PASW Statistics, Chicago, IL, USA). The Mann–Whitney U test was used to evaluate differences before and after treatment. P-values <0.05 were considered significant.
Results
Patient characteristics
A total of 78 patients with an NRS pain score >5 from OM were enrolled. The median patient age was 41 years (range 31–52 years). All patients were treated with chemoradiotherapy, in which the dose of intensity-modulated radiation therapy was 70 Gy/33 fractions. The characteristics of patients and treatments are summarized in Table 2.
None of the patients had received oral or slow-release opioid analgesics before they were enrolled in the study. All patients were treated with dexamethasone spray into the throat. Six patients were administered mild opioids (such as codeine), and 18 were administered a mouthwash (such as a 0.25% procaine or nonanalgesic mouthwash) prior to TDF therapy. The median time to the onset of moderate OM was 9 days (range 7–14 days) after beginning chemoradiotherapy, and the median time of onset of severe OM (NCI CTC grade 3) was 19 days (range 14–27 days). The number of patients with grade 3 mucositis was 32 (41.03%). Among patients with moderate OM pain, the median time to the onset of pain was 15 days after beginning chemoradiotherapy (range 10–21 days).
Efficacy
This study lasted for 3 weeks, and the median duration of TDF treatment was 9 days (range 3–20 days). The median number of patches used by the patients was three (range one to five). Two patients (2.56%) required a dose increase of TDF to 50 μg/hour.
The mean NRS pain score was reduced from 7.41±0.96 before treatment to 5.54±0.86 (P<0.001), 3.27±0.73 (P<0.001), 2.88±0.62 (P<0.001), and 2.82±0.68 (P<0.001) on days 1, 4, 7, and 10 after treatment, respectively. The median NRS pain score decreased to 5 after 24 hours of TDF treatment, and further declined to 3 after 72 hours. The median OM grades before TDF treatment and on days 1, 4, 7, 10 were 3, 3, 2, 2, and 1, respectively (Figure 1). No patient discontinued the TDF treatment, and all patients completed subsequent radiotherapy.
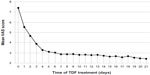  | Figure 1 Change of mean NRS score after treatment with TDF. |
Adverse events
The most common adverse reactions were nausea and vomiting (10.26%). Details of the treatment-related adverse events are shown in Table 3. No routine was used to prevent constipation due to lower incidence of TDF-induced constipation. All adverse events were NCI CTC grade 1, and were relieved after symptomatic treatment. No serious life-threatening adverse events were observed, and no symptoms of drug withdrawal or drug dependence were found after drug withdrawal. No patients had abnormal vital signs.
  | Table 3 Incidence of treatment-related adverse events |
QoL
The KPS scores of patients increased significantly after treatment compared with those before treatment (65.21±4.99 versus 79.39±2.39, respectively; P<0.001). In addition, SPAASMS values also showed significant improvement from before to after treatment, indicating an improvement in QoL (Table 4).
Discussion
TDF is an opioid that is an effective alternative to oral morphine. It provides a constant release of fentanyl through the skin for as long as 72 hours. It is not affected by gastrointestinal pH or food, and it has no hepatic first-pass effect, with a bioavailability of up to 92%. The duration of the analgesic effect is long, and the drug is easy to use and better tolerated than oral morphine.23 It is especially beneficial for patients who are unable to take oral medications.16,17 It has been reported that TDF has analgesic effects similar to PCA, and its use does not require a complex conversion schedule, which facilitates patient care.24–26 As early as 2000, TDF has been successfully used to treat severe OM pain caused by high-dose chemotherapy and after treatment, oral mucosal erythema was still present, but patients could ingest liquid food.27 Kim et al28 and Demarosi et al29 reported the effective use of TDF for the treatment of OM pain caused by high-dose chemotherapy in bone marrow stem cell-transplant recipients.
Current reports on the use of TDF for the treatment of OM pain have mainly focused on chemotherapy-induced pain.27–31 There are very limited reports on the effect of TDF for OM pain caused by concurrent chemoradiotherapy. Chang et al18 reported that TDF is effective and relatively easy to use for outpatient treatment of pain control in head and neck cancer patients following radiotherapy (57.7% with concurrent chemoradiotherapy). Our study showed that TDF could rapidly improve moderate-to-severe OM-related pain as a result of concurrent chemoradiotherapy in NPC patients. After treatment, KPS and SPAASMS values were significantly improved compared with those before treatment. Furthermore, patients’ oral hygiene was improved. No patients discontinued radiotherapy due to adverse events.
Strupp et al27 reported on the use of TDF to treat OM pain caused by postautologous hematopoietic stem cell-transplantation high-dose chemotherapy. A dose of 50 μg/hour was used in 94.6% of patients, and excellent analgesic effects were achieved. In our study, TDF at a dose of 25 μg/hour controlled OM pain caused by concurrent radiochemotherapy in the majority of patients; only 2.56% of patients required a TDF dose of 50 μg/hour. The possible reason that a dose of only 25 μg/hour was required by the majority of patients is that most cases of OM were mild, and only 41.03% of patients had grade 3 mucositis.
It has been reported that the incidence of adverse reactions to TDF is lower than that with extended-release oral opioids.32 Our study showed that the main adverse events of TDF were mild dizziness, mild gastrointestinal symptoms (nausea, vomiting, stomach upset, and constipation), and mild pruritus. Although it is difficult to assess whether nausea and vomiting were caused by chemoradiotherapy or TDF, all of the symptoms were relieved within a short period of time after symptomatic treatment. No severe adverse reactions, such as drowsiness and respiratory depression, were noted. Therefore, the results indicate that TDF is effective and safe.
Research has shown that after the first application of TDF in adults, serum fentanyl concentrations increase gradually and reach a steady level at 12 and 24 hours, with a flat plateau of the concentration curve.17 This suggests that the initial application of TDF can be combined with a short-acting analgesic, such as morphine, to provide pain relief until the effect of TDF occurs. In addition, the duration of action of TDF was short in a small number of patients, and thus the dosing interval needed to be adjusted. This might be related to individual variations of fentanyl metabolism.
This study has some limitations. First, the study was performed at a single center, and the sample size was small. Second, the study was not randomized or controlled. Third, there was only short-term observation in the study, and maybe the risk of side effects and complications would increase after TDF were used over several chemotherapy or radiotherapy cycles. Other limitations were inconsistent administration of other opioids in addition to TDF and only subjective means of measuring pain.
In conclusion, the results of this study suggest that TDF is an effective alternative to other opioids for the treatment of moderate and severe OM pain caused by concurrent chemoradiotherapy in patients with NPC. This drug is safe and effective, is well tolerated, and can significantly improve QoL. It is easy to use and is noninvasive, and its clinical application warrants further study.
Acknowledgments
This study was supported by a grant from the Sci-Tech Office of Guangdong Province (2008B060600019) and the Youth Foundation of the First Affiliated Hospital of Xiamen University (XYY2012005).
Disclosure
The authors report no conflicts of interest in this work.
References
Liu Q, Chen JO, Huang QH, Li YH. Trends in the survival of patients with nasopharyngeal carcinoma between 1976 and 2005 in Sihui, China: a population-based study. Chin J Cancer. 2013;32(6):325–333. | |
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. | |
Mao YP, Liang SB, Liu LZ, et al. The N staging system in nasopharyngeal carcinoma with Radiation Therapy Oncology Group guidelines for lymph node levels based on magnetic resonance imaging. Clin Cancer Res. 2008;14(22):7497–7503. | |
Chen Y, Sun Y, Liang SB, et al. Progress report of a randomized trial comparing long-term survival and late toxicity of concurrent chemoradiotherapy with adjuvant chemotherapy versus radiotherapy alone in patients with stage III to IVB nasopharyngeal carcinoma from endemic regions of China. Cancer. 2013;119(12):2230–2238. | |
Al-Sarraf M, LeBlanc M, Giri PG, et al. Chemoradiotherapy versus radiotherapy in patients with advanced nasopharyngeal cancer: phase III randomized Intergroup study 0099. J Clin Oncol. 1998;16(4):1310–1317. | |
Lee AW, Tung SY, Ngan RK, et al. Factors contributing to the efficacy of concurrent-adjuvant chemotherapy for locoregionally advanced nasopharyngeal carcinoma: combined analyses of NPC-9901 and NPC-9902 trials. Eur J Cancer. 2011;47(5):656–666. | |
Wee J, Tan EH, Tai BC, et al. Randomized trial of radiotherapy versus concurrent chemoradiotherapy followed by adjuvant chemotherapy in patients with American Joint Committee on Cancer/International Union against cancer stage III and IV nasopharyngeal cancer of the endemic variety. J Clin Oncol. 2005;23(27):6730–6738. | |
Worthington HV, Clarkson JE, Bryan G, et al. Interventions for preventing oral mucositis for patients with cancer receiving treatment. Cochrane Database Syst Rev. 2011;(4):CD000978. | |
Peterson DE, Bensadoun RJ, Roila F, et al. Management of oral and gastrointestinal mucositis: ESMO Clinical Practice Guidelines. Ann Oncol. 2011;22(Suppl 6):vi78–vi84. | |
Hong JP, Lee SW, Song SY, et al. Recombinant human epidermal growth factor treatment of radiation-induced severe oral mucositis in patients with head and neck malignancies. Eur J Cancer Care (Engl). 2009;18(6):636–641. | |
Naidu MU, Ramana GV, Rani PU, Mohan IK, Suman A, Roy P. Chemotherapy-induced and/or radiation therapy-induced oral mucositis – complicating the treatment of cancer. Neoplasia. 2004;6(5):423–431. | |
Saroja G, Devi PS, Namrata R. Oral morphine solution as an oral rinse or mouth gargle for mucositis pain. Indian J Palliat Care. 2010;16(1):54–55. | |
Saunders DP, Epstein JB, Elad S, et al. Systematic review of antimicrobials, mucosal coating agents, anesthetics, and analgesics for the management of oral mucositis in cancer patients. Support Care Cancer. 2013;21(11):3191–3207. | |
Peterson DE, Bensadoun RJ, Roila F. Management of oral and gastrointestinal mucositis: ESMO clinical recommendations. Ann Oncol. 2008;19 Suppl 2:ii122–ii125. | |
Chen WH, Liu K, Tan PH, Chia YY. Effects of postoperative background PCA morphine infusion on pain management and related side effects in patients undergoing abdominal hysterectomy. J Clin Anesth. 2011;23(2):124–129. | |
Muijsers RB, Wagstaff AJ. Transdermal fentanyl: an updated review of its pharmacological properties and therapeutic efficacy in chronic cancer pain control. Drugs. 2001;61(15):2289–2307. | |
Ahmedzai S, Brooks D. Transdermal fentanyl versus sustained-release oral morphine in cancer pain: preference, efficacy, and quality of life. The TTS-Fentanyl Comparative Trial Group. J Pain Symptom Manage. 1997;13(5):254–261. | |
Chang JT, Lin CY, Lin JC, Lee MS, Chen YJ, Wang HM. Transdermal fentanyl for pain caused by radiotherapy in head and neck cancer patients treated in an outpatient setting: a multicenter trial in Taiwan. Jpn J Clin Oncol. 2010;40(4):307–312. | |
National Cancer Institute. Common terminology criteria for adverse events v3.0 (CTCAE). 2006. Available from: http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf. Accessed May 21, 2008. | |
International Commission on Radiation Units and Measurements (ICRU). Prescribing, Recording and Reporting Photon Beam Therapy. ICRU Report 50. Bethesda: ICRU; 1993. | |
International Commission on Radiation Units and Measurements (ICRU). Prescribing, Recording and Reporting Photon Beam Therapy [supplement to ICRU Report 50]. ICRU Report 62. Bethesda: ICRU; 1999. | |
Farzana M, Shahead C, Buettner P. Measuring clinical outcomes of chronic pain patients. 2011. Available from: http://www.practicalpainmanagement.com/resources/diagnostic-tests/measuring-clinical-outcomes-chronic-pain-patients. Accessed December 12, 2013. | |
van Seventer R, Smit JM, Schipper RM, et al. Comparison of TTS-fentanyl with sustained-release oral morphine in the treatment of patients not using opioids for mild-to-moderate pain. Curr Med Res Opin. 2003;19(6):457–469. | |
Minkowitz HS, Rathmell JP, Vallow S, Gargiulo K, Damaraju CV, Hewitt DJ. Efficacy and safety of the fentanyl iontophoretic transdermal system (ITS) and intravenous patient-controlled analgesia (IV PCA) with morphine for pain management following abdominal or pelvic surgery. Pain Med. 2007;8(8):657–668. | |
Tawfik MO, Bryuzgin V, Kourteva G. Use of transdermal fentanyl without prior opioid stabilization in patients with cancer pain. Curr Med Res Opin. 2004;20(3):259–267. | |
Pillitteri LC, Clark RE. Comparison of a patient-controlled analgesia system with continuous infusion for administration of diamorphine for mucositis. Bone Marrow Transplant. 1998;22(5):495–498. | |
Strupp C, Sudhoff T, Germing U, et al. Transdermal fentanyl during high-dose chemotherapy and autologous stem cell support. Oncol Rep. 2000;7(3):659–661. | |
Kim JG, Sohn SK, Kim DH, et al. Effectiveness of transdermal fentanyl patch for treatment of acute pain due to oral mucositis in patients receiving stem cell transplantation. Transplant Proc. 2005;37(10):4488–4491. | |
Demarosi F, Lodi G, Soligo D, et al. Transdermal fentanyl in HSCT patients: an open trial using transdermal fentanyl for the treatment of oral mucositis pain. Bone Marrow Transplant. 2004;33(12):1247–1251. | |
Cai Q, Huang H, Sun X, et al. Efficacy and safety of transdermal fentanyl for treatment of oral mucositis pain caused by chemotherapy. Expert Opin Pharmacother. 2008;9(18):3137–3144. | |
Stiff P, Mumby P, Miler L, et al. Autologous hematopoietic stem cell transplants that utilize total body irradiation can safely be carried out entirely on an outpatient basis. Bone Marrow Transplant. 2006;38(11):757–764. | |
Clark AJ, Ahmedzai SH, Allan LG, et al. Efficacy and safety of transdermal fentanyl and sustained-release oral morphine in patients with cancer and chronic non-cancer pain. Curr Med Res Opin. 2004;20(9):1419–1428. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.