Back to Journals » Patient Preference and Adherence » Volume 14
Transdermal Asenapine in Schizophrenia: A Systematic Review
Authors Carrithers B, El-Mallakh RS
Received 20 May 2020
Accepted for publication 26 June 2020
Published 25 August 2020 Volume 2020:14 Pages 1541—1551
DOI https://doi.org/10.2147/PPA.S235104
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Johnny Chen
Brennan Carrithers, Rif S El-Mallakh
Department of Psychiatry and Behavioral Sciences, University of Louisville School of Medicine, Louisville, Kentucky 40202, USA
Correspondence: Rif S El-Mallakh
Department of Psychiatry and Behavioral Sciences, University of Louisville School of Medicine, 401 East Chestnut Street, Suite 610, Louisville, Kentucky 40202, USA
Tel +1 502 588 – 4450
Fax +1 502 588 - 9539
Email [email protected]
Background: Asenapine is a novel antipsychotic that has demonstrated efficacy in controlling psychosis in schizophrenia and mania in bipolar illness. It must be administered as a sublingual formulation because it is nearly completely metabolized in the first pass through the liver. Recently, a transdermal formulation of asenapine has been approved for schizophrenia by the Food and Drug Administration.
Methods: A systematic review of transdermal asenapine was done utilizing the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) model.
Discussion: There are several formulations of transdermal asenapine but only Secuado® has been approved for clinical use. Total bioavailability is 35%. Peak plasma concentration (Cmax) is 4 ng/mL and occurs within 1 hr (Tmax); elimination half-life (t1/2) is 24 hrs (range 13.4 to 39.2 h). Asenapine is highly bound (95%) to albumin and α1-acid glycoprotein. It has a unique receptor profile in which it functions as an antagonist at multiple receptors with affinity that is higher than D2 (Ki = 1.3) including D3, D4, 5HT2A, 5HT2C, 5HT2B, 5HT7, 5HT6, H1, and α 2. This profile suggests that asenapine may be of particular value off label for bipolar depression, anxiety, and aggression. Transdermal asenapine was only tested in one randomized, placebo-controlled study of acute psychosis in schizophrenia. It was superior to placebo at week 6 with nearly one-third of patients experiencing > 30% improvement in total PANSS score which translates in a number needed to treat (NNT) of 9.
Keywords: asenapine, schizophrenia, transdermal, topical, antipsychotic
Introduction
Background
Asenapine is a second-generation antipsychotic that was approved in the United States by the Food and Drug Administration (FDA) in August 2009 for the acute treatment of adults with schizophrenia and manic or mixed episodes associated with bipolar I disorder with or without psychotic features.1 Because of its nearly complete first-pass metabolism when taken orally, asenapine was initially approved as a sublingual formulation – a route that bypassed the first-pass effect. But there are other ways to avoid a devastating first-pass effect.1
In October 2019, a transdermal system of delivery for asenapine (brand name Secuado®) was approved for schizophrenia (but was not studied for bipolar disorder) by the FDA.2 While the active ingredient is still asenapine, the novel route of administration does alter some aspects of how the drug is used. The objective of this article is to summarize the pharmacokinetics, efficacy, and safety of transdermal asenapine in the treatment of schizophrenia.
Methods
Protocol and Registration
This study utilized the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) model.
Information Sources
Literature was collected from a broad range of databases that include OVID MED, PubMed, PsycInfo, and clinicaltrials.gov.
Search
Search terms that were used include the generic drug name, asenapine, in combination with each of the following keywords: transdermal, topical, transcutaneous, percutaneous, patch, film, liposome, nanoparticle, emulsion, gel, and solution. Reference lists from all relevant literature were hand-searched for clinical trials and patents that fit inclusion criteria. Additionally, the brand name, Secuado®, was used as a key search term. The manufacturer of Secuado, Noven Pharmaceuticals, was also contacted in order to obtain unpublished studies.
Eligibility Criteria and Study Selection
Randomized clinical trials and patents, including unpublished results that have been reviewed by nationally recognized clinical experts (eg, USFDA), were eligible for inclusion. There were no limitations on language or date of publication. Literature was considered applicable if it contained primary data concerning the efficacy, tolerability, and/or pharmacokinetics of ASP administered via transdermal route.
Data Selection Process
Data extraction was performed in duplicate by 2 authors.
Results
Study Selection
After conducting the search process, 51 articles were found. After screening and assessing for eligibility and excluding duplicates, 8 studies were included in this systematic review.
Results of Individual Studies
Table 1 summarizes the most relevant studies examining transdermal asenapine.
 |  |  |
Table 1 Summary of the Most Relevant Studies Examining Transdermal Asenapine |
Discussion
Chemistry
Asenapine is a tertiary amine belonging to the class dibenzo-oxepino pyrroles; the chemical name is trans-5-chloro- 2-methyl-2,3,3a,12b-tetrahydro-1H-dibenz[2,3:6,7] oxepino [4,5-c] pyrrole (Figure 1). It has a relatively small molecular weight (M = 285.8 g/mol), is lipid soluble (Log P = 4.77), and nonionized with a formal charge (FC) of zero (PubChem CID = 3,036,780 [National Center for Biotechnology Information. PubChem Database. Asenapine, CID=3,036,780, https://pubchem.ncbi.nlm.nih.gov/compound/Asenapine {accessed on Mar. 18, 2020}]). The brand name for approved transdermal asenapine is Secuado®.
 |
Figure 1 The chemical structure of asenapine. (image courtesy PubChem). |
Pharmacokinetics
Asenapine is rapidly metabolized by the liver via direct glucuronidation by uridine-diphosphoglucuronate glucuronosyl transferase 1A4 (UGT1A4) and oxidative metabolism by cytochrome P450 isoenzymes (predominantly CYP 1A2 and to a lesser degree CYP3A4 and CYP2D6).11–13 First-pass effects exceed 95%, so that bioavailability (F or fraction in general circulation) is < 2% of oral dose.13,14 Sublingual administration to escape immediate hepatic catabolism and deactivation results in 35% bioavailability in systemic circulation.13 Peak plasma concentration of sublingual formulation (Cmax) is 4 ng/mL and occurs within 1 hr (Tmax);11 elimination half-life (t1/2) is 24 hrs (range 13.4 to 39.2 h).14–16 Asenapine is highly bound (95%) to albumin and α1-acid glycoprotein.14 Asenapine has weak affinity to the permeability glycoprotein (P-gp), and so would be unlikely to cause an inordinate increase in prolactin like risperidone, paliperidone, and amisulpride.17
A transdermal system of delivery could offer several alternatives relative to a dissolvable tablet. In addition to being convenient, painless, non-invasive, and able to bypass first-pass metabolism by the liver, it is also unaffected by food or drink intake surrounding the time of administration.18,19 While the sublingual route is acknowledged for its rapid absorption and onset, a transdermal system provides a steadier, sustained delivery (Cmax ~ 1.72 ng/mL), with a controlled release over a longer period of time (Tmax ~16 hrs, t1/2 = 30 hrs).20,21 The peak to trough ratio of sublingual asenapine is the highest among antipsychotics (> 3) because of the rapid initial absorption,22 but this ratio is only 1:1 for transdermal asenapine.23 Application of the patch to the abdomen, upper back, arms, or hips are all acceptable. The location of the patch should be varied with subsequent applications. Placement over broken skin should be avoided. It is important to note that heat can double the absorption rate from the patch to a Tmax of approximately 8 hrs.21 This maybe an issue in winter if patients use a heated blanket, but is not an issue with environmental heat (eg, on a hot summer day).
Pharmacodynamics
Asenapine is a novel second-generation antipsychotic with a unique receptor profile (Table 2) that has been approved in its sublingual formulation for treatment of people with schizophrenia and bipolar disorder.24–27 Its exact mechanism of action in schizophrenia and bipolar disorder is unknown, but it has a unique human receptor signature, with binding affinity and receptor profiles that differ considerably from other antipsychotic drugs. It has antagonist activity for the D3, D4, 5HT2A, 5HT2C, 5HT2B, 5HT7, and 5HT6, histamine type 1 (H1), and alpha adrenergic type 2 (α2)-receptors, all at higher affinity than to the dopamine type 2 (D2) receptor (Table 2).28,29 It has negligible partial agonist activity at the serotonin (5HT) type 1A (5HT1A) receptor (with intrinsic activity of approximately 25%30), so that it is a functional antagonist at 5HT1A. Affinity, or attractiveness of the drug to neurotransmitter receptor, is measured by the inhibitory constant (Ki). Ki values represent the concentration of the drug that is required to displace the intrinsic neurotransmitter off of the receptor. Dosing strategies for schizophrenia, for either sublingual or transdermal administration, aim to achieve roughly 75% D2 receptor occupancy.31 Consequently, any receptors with a Ki value that are higher than the Ki for D2 (ie, lower affinity), will not be occupied sufficiently to mediate any measurable effects in routine clinical care; for asenapine at a typical dose of 5 mg twice daily (BID), any receptor with Ki ≥ 2.0 would fall into that category (eg, 5HT1A and 5HT1B). However, those receptors may be adequately occupied at 10 mg BID.
 |
Table 2 The Inhibitory Constant of Asenapine and Potential Neurotransmitters |
The widespread antagonism of postsynaptic serotonin, along with the relatively higher D4 versus D2 affinity32 may mediate the observed benefit of asenapine in agitation and aggression in severely ill patients, and affective instability in borderline personality disorder.33–36 Similarly, blockade of 5HT7 has been associated with antidepressant action in animal models37,38 and this is believed to mediate the antidepressant effect of lurasidone in bipolar depression.39 Consequently, it is not surprising that sublingual asenapine has also demonstrated antidepressant efficacy in bipolar depression.40 Transdermal asenapine has only been examined in schizophrenia.
Transdermal Technology
The stratum corneum (ie, outermost layer of skin) is a difficult barrier for drugs to penetrate. It is composed of 10–15 layers of epidermal cells that are tightly packed with keratin and is lipid-rich to exclude unwanted toxins and prevent unnecessary water loss.41 Various methods, both physical and chemical, have been employed on transdermal drug delivery systems in order to augment permeation through the skin. Applying a voltage gradient (ie, iontophoresis), ultrasound waves (ie, sonophoresis), and skin ablation via heat, lasers, or microneedles (ie, electropermeabilization) are all physical modes that can be used to enhance percutaneous drug delivery. Chemical enhancers act on the stratum corneum by either increasing partitioning of the tightly packed cell layer or increasing the diffusion ability of the drug. Examples of chemical penetration enhancers include polyalcohols, pyrrolidones, fatty acids, alkanes, surfactants, terpenes, and others.7,42-44. Each one of these strategies is associated with its own potential adverse reactions, but all such consequences are quite local in scope.
Several groups have been working to develop transdermal administration of asenapine. Secuado® is simply the first on the American market. It was developed by Noven Pharmaceuticals based in Miami, Florida, a subsidiary of the Japanese Hisamitsu Pharmaceutical Company. Hisamitsu specializes in transdermal technology. Worldwide there are two antipsychotics available in transdermal formulation: blonanserin for schizophrenia in Japan,45,46 and asenapine for schizophrenia in the United States.
The patch technology for Secuado® is outlined in a series of patents, eg,9,10 The patch (area of 8 cm2) uses reservoir-technology comprised of a support layer, an asenapine plus adhesive agent layer containing isopropyl palmitate as a transdermal absorption enhancer, and an adhesive base agent.9,10 Sodium diacetate was added to the adhesive layer in order to further increase the skin permeability to asenapine constantly over time.
Sonobe et al47 filed a patent describing different adhesive layer formulations that decreased drug degradation, improved drug release, or increased adhesion even when moisture was applied. It was found that by using a rubber-based adhesive agent, the skin permeability of asenapine could be enhanced; while at the same time, this novel system decreased adhesive forces. The given patent describes a series of studies using hairless mice to confront this problem. The findings suggest that a patch formulation comprising free-asenapine, a rubber-based adhesive agent, and a maleic acid alkali salt was able to effectively penetrate mouse skin while still retaining adhesive capabilities.
A group from Germany led by Mohr has filed at least three patents.4–6 They also use a reservoir-type patch (area of 10 cm2) consisting of a backing layer, an asenapine-containing matrix layer (asenapine + silicone polymer [polysiloxones/polyisobutylenes] + tocopherol [stabilizer] + polyvinylpyrrolidone [crystallization inhibitor]), and optionally an additional skin contact layer,4,5 or an acrylic adhesive layer.6 This system was tested in animals (Goettingen minipigs4) and humans.5 They actually performed Phase I trials demonstrating the adequacy of asenapine drug levels with their system.6
Solomon8 studied a variety of transdermal routes in pigs (sprays, aerosols, patches, films, gels, creams, ointments, lotions, and foams) with the spray method showing the shortest Tmax (10–20 min), in vivo; this is comparable to the Tmax of sublingual asenapine. Additional in vitro experiments showed the gel formulation to have the highest maximum permeation.
A group in India used Asenapine maleate and combined chemical and nanocarriers (transfersomes) to improve transdermal absorption. This method of increased bioavailability with a physical process resulted in transdermal bioavailability the exceeded oral bioavailability.7 It is not clear how this method compares with what is already on the market.
Transdermal Asenapine Dosing
Secuado®, the transdermal formulation of asenapine, is designed as a patch that is applied every 24 hrs on the torso. The recommended dosing is 3.8 mg/24 hrs patch once daily; based on efficacy and tolerability, the dose may be increased as clinically indicated to 5.7 mg/24 hrs or 7.6 mg/24 hrs after 1 week at a lower dose.21 The transdermal asenapine patch of 3.8 mg/24 contains a total of 6.4 mg of asenapine and is equivalent to a sublingual asenapine dose of 5 mg BID. The 7.6 mg/24 hrs patch contains 12.8 mg of asenapine and is equivalent to 10 mg BID.21
Oral hypoesthesia and bitter taste are among the most frequently reported side-effects associated with sublingual asenapine and can contribute to patient noncompliance.48 However, skin irritation is a common adverse reaction of topical administration (14–15%),21 and could also lead to patient discomfort and noncompliance. Sublingual versus transdermal route of administration does not affect the volume of distribution of asenapine (Vd = ~20 to 25 L/kg), which is relatively large due to its extensive extravascular spread.
Clinical Studies: Efficacy
There was one Phase 3, randomized, double-blind, fixed-dose, placebo-controlled inpatient study that evaluated the efficacy and tolerability of transdermal asenapine in acutely ill subjects with schizophrenia.3 It enrolled 616 adults with schizophrenia in 59 centers in the United States, Russia, Bulgaria, Serbia, and Ukraine. A total of 489 participants completed the trial.3 Subjects were treated for 6 weeks with high-dose asenapine patch (7.6mg/24hrs; equivalent to 10mg BID of the sublingual formulation), low-dose asenapine patch (3.8mg/24hrs; equivalent to 5mg BID of the sublingual formulation) or placebo. All subjects had two patches placed daily (either active or placebo) to maintain the blind.23 Additionally, subjects were seen 30 days after study termination. The efficacy outcomes measured include the Positive and Negative Syndrome Scale (PANSS) and Clinical Global Impressions-Severity (CGI-S) rating scales. PANSS is a 30 item scale used to quantify the severity of positive symptoms, negative symptoms, and general psychopathy in subjects with schizophrenia.49–51 The primary endpoint changed from baseline in the sum of all participants’ PANSS score to Week 6.
Both low dose and high-dose transdermal asenapine separate significantly from placebo at week two.52 That persists until the study end at week 6 (Figure 2).23,52 Nearly one-third of asenapine-treated patients responded (ie, experienced > 30% improvement in total PANSS score by week 6) (Figure 3).53 This is comparable to changes seen with sublingual asenapine in schizophrenia.26,27 This translates in a number needed to treat (NNT) of 9 for low-dose transdermal asenapine and 10 for high-dose transdermal asenapine.53 This is comparable to an NNT for acute psychosis in schizophrenia with sublingual asenapine (administered at 5 mg BID).54
 |
Figure 2 Change in PANSS score in subjects with acute schizophrenia and baseline score of 97.4 (placebo), 97.0 (low dose), and 95.6 (high dose). Data From Citrome et al’s study.53 |
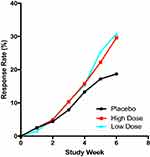 |
Figure 3 Response rates over time in in subjects with acute schizophrenia treated with low-dose transdermal asenapine (3.8 mg/24 hours), high-dose transdermal asenapine (7.6 mg/24 hours), and placebo. Only week 6 is significantly higher than placebo for both doses (P < 0.05). Data from Citrome et al’s study.3 |
The key secondary endpoint was the change from baseline in the sum of Clinical Global Impression – Severity (CGI-S) scores at Week 6.55 All groups were equally severe at baseline (CGI mean = 4.9). Placebo treated patients experienced the smallest improvement by week 6 (4.0 ± SD 1.01), but both the low-dose (3.6 ± 0.93) and high-dose (3.7 ± 0.96) groups experienced significantly more improvement (−0.8, −1.2, and −1.1, respectively, P < 0.001 vs placebo).23,52
While sublingual asenapine has been studied and approved for acute mania in bipolar illness,24,25 transdermal asenapine has not been examined in this patient population group.
Clinical Studies: Safety
Transdermal asenapine was generally well-tolerated. Some treatment-emergent adverse events (TEAEs) were experienced by nearly half of all subjects (51.5%, 53.9%, and 55.4% of placebo-, low-dose-, and high-dose-treated subjects, respectively).52 Those occurring at a rate of at least 5% are presented in Table 3.52 Serious adverse events were rare (1.9%, 1.5%, and 1.0% in placebo-, low-dose-, and high-dose-treated subjects, respectively), as were discontinuations due to adverse events (6.8%, 4.9%, and 7.8% of placebo-, low-dose-, and high-dose-treated subjects, respectively).52 Approximately a sixth of patients developed some kind of mild to moderate skin reaction at the patch site (14.2% high-dose; 15.2% low-dose; 4.4% placebo; see Table 3), but < 0.5% of patients stopped the study due to a skin reaction. No information is available regarding the effect on skin with long-term exposure. There were no human deaths in the development program.23
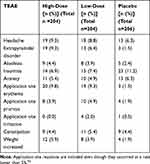 |
Table 3 Treatment-Emergent Adverse Effects (TEAE) That Occurred in the Phase 3 Trial of Transdermal Asenapine at a Rate of 5% of More in Subjects Receiving at Least One Dose |
Weight gain occurred in roughly 5% of patients receiving transdermal asenapine over the 6 weeks of the study (Table 3). There is no long-term exposure data with transdermal asenapine, but long-term safety studies with sublingual asenapine demonstrate weight gain.56 In a naturalistic, post-marketing study of 20 patients with different diagnoses receiving sublingual asenapine for an average of 7.6 months, patients gained an average weight of 0.32 kg/month (0.7 lbs/month).57
In general, safety considerations with transdermal asenapine are the same as with sublingual asenapine. Asenapine is metabolized predominantly by direct glucuronidation, so there is little drug–drug interaction. However, it is also broken down by CYPs 1A2, 3A4, and 2D6 and so can have variations in level in response to enzyme inducers or inhibitors, or to smoking. Similarly, same considerations as sublingual asenapine regarding physical illness, such as severe liver disease or kidney disease need to be kept in mind. It is perhaps wise to avoid transdermal asenapine in the presence of significant skin disease.”
Summary
Asenapine is a unique second-generation antipsychotic that is nearly completely destroyed in the first pass through the liver, so that dosing strategies must bypass the gut. A sublingual formulation has been available. Recently, a topical transdermal formulation has been approved in the United States for schizophrenia. Doing the transdermal formulation results in plasma levels and receptor occupancy that are equivalent to the sublingual formulation. Clinical response in terms of efficacy and adverse consequences to transdermal asenapine is also comparable to sublingual asenapine. The dermal route avoids some of the problematic issues with sublingual administration (complex instruction, bitter taste, and potential for mouth ulcers58), but replaces them with the potential for topical reactions (Table 3). What might be considered advantages of a specific formulation for a specific patient may be disadvantages for another patient. Nonetheless, this is the only topical antipsychotic available in the United States and offers patients and clinicians with an additional option to maximize patient adherence and satisfaction.
Disclosure
Dr Rif S El-Mallakh reports personal fees from Alkermes, Indivior, Intra-Cellular Therapeutics, Janssen, Lundbeck, Otsuka, Sunovion, and Teva, outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. United Ststes Food and Drug Administration (USFDA). Press Announcements: FDA Approves Saphris to Treat Schizophrenia and Bipolar Disorder; 2009. Available from: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm177401.html.
2. Citrome L, Zeni CM, Correll CU. Patches: established and emerging transdermal treatments in psychiatry. J Clin Psychiatry. 2019;80(4):18nr12554. doi:10.4088/JCP.18nr12554.
3. Citrome L, Walling D, Zeni C, Komaroff M, Park A Efficacy and safety of an asenapine transdermal system (HP-3070) for treatment of adults with schizophrenia: a Phase 3 randomized, placebo-controlled, inpatient study. Presented at: Psych Congress 2019; October 3-6, 2019; San Diego, CA. Poster 122.
4. Mohr P, Rietscher R, Eifler R, Bourquain O, inventors. LTS Lohmann Therapie-Systeme AG, Andernach, Germany, applicant. Transdermal therapeutic system containing asenapine and polysiloxane or polyisobutylene. WO2018115010A1; 2018.
5. Mohr P, Rietscher R, Eifler R, Bourquain O, inventors. LTS Lohmann Therapie-Systeme AG, Andernach, Germany, applicant. Transdermal therapeutic system containing asenapine and silicone acrylic hybrid polymer. WO2019002204A1; 2019.
6. Mohr P, Rietscher R, Eifler R, Bourquain O, inventors. LTS Lohmann Therapie-Systeme AG, Andernach, Germany, appli- cant. Transdermal therapeutic system containing asenapine. WO2018115001A1; 2018.
7. Shreya AB, Managuli RS, Menon J, et al. Nano-transfersomal formulations for transdermal delivery of asenapine maleate: in vitro and in vivo performance evaluations. J Liposome Res. 2016;26(3):221–232. doi:10.3109/08982104.2015.1098659
8. Solomon WD; inventor. Transdermal Compositions of Asenapine for the Treatment of Psychiatric Disorders. WO2010127674A1. Copenhagen, Denmark: Sunin K/S; 2010.
9. Suzuki M, Okutsu H, Yasukochi T, Takada Y; inventors. Assignee. Patch. US20170172981A1. Tosu-shi: Hisamitsu Pharmaceutical Co., Inc.; 2017.
10. Suzuki M, Okutsu H, Yasukochi T, Takada Y; inventors. Assignee. Patch US20180360968A1. Tosu-shi, Japan: Hisamitsu Pharmaceutical Co., Inc; 2018.
11. van de Wetering-krebbers SFM, Jacobs PL, Kemperman GJ, et al. Metabolism and excretion of asenapine in healthy male subjects. Drug Metab Dispos. 2011;39(4):580–590. doi:10.1124/dmd.110.036715
12. Lu D, Xie Q, Wu B. N-glucuronidation catalyzed by UGT1A4 and UGT2B10 in human liver microsomes: assay optimization and substrate identification. J Pharm Biomed Anal. 2017;145:692–703. doi:10.1016/j.jpba.2017.07.037
13. Reyad A, Asenapine: MR. Pharmacological aspects and role in psychiatric disorders. Psychiatr Danub. 2019;31(2):157–161. doi:10.24869/psyd.2019.157
14. Citrome L. Asenapine review, part I: chemistry, receptor affinity profile, pharmacokinetics and metabolism. Expert Opin Drug Metab Toxicol. 2014;10(6):893–903. doi:10.1517/17425255.2014.908185
15. Center for Drug Evaluation and Research (CDER). SAPHRIS® (asenapine) Sublingual Tablets, Chemistry Review(s) – access data FDA; NDA 22-117, 2009. Available from: http://www.accessdata.fda.gov/drugsatfda_docs/nda/2009/022117s000_ChemR.pdf.
16. Hua S. Advances in nanoparticulate drug delivery approaches for sublingual and buccal administration. Front Pharmacol. 2019;10:1328. doi:10.3389/fphar.2019.01328
17. El-Mallakh RS, Watkins J. Prolactin elevations and the permeability glycoprotein. Prim Care Companion CNS Disord. 2019;21(3):18nr02412. doi:10.4088/PCC.18nr02412
18. Prausnitz MR, Langer R. Transdermal drug delivery. Nat Biotechnol. 2008;26(11):1261–1268. doi:10.1038/nbt.1504
19. Abruzzo A, Cerchiara T, Luppi B, Bigucci F. Transdermal delivery of antipsychotics: rationale and current status. CNS Drugs. 2019;33:849–865. doi:10.1007/s40263-019-00659-7
20. Kaminsky B, Bostwick J, Guthrie S. Alternative routes of administration of antidepressants and antipsychotic medications. Ann Pharmacother. 2015;49(7):808–817. doi:10.1177/1060028015583893
21. Secuado (asenapine) [prescribing information]. Miami, FL. Noven Therapeutics, LLC; 2019. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/212268s000lbl.pdf.
22. de Greef R, Maloney A, Olsson-Gisleskog P, Schoemaker J, Panagides J. Dopamine D2 occupancy as a biomarker for antipsychotics: quantifying the relationship with efficacy and extrapyramidal symptoms. AAPS J. 2011;13(1):121–130. doi:10.1208/s12248-010-9247-4
23. Food and Drug Administration. NDA/BLA Multi-disciplinary Review and Evaluation NDA 212268 Secuado (asenapine) transdermal system. 2018. Available from: https://www.fda.gov/media/124109/download.
24. McIntyre RS, Cohen M, Zhao J, Alphs L, Macek TA, Panagides J. A 3-week, randomized, placebo-controlled trial of asenapine in the treatment of acute mania in bipolar mania and mixed states. Bipolar Disord. 2009;11(7):673–686. doi:10.1111/j.1399-5618.2009.00748.x
25. McIntyre RS, Cohen M, Zhao J, Alphs L, Macek TA, Panagides J. Asenapine in the treatment of acute mania in bipolar I disorder: a randomized, double-blind, placebo-controlled trial. J Affect Disord. 2010;122(1–2):27–38. doi:10.1016/j.jad.2009.12.028
26. Kinoshita T, Bai YM, Kim JH, Miyake M, Oshima N. Efficacy and safety of asenapine in Asian patients with an acute exacerbation of schizophrenia: a multicentre, randomized, double-blind, 6-week, placebo-controlled study. Psychopharmacology (Berl). 2016;233(14):2663–2674. doi:10.1007/s00213-016-4295-9
27. Landbloom R, Mackle M, Wu X, et al. Asenapine for the treatment of adults with an acute exacerbation of schizophrenia: results from a randomized, double-blind, fixed-dose, placebo-controlled trial with olanzapine as an active control. CNS Spectr. 2017;22(4):333–341. doi:10.1017/S1092852916000377
28. Shahid M, Walker GB, Zorn SH, Wong E. Asenapine: A novel psychopharmacological agent with a unique human receptor signature. J Psychopharmacol. 2009;23(1):65–73. doi:10.1177/0269881107082944
29. Marazziti D, Piccini A, Baroni S, et al. Current trends on antipsychotics: focus on asenapine. Curr Medicinal Chem. 2019;23(21):2204–2216. doi:10.2174/0929867323666160525115014
30. Ghanbari R, El Mansari M, Shahid M, Blier P. Electrophysiological characterization of the effects of asenapine at 5-HT1A, 5-HT2A, alpha2-adrenergic and D2 receptors in the rat brain. Eur Neuropsychopharmacol. 2009;19(3):177–187. doi:10.1016/j.euroneuro.2008.11.001
31. Farde L, Nordström A-L, Wiesel F-A, Pauli S, Halldin C, Sedvall G. Positron emission tomographic analysis of central D1- and D2-dopamine receptor occupancy in patients treated with classical neuroleptics and clozapine—relation to extrapyramidal side effects. Arch Gen Psychiatry. 1992;49:538–544. doi:10.1001/archpsyc.1992.01820070032005
32. El-Mallakh RS, McKenzie C. The dopamine D4/D2 receptor antagonist affinity ratio as a predictor of anti-aggression medication efficacy. Med Hypotheses. 2013;80(5):530–533. doi:10.1016/j.mehy.2012.10.014
33. Pratts M, Citrome L, Grant W, Leso L, Opler LA. A single-dose, randomized, double-blind, placebo-controlled trial of sublingual asenapine for acute agitation. Acta Psychiatr Scand. 2014;130(1):61–68. doi:10.1111/acps.12262
34. Amon JS, Johnson SB, El-Mallakh RS. Asenapine for the control of physical aggression: a prospective naturalist pilot study. Psychopharmacol Bull. 2017;47(1):27–32.
35. Bozzatello P, Rocca P, Uscinska M, Bellino S. Efficacy and tolerability of asenapine compared with olanzapine in borderline personality disorder: an open-label randomized controlled trial. CNS Drugs. 2017;31(9):809–819. doi:10.1007/s40263-017-0458-4
36. Citrome L, Landbloom R, Chang CT, Earley W. Effects of asenapine on agitation and hostility in adults with acute manic or mixed episodes associated with bipolar I disorder. Neuropsychiatr Dis Treat. 2017;13:2955–2963. doi:10.2147/NDT.S149376
37. Hedlund PB, Huitron-Resendiz S, Henriksen SJ, Sutcliffe JG. 5-HT7 receptor inhibition and inactivation induce antidepressant-like behavior and sleep pattern. Biol Psychiatry. 2005;58:831–837. doi:10.1016/j.biopsych.2005.05.012
38. Wesolowska A, Nikiforuk A, Stachowicz K, Tatarczynska E. Effect of the selective 5-HT7 receptor antagonist SB 269970 in animal models of anxiety and depression. Neuropharmacology. 2006;51:578–586. doi:10.1016/j.neuropharm.2006.04.017
39. Wang H, Xiao L, Wang HL, Wang GH. Efficacy and safety of lurasidone versus placebo as adjunctive to mood stabilizers in bipolar I depression: A meta-analysis. J Affect Disord. 2020;264:227–233. doi:10.1016/j.jad.2019.11.031
40. El-Mallakh RS, Nuss S, Gao D, et al. Asenapine in the treatment of bipolar depression. Psychopharmacol Bull. 2020;50(1):8–18.
41. Fox L, Gerber M, Plessis JD, Hamman J. Transdermal drug delivery enhancement by compounds of natural origin. Molecules. 2011;16:10507–10540. doi:10.3390/molecules161210507
42. Paudel KS, Milewski M, Swadley CL, Brogden NK, Ghosh P, Sitinchcomb AL. Challenges and opportunities in dermal/transdermal delivery. Ther Deliv. 2010;1:109–131. doi:10.4155/tde.10.16
43. Borovinskaya M, Robert B, Plakogiannis FM. Evaluation of in vitro percutaneous absorption of olanzapine and fluoxetine HCl: enhancement properties of olanzapine. Drug Dev Ind Pharm. 2012;38(2):227–234. doi:10.3109/03639045.2011.597765
44. Ng KW. Penetration enhancement of topical formulations. Pharmaceutics. 2018;10(2):E51. doi:10.3390/pharmaceutics10020051
45. Tenjin T, Miyamoto S, Ninomiya Y, et al. Profile of blonanserin for the treatment of schizophrenia. Neuropsychiatr Dis Treat. 2013;9:587–594. doi:10.2147/NDT.S34433
46. Sumitomo Dainippon Pharma Co., Ltd., Long-term study of DSP- 5423P in Patients with Schizophrenia. ClinicalTrials.gov identifier NCT02335658; 2014. Available from: https://clinicaltrials.gov/ct2/show/study/NCT02335658?term=DSP5423P&cntry=JP&rank=1.
47. Sonobe A, Yasukochi T, Takada Y; inventors. Assignee. Patch US20180289631A1. Tosu-shi, Japan: Hisamitsu Pharmaceutical Co., Inc.; 2018.
48. Minassian A, Young J. Evaluation of the clinical efficacy of asenapine in schizophrenia. Expert Opin Pharmacother. 2011;11(12):2107–2115. doi:10.1517/14656566.2010.506188
49. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261–276. doi:10.1093/schbul/13.2.261
50. Kay SR, Opler LA, Lindenmayer JP. Reliability and validity of the positive and negative syndrome scale for schizophrenia. Psychiatry Res. 1988;23(1):99–110. doi:10.1016/0165-1781(88)90038-8
51. Østergaard SD, Foldager L, Mors O, Bech P, Correll CU. The validity and sensitivity of PANSS-6 in treatment-resistant schizophrenia. Acta Psychiatr Scand. 2018;138(5):420–431. doi:10.1111/acps.12952
52. Citrome L, Walling D, Zeni C, Komaroff M, Park A Efficacy and safety of an asenapine transdermal system (Asenapine Transdermal System, HP-3070) in the treatment of adults with schizophrenia: a Phase 3 randomized, double-blind, placebo-controlled, 6-week inpatient study. Presented at the 57th Annual Meeting of the American College of Neuropsychopharmacology, December 9–13, 2018, Hollywood, Florida
53. Citrome L, Walling D, Zeni C, Komaroff M, Park A HP-3070 asenapine transdermal system in adults with schizophrenia: categorical response and clinical relevance as assessed in a Phase 3 RCT. Presented at the Annual American Psychiatric Association Meeting, May 18–22, 2019, San Francisco, CA, USA
54. Citrome L. Asenapine for schizophrenia and bipolar disorder: a review of the efficacy and safety profile for this newly approved sublingually absorbed second-generation antipsychotic. Int J Clin Pract. 2009;63(12):1762–1784. doi:10.1111/j.1742-1241.2009.02228.x
55. Leucht S, Barabássy Á, Laszlovszky I, et al. Linking PANSS negative symptom scores with the Clinical Global Impressions Scale: understanding negative symptom scores in schizophrenia. Neuropsychopharmacology. 2019;44(9):1589–1596. doi:10.1038/s41386-019-0363-2
56. Durgam S, Landbloom RP, Mackle M, Wu X, Mathews M, Nasrallah HA. Exploring the long-term safety of asenapine in adults with schizophrenia in a double-blind, fixed-dose, extension study. Neuropsychiatr Dis Treat. 2017;13:2021–2035. doi:10.2147/NDT.S130211
57. Worthington MA, El-Mallakh RS. A naturalistic retrospective review of weight gain in bipolar patients treated with second generation antipsychotics. J Clin Psychopharmacol. 2015;35(2):192–193. doi:10.1097/JCP.0000000000000271
58. Sweet G
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
