Back to Journals » Neuropsychiatric Disease and Treatment » Volume 15
Transcranial magnetic stimulation for the treatment of anxiety disorder
Authors Rodrigues PA , Zaninotto AL , Neville IS , Hayashi CY , Brunoni AR , Teixeira MJ , Paiva WS
Received 13 January 2019
Accepted for publication 26 April 2019
Published 23 September 2019 Volume 2019:15 Pages 2743—2761
DOI https://doi.org/10.2147/NDT.S201407
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Roger Pinder
Priscila Aparecida Rodrigues,1 Ana Luiza Zaninotto,1,2 Iuri Santana Neville,1 Cintya Yukie Hayashi,1 André R Brunoni,3 Manoel Jacobsen Teixeira,1 Wellingson Silva Paiva1
1Department of Neurology, University of São Paulo, São Paulo, Brazil; 2Laboratory of Neuromodulation, Harvard Medical School, Boston, MA, USA; 3Institute of Psychiatry, University of São Paulo, São Paulo, Brazil
Correspondence: Priscila Aparecida Rodrigues
Caring - Clinic of Psychology and Neuropsychology, Avenue Vereador Narciso Yague, Guimarães, Number 1145 – Sala 1610, Mogi das Cruzes, São Paulo 08780-500, Brazil
Tel +55 1 199 654 2972
Email [email protected]
Abstract: Anxiety is currently one of the main mood changes and can impair the quality of life of the individual when associated with other neurological or psychiatric disorders. Neuromodulation has been highlighted as a form of treatment of several pathologies, including those involving anxiety symptoms. Among the neuromodulatory options with the potential to improve mood changes, we highlight repetitive transcranial magnetic stimulation (rTMS). rTMS is a viable therapeutical option for neuropsychiatric dysfunctions of high prevalence and is important for the understanding of pathological and neuropsychological adaptation processes. Even with this potential, and high relevance of intervention, we observe the scarcity of literature that covers this subject. The objective of this study was to carry out a survey of the current literature, using scientific databases for the last five years. We found 32 studies reporting the effects of rTMS on anxiety, 7 on anxiety disorders and 25 on anxiety symptoms as comorbidities of neurological or psychiatric disorders. This survey suggests the need for further studies using TMS for anxiety in order to seek strategies that minimize these anxiety effects on the quality of life of the victims of this disorder.
Keywords: transcranial magnetic stimulation, anxiety disorders, review, treatment
Introduction
Individuals may experience anxiety as a warning sign in unknown situations, especially in response to fear and anticipation of danger.1 However, it is considered pathological when it directly affects the individual’s quality of life, affecting social relations, cognitive function, and the wake–sleep cycle.2–4 Anxiety disorders represent one of the major psychiatric disorders today that can impair the quality of life of adults.2–4
The Diagnostic and Statistical Manual of Mental Disorders – Fifth Edition (DSM-5) defines the presence of an anxiety disorder when some criteria are met, for example: symptoms occurring more than six months, excessive anxiety and worry, panic attacks, restlessness or feeling nervous, fatigue, irritability, sleep and eating disorder. Based on the symptomatology, anxiety disorders can be classified in: Generalized Anxiety Disorder (GAD), Social Anxiety Disorder, Panic Disorder (PD), Agoraphobia, Separation Anxiety Disorder, Selective Mutism and Specific Phobias. In addition to anxiety disorders, anxiety can be a symptom or comorbidity in several other pathologies, such as Major Depression,5 Obsessive Compulsive Disorder,6 TBI,7 among others.
Neuromodulation minimizes the impact of mood changes8–11 and repetitive transcranial magnetic stimulation (rTMS) is receiving attention in the last decade.12,17 TMS is a non-invasive method of stimulating the motor cortex neurons through the scalp and skull based on the principle of electromagnetic induction.18,19 TMS was approved by the Food and Drug Administration (FDA) in 2008 as an alternative treatment for Major Depression Disorder,20 and has been shown to decrease the symptoms of Post Traumatic Stress Disorder (PTSD),21 Obsessive Compulsive Disorder (OCD),22 and Anxiety Disorders.14
Non-invasive brain stimulation techniques allow researchers to study in real-time the human brain activity, characterize the balance of excitation and cortical inhibition, and help guide plastic changes.8 With TMS, you can apply repetitive induced current pulses that can increase or decrease cortical excitability and stimulate the process of neuronal plasticity.9,23,24 A coil of wire is placed on the scalp generating an electric current that flows through the target area, inducing neuronal depolarization. Possible effects are depended on subject’s age, pharmacological treatment, number of technical parameters, including the intensity and number of stimulations (ie, frequency), coil orientation, focus, and depth of stimulation.9,25,26
Although TMS pulses (simple and repetitive) are considered safe, it is contraindicated for people who use a pacemaker or other implantable electronic devices.21,27 Patients with bone defects and craniotomy pose another concern, because the conductance of the structures would be modified.21,24 Some adverse effects might occur, such as headache and minor muscle spasms at the stimulation site.21,25,28,29
Recent literature reviews discussed the use of TMS as an intervention strategy for anxiety. Vicario et al's30 study addressed the use of TMS and transcranial direct current stimulation (tDCS) and other studies portray its efficacy for other psychiatric disorders, without major insights.14,30–32 However, there is a gap in the literature regarding rTMS efficacy in anxiety symptoms (primary and secondary outcomes) in clinical trials. Our study focused on the effectiveness of TMS for Anxiety Disorders and as an intervention for Anxiety Symptoms arising from other pathologies. We make the division between anxiety arising from Neurological Disorders and Psychiatric Disorders. Our hypothesis was that high-frequency rTMS on the dorsolateral prefrontal cortex (DLPFC) will decrease anxiety symptoms in patients with anxiety disorders, considering anxiety as the primary outcome.
Methods
We conducted a literature online search including Web of Science Medline/PubMed MEDLINE databases. We have included publications from any time until March 2019. We included clinical trial and open-label using the keywords: “TMS”, “transcranial magnetic stimulation”, “noninvasive brain stimulation”, and “anxiety”. The first exclusion was by title and followed by abstracts and full texts. Abstracts and full text, and studies were included if fulfilled, the inclusion criteria: (a) use of rTMS as intervention; (b) anxiety was assessed as primary or secondary outcome; (c) sample was adults; (d) published in peer-review journals; (e) full text written in English. The exclusion criteria: (a) animal studies; (b) case report; (c) systematic review or meta-analysis; (d) paper not written in English; (e) study with healthy; and (f) studies that did not report the use of rTMS in anxiety symptoms. Searching and data analysis were performed by Rodrigues PA and Zaninotto AL. This method follows PRISMA guidelines.
Results
Figure 1 shows the systematic review that initially 358 papers were found (Pubmed =172; Web of Science =186). We found 32 studies that fulfilled our eligibility criteria.
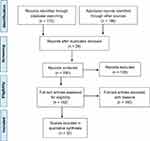 |
Figure 1 Flow diagram of the papers selected in our study, following PRISMA statement guidelines. |
For a better understanding of the results, we divided the target population of the trials in two categories: Category 1) patients with Anxiety Disorders, according to DSM-5 (Table 1); Category 2) Anxiety symptoms, from neurological and psychiatric disorders that also evaluated anxiety symptoms as comorbidity (Table 2).
 |
Table 1 Patients with anxiety disorders, according to DSM 5 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
Table 2 Anxiety symptoms, from neurological and psychiatric disorders |
Treatment for anxiety disorders
In our search, five studies were randomized double-blind clinical trial (RCT) and two studies were open-label. Seven papers described Anxiety Disorders as the main outcome for rTMS,5,33–38 one was related to Anxiety Disorders, two were Panic Disorder (PD), and the other four were GAD (Table 1). The sample size of these studies ranged from 13 to 25 patients.
The right dorsolateral prefrontal cortex (DLPFC) was the most frequent stimulation region and in one study they stimulated the left region of the DLPFC. The predominant frequency used was 1 Hz, although one study compared the use of 1 hz and 10 hz and another used only 20 Hz.
The number of sessions ranged between 10 and 30 and the number of pulses per stimulation ranged from 750 to 3600. Most papers used an intensity of 110% of the rest motor threshold (RMT), one used 80% and other used 90% of RMT and one study did not specify.
Treatment for other psychiatric neurological diseases
We found 23 studies reporting rTMS as an alternative for the treatment of neurological or psychiatric disorders and as secondary intervention to anxiety symptoms (Table 2).6,21,39–61
Papers that cited symptoms of anxiety as comorbidity were grouped in Neurological Disorders (Table 3)40,45,47,51,54,55,59 and Psychiatric Disorders (Table 4).6,21,39,41–44,46,48–50,52,53,56–58,60,61 We found that MDD is the psychiatric disorder with the highest occurrence appearing in seven papers while pain is the highest Neurological Disorders occurrence appearing in three papers.
 |
Table 3 Symptoms of anxiety present in neurological disorders |
Fourteen papers reported the stimulation in the left DLPFC, three papers both left and right DLPFC and in one study the left auditory cortex was stimulated. Two papers focused the stimulation in the left temporoparietal region and left primary motor cortex. The stimulation target of right DLPFC appeared in three papers, in addition to those already mentioned. These regions were stimulated in one paper each: primary motor cortex contralateral to the amputated leg, motor cortex area of the frontal lobe, and the epileptogenic focus. Two studies described stimulation in the left temporoparietal region and left primary motor cortex.
High pulse frequency (>5 Hz) used in rTMS protocols can have an excitatory effect while a low frequency (1 Hz) has an inhibitory effect. These effects are not limited to the target of stimulation, favoring the improvement of mood symptoms since there is complex connectivity of the cerebral cortex with other deep brain regions, favoring, ultimately, the improvement of mood symptoms. Most studies analyzed in this review used 10 stimulation sessions and half used excitatory stimulation for all subjects with rTMS frequency range between 5 Hz and 20 Hz. In the treatment of anxious symptoms, studies reported using between 4 and 31 sessions with 200–5000 pulses per stimulation and an intensity of 20–130% of the RMT.
Measures of anxiety symptoms
One of the criteria used to include the paper in our systematic review was to contain measures to assess the symptoms of anxiety pre and post rTMS sessions. The Hamilton Rating Scale for Anxiety (HRSA) was the main scale found in this review; overall, this scale appeared in 14 papers; secondly the Beck Anxiety Inventory (BAI) and the State-Trait Anxiety Inventory (STAI), both appearing in four papers. In Table 5, we observe the other scales of assessment of the symptoms of anxiety found and their frequency in the papers.
 |
Table 4 Symptoms of anxiety present in psychiatric disorders |
 |
Table 5 Anxiety scales found in papers |
Clinical findings
We observed, in general, that most of the studies found satisfactory results with the use of rTMS in Anxiety Disorders and Anxiety as comorbidity.
In the studies on Anxiety Disorders, it was observed that three papers reported a sustained effect of response on the improvement of anxious symptoms, one paper reported improvement of symptoms, although this response did not sustain in the long term and one paper reported that there was no significant improvement.
The papers on Anxious Symptoms showed that most of the studies obtained the sustained effect of response on the anxiety symptoms observed in 21 studies. Two studies showed improvement but not sustained over time and one of them did not find a positive response to the improvement in anxiety symptoms.
Tolerability and safety
Adverse effects were minimal, showing that there is safety in the application of rTMS in anxious symptoms independent of the primary outcome.
In the papers on Anxiety Disorders, only one study reported that one participant in the active group had seizures and three participants reported transient dizziness; in the other papers, no adverse effects were reported.
In the papers on Anxious Symptoms, mild side effects were observed. The most frequent effect was a headache that appeared in 12 studies. In two studies, some patients were withdrawn because of side effects or treatment intolerance. Table 6 has the description and frequency of other side effects found in the papers on Anxious Symptoms.
 |
Table 6 Side effects of rTMS found in papers |
Discussion
The rTMS is already established as a non-invasive valid alternative to measure plastic alterations of the cerebral cortex for the treatment of depression,2 borderlines,41 OCD,6 among other pathologies. We observed in this review that anxiety is one of the main symptoms related to current mood disorders, with a direct impact on individuals’ quality of life.7
TMS have a direct correlation between the parameters associated with cortical excitability and plasticity, suggesting the existence of mechanisms that partially overlap and probably act in the same neurophysiological framework.62
Pennisi et al suggest that lesion in the ischemic subcortical and prefrontal region might have implications in cognition and mood and may result in functional changes of the intracortical system, in addition to associating an increase in global cortical excitability, together with a significant worsening of frontal lobe abilities, but without substantial functional impairment.62
Cortical plasticity plays a clear and fundamental role in patients with depression, while this still seems to be firmly established in anxiety disorders.63
Studies observed that impaired brain plasticity may be one of the pathophysiological mechanisms underlying cognitive decline and major depression.63 Some degree of cognitive impairment is often observed across the clinical spectrum of mood disorders, and between depression and cognition often bidirectional.63 Depressed patients have significant differences between the brain hemispheres, the intracortical neurochemical circuit (inhibitory or excitatory) might be unbalanced, the excitability of the motor cortex and TMS may indicate a disruption of plasticity.63 The data suggest that the motor cortex is more refractory to modulatory inputs from other non-motor areas within the CNS in depressed individuals.63 In addition, several studies have shown that functional abnormalities in cortical connections may play crucial roles in patients with depression and other mood disorders.63
Studies with depressive disorder usually predict anxiety symptoms as a secondary outcome. It is possible to observe how this mechanism occurs in cases of anxiety disorders, since it is directly related to the functioning of neurotransmitters and to the cerebral circuits. TMS may interfere directly in this functioning and may influence the regulation of anxiety as a symptom arising from another psychopathology or as an anxiety disorder.63
Other studies14,30,32 have found that TMS have promising results as a treatment for anxiety disorders; we observed comparable results, showing TMS intervention having positive effects on anxiety symptoms. However, there is still no standard intervention protocol for the use of TMS, neither for anxiety or depression disorders, as a comorbidity of psychiatric and neurological disorders.14,30,32
A few studies describe protocols for TMS application on anxiety disorders, Dilkov et al33 evidenced the effects of rTMS on participants with GAD. Most participants who received treatment from 25 rTMS sessions had a clinically significant decrease in anxiety symptoms as identified by the efficacy symptom scale.33
Another study also observed positive results in subjects with GAD after 30 rTMS sessions according to pre- and post-treatment comparative assessment scales.35 However, the Sham coil group also had reduced anxiety symptoms; they had a limited sample size and further assessment is necessary.35
The authors used 2 sessions of rTMS in the prefrontal ventromedial cortex region for the treatment of Acrophobia and fear of irrational stature.64 Their study used virtual reality technology to assist in the therapy and they observed a better response for fear and symptoms.64
There is no clear pattern for using high or low frequencies of TMS stimulation in which regions of the brain. One review study cited the use of both high- and low-frequency TMS stimulation under the right and left stimulation target DLPFC.30 Iannone et al14 cited papers that used both high and low frequencies of TMS on anxiety disorders. Another review study found that the right and left DLPFC were targets for TMS stimulation in most papers.31 However, when searching for anxiety as a secondary outcome, the stimulation region was dependable on the primary outcome of the TMS intervention. TMS stimulation for pathologies involving motor aspects poses another difficulty.65 In these cases, the stimulation target was primarily in specific regions of the motor cortex.65
Patients with Restless Legs Syndrome (RLS) who receive TMS intervention had improvements on anxiety symptoms with the improvement of the RLS symptoms.47 A similar study found that using TMS to stimulate the motor cortex area alleviated motor sensory complaints of RLS patients. TMS excitation and inhibition rates indicate intracortical injury and corticospinal imbalance, involving GABAergic and glutamatergic circuits, as well as impairment of the short-term mechanisms of plasticity.65 The activation induced by rTMS with the consequent increase in dopamine release may have contributed to the clinical and neurophysiological outcome in those patients.65 The occurrence of anxiety in patients with RLS is related to an abnormal sensorimotor integration, suggesting an interrupted connectivity in the RLS.66 Using TMS in the sensory cortex motor region may promote improvements of physical symptoms and therefore reduction in anxiety.66
TMS has also been shown to be effective as a sustained response effect compared to drug intervention. It is observed that drug-resistant patients on MDD treatment achieved improvement of symptoms with the use of TMS after a period of six months post-intervention, while this period is reduced when only medication is used.67
Although there is no consensus in the TMS intervention parameters for anxiety disorders and symptoms due to neurological or psychiatric disorders, we observed that the DLPFC region is preferred among researcher’s stimulation target. TMS have promising results in high frequency, promoting excitatory stimulation, and low frequency, promoting inhibitory stimulation. The number of sessions should range from 10 to 20 sessions so that the sustained response effect is possible. The stimulation of the motor cortex, especially in the sensorimotor region, also shows promising results in the stimulation of anxiety symptoms due to neurological disorders, mainly focusing on motor aspects.
Conclusion
We observed in this review that TMS might be a satisfactory intervention measure to improve anxiety, although there are a limited number of reports on the use of this intervention. In addition, satisfactory results have also noted that TMS is a safe treatment strategy with low side effect. Further studies using TMS could direct the treatment with psychological or psychiatric intervention.
Disclosure
Miss Cintya Yukie Hayashi reports grant from Coordination for the Improvement of Higher Education Personnel (CAPES) – Brazil, outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Cabrera CC, Sponholz Júnior A. Ansiedade e insônia. In: Prática Psiquiátrica No Hospital Geral: Interconsulta E Emergência. Porto Alegre, Brazil: Artmed Editora;2005:283–304.
2. Young AS, Klap R, Sherbourne CD, Wells KB. The quality of care for depressive and anxiety disorders in the United States. Arch Gen Psychiatry. 2001;58(1):55–61. doi:10.1001/archpsyc.58.1.55
3. Borkovec TD, Newman MG, Pincus AL, Lytle R. A component analysis of cognitive-behavioral therapy for generalized anxiety disorder and the role of interpersonal problems. J Consult Clin Psychol. 2002;70(2):288. doi:10.1037/0022-006X.70.2.288
4. Balconi M, Ferrari C. Left DLPFC rTMS stimulation reduced the anxiety bias effect or how to restore the positive memory processing in high-anxiety subjects. Psychiatry Res. 2013;209:554–559. doi:10.1016/j.psychres.2013.03.032
5. White D, Tavakoli S. Repetitive transcranial magnetic stimulation for treatment of major depressive disorder with comorbid generalized anxiety disorder. Ann Clin Psychiatry. 2015;27(3):192–196.
6. Elbeh KAM, Elserogy YMB, Khalifa HE, Ahmed MA, Hafez MH, Khedr EM. Repetitive transcranial magnetic stimulation in the treatment of obsessive-compulsive disorders: double blind randomized clinical trial. Psychiatry Res. 2016;238:264–269. doi:10.1016/j.psychres.2016.02.031
7. Gould KR, Ponsford JL, Spitz G. Association between cognitive impairments and anxiety disorders following traumatic brain injury. J Clin Exp Neuropsychol. 2014;36(1):1–14. doi:10.1080/13803395.2013.863832
8. Bashir S, Mizrahi I, Weaver K, Fregni F, Pascual-Leone A. Assessment and modulation of neural plasticity in rehabilitation with transcranial magnetic stimulation. PM&R. 2010;2(12):S253–S268. doi:10.1016/j.pmrj.2010.10.015
9. Kobayashi M, Pascual-Leone A. Transcranial magnetic stimulation in neurology. Lancet Neurol. 2003;2(3):145–156.
10. Barker AT. An introduction to the basic principles of magnetic nerve stimulation. J Clin Neurophysiol. 1991;8(1):26–37.
11. Hallett M. Transcranial magnetic stimulation and the human brain. Nature. 2000;406(6792):147–150. doi:10.1038/35018000
12. Kim YI, Kim SM, Kim H, Han DH. The effect of high-frequency repetitive transcranial magnetic stimulation on occupational stress among health care workers: a pilot study. Psychiatry Investig. 2016;13(6):622–629. doi:10.4306/pi.2016.13.6.622
13. Sagliano L, D’Olimpio F, Panico F, Gagliardi S, Trojano L. The role of the dorsolateral prefrontal cortex in early threat processing: a TMS study. Soc Cogn Affect Neurosci. 2016;11(12):1992–1998. doi:10.1093/scan/nsw105
14. Iannone A, Cruz APM, Brasil-Neto JP, Boechat-Barros R. Transcranial magnetic stimulation and transcranial direct current stimulation appear to be safe neuromodulatory techniques useful in the treatment of anxiety disorders and other neuropsychiatric disorders. Arq Neuropsiquiatr. 2016;74(10):829–835. doi:10.1590/0004-282X20160115
15. Luber BM, Davis S, Bernhardt E, et al. Using neuroimaging to individualize TMS treatment for depression: toward a new paradigm for imaging-guided intervention. NeuroImage. 2017;148:1–7. doi:10.1016/j.neuroimage.2016.12.083
16. Vidor LP, Torres ILS, Medeiros LF, et al. Association of anxiety with intracortical inhibition and descending pain modulation in chronic myofascial pain syndrome. BMC Neurosci. 2014;15:42. doi:10.1186/1471-2202-15-42
17. Mak ADP, Chan SSM, Lam LCW, et al. Preliminary results from a randomized sham-controlled trial of augmentative neuro-navigated right-dorsolateral prefrontal cortex low-frequency repetitive transcranial magnetic stimulation for antidepressant non-responding bipolar depression. Brain Stimul. 2017;10(2):378. doi:10.1016/j.brs.2017.01.121
18. Bashir S, Vernet M, Yoo WK, Mizrahi I, Theoret H, Pascual-Leone A. Changes in cortical plasticity after mild traumatic brain injury. Restor Neurol Neurosci. 2012;30(4):277–282. doi:10.3233/RNN-2012-110207
19. Li SS, Zaninotto AL, Neville IS, Paiva WS, Nunn D, Fregni F. Clinical utility of brain stimulation modalities following traumatic brain injury: current evidence. Neuropsychiatr Dis Treat. 2015;11:1573–1586. doi:10.2147/NDT.S65816
20. Weir K, Gray S. Can magnets cure depression. Transcranial magnetic stimulation is gaining ground as a therapy for treatment-resistant depression. APA. 2015;46(2):50.
21. Watts BV, Landon B, Groft A, Young-Xu Y. A sham controlled study of repetitive transcranial magnetic stimulation for posttraumatic stress disorder. Brain Stimul. 2012;5(1):38–43. doi:10.1016/j.brs.2011.02.002
22. Ma X, Huang Y, Liao L, Jin Y. A randomized double-blinded sham-controlled trial of a electroencephalogram-guided transcranial magnetic stimulation for obsessive-compulsive disorder. Chin Med J. 2014;127(4):601–606.
23. Hegde S. Music-based cognitive remediation therapy for patients with traumatic brain injury. Front Neurol. 2014;5. doi:10.3389/fneur.2014.00034
24. Nielson DM, McKnight CA, Patel RN, Kalnin AJ, Mysiw WJ. Preliminary guidelines for safe and effective use of repetitive transcranial magnetic stimulation in moderate to severe traumatic brain injury. Arch Phys Med Rehabil. 2015;96(4):S138–S144. doi:10.1016/j.apmr.2014.09.010
25. Araújo HA, Iglesio RF, de Camargo Correia GS, et al. Estimulação magnética transcraniana e aplicabilidade clínica: perspectivas na conduta terapêutica neuropsiquiátrica. Rev Med. 2011;90(1):3–14. doi:10.11606/issn.1679-9836.v90i1p3-14
26. Barker A, Freeston I, Jalinous R, Jarratt J. Magnetic stimulation of the human brain and peripheral nervous system: an introduction and the results of an initial clinical evaluation. Neurosurgery. 1987;20(1):100–109. doi:10.1097/00006123-198701000-00024
27. Lefaucheur JP. Stroke recovery can be enhanced by using repetitive transcranial magnetic stimulation (rTMS). Clin Neurophysiol. 2006;36(3):105–115. doi:10.1016/j.neucli.2006.08.011
28. Miniussi C, Rossini PM. Transcranial magnetic stimulation in cognitive rehabilitation. Neuropsychol Rehabil. 2011;21(5):579–601. doi:10.1080/09602011.2011.562689
29. Ricci R, Ramsey D, Johnson K, et al. A pilot feasibility study of daily rTMS to modify corticospinal excitability during lower limb immobilization. Ther Clin Risk Manag. 2008;4(5):1127–1134. doi:10.2147/tcrm.s2719
30. Vicario CM, Salehinejad MA, Felmingham K, Martino G, Nitsche MA. A systematic review on the therapeutic effectiveness of non-invasive brain stimulation for the treatment of anxiety disorders. Neurosci Biobehav Rev. 2018;96:219–231.
31. Paes F, Machado S, Arias-Carrion O, et al. The value of repetitive transcranial magnetic stimulation (rTMS) for the treatment of anxiety disorders: an integrative review. CNS Neurol Disord Drug Targets. 2011;10(5):610–620.
32. Lefaucheur JP, André-Obadia N, Antal A, et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS). Clin Neurophysiol. 2014;125(11):2150–2206. doi:10.1016/j.clinph.2014.05.021
33. Dilkov D, Hawken ER, Kaludiev E, Milev R. Repetitive transcranial magnetic stimulation of the right dorsal lateral prefrontal cortex in the treatment of generalized anxiety disorder: a randomized, double-blind sham controlled clinical trial. Prog Neuro-Psychopharmacol Biol Psychiatry. 2017;78:61–65. doi:10.1016/j.pnpbp.2017.05.018
34. Mantovani A, Aly M, Dagan Y, Allart A, Lisanby SH. Randomized sham controlled trial of repetitive transcranial magnetic stimulation to the dorsolateral prefrontal cortex for the treatment of panic disorder with comorbid major depression. J Affect Disord. 2013;144(1–2):153–159. doi:10.1016/j.jad.2012.05.038
35. Diefenbach GJ, Bragdon LB, Zertuche L, et al. Repetitive transcranial magnetic stimulation for generalised anxiety disorder: a pilot randomised, double-blind, sham-controlled trial. Br J Psychiatry. 2016;209(3):222–228. doi:10.1192/bjp.bp.115.168203
36. Prasko J, Zalesky R, Bares M, et al. The effect of repetitive transcranial magnetic stimulation (rTMS) add on serotonin reuptake inhibitors in patients with panic disorder: a randomized, double blind sham controlled study. Neuroendocrinol Lett. 2007;28(1):33–38.
37. Zhang T, Zhu J, Xu L, et al. Add-on rTMS for the acute treatment of depressive symptoms is probably more effective in adolescents than in adults: evidence from real-world clinical practice. Brain Stimul. 2019;12(1):103–109. doi:10.1016/j.brs.2018.09.007
38. Lu R, Zhang C, Liu Y, Wang L, Chen X, Zhou X. The effect of bilateral low-frequency rTMS over dorsolateral prefrontal cortex on serum brain-derived neurotropic factor and serotonin in patients with generalized anxiety disorder. Neurosci Lett. 2018;684:67–71. doi:10.1016/j.neulet.2018.07.008
39. Kaur M, Naismith SL, Lagopoulos J, et al. Sleep-wake, cognitive and clinical correlates of treatment outcome with repetitive transcranial magnetic stimulation for young adults with depression. Psychiatry Res. 2019;271:335–342. doi:10.1016/j.psychres.2018.12.002
40. Lin H, Li W, Ni J, Wang Y. Clinical study of repetitive transcranial magnetic stimulation of the motor cortex for thalamic pain. Medicine. 2018;97(27):e11235.
41. Reyes-López J, Ricardo-Garcell J, Armas-Castañeda G, et al. Clinical improvement in patients with borderline personality disorder after treatment with repetitive transcranial magnetic stimulation: preliminary results. Rev Bras Psiquiatr. 2018;40(1):97–104. doi:10.1590/1516-4446-2016-2112
42. Durmaz O, Ebrinc S, Ates MA, Algul A. Evaluation of repetitive transcranial magnetic stimulation for treatment resistant major depression and the impact of anxiety symptoms on outcome. Psychiatry Clin Psychopharmacol. 2017;27(1):14–18. doi:10.1080/24750573.2017.1293239
43. Noh TS, Kyong JS, Chang MY, et al. Comparison of treatment outcomes following either prefrontal cortical-only or dual-site repetitive transcranial magnetic stimulation in chronic tinnitus patients: a double-blind randomized study. Otol Neurotol. 2017;38(2):296–303. doi:10.1097/MAO.0000000000001266
44. Tovar-Perdomo S, McGirr A, Van Den Eynde F, Dos Santos NR, Berlim MT. High frequency repetitive transcranial magnetic stimulation treatment for major depression: dissociated effects on psychopathology and neurocognition. J Affect Disord. 2017;217:112–117. doi:10.1016/j.jad.2017.03.075
45. Malavera A, Silva FA, Fregni F, Carrillo S, Garcia RG. Repetitive transcranial magnetic stimulation for phantom limb pain in land mine victims: a double-blinded, randomized, sham-controlled trial. J Pain. 2016;17(8):911–918. doi:10.1016/j.jpain.2016.05.003
46. Bilici S, Yigit O, Taskin U, Gor AP, Yilmaz ED. Medium-term results of combined treatment with transcranial magnetic stimulation and antidepressant drug for chronic tinnitus. Eur Archiv Oto-Rhino-Laryngol. 2015;272(2):337–343. doi:10.1007/s00405-013-2851-z
47. Lin YC, Feng Y, Zhan SQ, et al. Repetitive transcranial magnetic stimulation for the treatment of restless legs syndrome. Chin Med J. 2015;128(13):1728. doi:10.4103/0366-6999.159344
48. Oznur T, Akarsu S, Celik C, et al. Is transcranial magnetic stimulation effective in treatment-resistant combat related posttraumatic stress disorder? Neurosciences (Riyadh). 2014;19(1):29–32.
49. Diefenbach GJ, Bragdon L, Goethe JW. Treating anxious depression using repetitive transcranial magnetic stimulation. J Affect Disord. 2013;151(1):365–368. doi:10.1016/j.jad.2013.05.094
50. Pretalli JB, Nicolier M, Chopard G, et al. Resting motor threshold changes and clinical response to prefrontal repetitive transcranial magnetic stimulation in depressed patients. Psychiatry Clin Neurosci. 2012;66(4):344–352. doi:10.1111/j.1440-1819.2012.02341.x
51. Sun W, Mao W, Meng X, et al. Low‐frequency repetitive transcranial magnetic stimulation for the treatment of refractory partial epilepsy: a controlled clinical study. Epilepsia. 2012;53(10):1782–1789. doi:10.1111/j.1528-1167.2012.03626.x
52. Berlim MT, McGirr A, Beaulieu MM, Turecki G. High frequency repetitive transcranial magnetic stimulation as an augmenting strategy in severe treatment-resistant major depression: a prospective 4-week naturalistic trial. J Affect Disord. 2011;130(1–2):312–317. doi:10.1016/j.jad.2010.10.011
53. Boggio PS, Rocha M, Oliveira MO, et al. Noninvasive brain stimulation with high-frequency and low-intensity repetitive transcranial magnetic stimulation treatment for posttraumatic stress disorder. J Clin Psychiatry. 2010;71(8):992. doi:10.4088/JCP.08m04638blu
54. Epstein CM, Evatt ML, Funk A, et al. An open study of repetitive transcranial magnetic stimulation in treatment-resistant depression with Parkinson’s disease. Clin Neurophysiol. 2007;118(10):2189–2194. doi:10.1016/j.clinph.2007.07.010
55. Passard A, Attal N, Benadhira R, et al. Effects of unilateral repetitive transcranial magnetic stimulation of the motor cortex on chronic widespread pain in fibromyalgia. Brain. 2007;130(10):2661–2670. doi:10.1093/brain/awm189
56. Rossi S, De Capua A, Ulivelli M, et al. Effects of repetitive transcranial magnetic stimulation on chronic tinnitus. A randomised, cross over, double blind, placebo-controlled study. Journal of Neurology, Neurosurgery & Psychiatry. 2007. doi:10.1136/jnnp.2006.105007
57. Cohen H, Kaplan Z, Kotler M, Kouperman I, Moisa R, Grisaru N. Repetitive transcranial magnetic stimulation of the right dorsolateral prefrontal cortex in posttraumatic stress disorder: a double-blind, placebo-controlled study. Am J Psychiatry. 2004;161(3):515–524. doi:10.1176/appi.ajp.161.3.515
58. Loo CK, Sachdev PS, Haindl W, et al. High (15 Hz) and low (1 Hz) frequency transcranial magnetic stimulation have different acute effects on regional cerebral blood flow in depressed patients. Psychol Med. 2003;33(6):997–1006.
59. Münchau A, Bloem BR, Thilo KV, Trimble MR, Rothwell JC, Robertson MM. Repetitive transcranial magnetic stimulation for Tourette syndrome. Neurology. 2002;59(11):1789–1791. doi:10.1212/01.wnl.0000036615.25044.50
60. George MS, Nahas Z, Molloy M, et al. A controlled trial of daily left prefrontal cortex TMS for treating depression. Biol Psychiatry. 2000;48(10):962–970. doi:10.1016/s0006-3223(00)01048-9
61. Rollnik JD, Huber TJ, Mogk H, et al. High frequency repetitive transcranial magnetic stimulation (rTMS) of the dorsolateral prefrontal cortex in schizophrenic patients. Neuroreport. 2000;11(18):4013–4015. doi:10.1097/00001756-200012180-00022
62. Pennisi G, Bella R, Lanza G. Motor cortex plasticity in subcortical ischemic vascular dementia: what can TMS say? Clin Neurophysiol. 2015;126(5):851–852. doi:10.1016/j.clinph.2014.09.001
63. Cantone M, Bramanti A, Lanza G, et al. Cortical plasticity in depression: a neurochemical perspective from transcranial magnetic stimulation. ASN Neuro. 2017;9(3):1759091417711512. doi:10.1177/1759091417711512
64. Herrman MJ, Katzorke A, Busch Y, et al. Medial prefrontal cortex stimulation accelerates therapy response of exposure therapy in acrophobia. Brain Stimul. 2017;10(2):291. doi:10.1016/j.brs.2016.11.007
65. Lanza G, Cantone M, Aricò D, et al. Clinical and electrophysiological impact of repetitive low-frequency transcranial magnetic stimulation on the sensory–motor network in patients with restless legs syndrome. Ther Adv Neurol Disord. 2018;11:1756286418759973. doi:10.1177/1756286418759973
66. Lanza G, Bachmann CG, Ghorayeb I, Wang Y, Ferri R, Paulus W. Central and peripheral nervous system excitability in restless legs syndrome. Sleep Med. 2017;31:49–60. doi:10.1016/j.sleep.2016.05.010
67. Concerto C, Lanza G, Cantone M, et al. Repetitive transcranial magnetic stimulation in patients with drug-resistant major depression: a six-month clinical follow-up study. Int J Psychiatry Clin Pract. 2015;19(4):252–258. doi:10.3109/13651501.2015.1084329
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
