Back to Journals » Neuropsychiatric Disease and Treatment » Volume 16
Transcranial Direct Current Stimulation in Patients with Anxiety: Current Perspectives
Authors Stein DJ , Fernandes Medeiros L, Caumo W , Torres ILS
Received 18 October 2019
Accepted for publication 21 December 2019
Published 14 January 2020 Volume 2020:16 Pages 161—169
DOI https://doi.org/10.2147/NDT.S195840
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Roger Pinder
Dirson João Stein, 1, 2,* Liciane Fernandes Medeiros, 1, 3, 4,* Wolnei Caumo, 2 Iraci LS Torres 1– 3
1Laboratório de Farmacologia da Dor e Neuromodulação, Investigações Pré-Clínicas, Centro de Pesquisa Experimental, Hospital de Clínicas de Porto Alegre (HCPA), Porto Alegre, RS, Brazil; 2Programa de Pós-Graduação em Medicina: Ciências Médicas, Universidade Federal do Rio Grande do Sul (UFRGS), Porto Alegre, RS, Brazil; 3Programa de Pós-Graduação em Ciências Biológicas: Farmacologia e Terapêutica, UFRGS, Porto Alegre, RS, Brazil; 4Programa de Pós-Graduação em Saúde e Desenvolvimento Humano, Universidade La Salle, Canoas, RS, Brazil
*These authors contributed equally to this work
Correspondence: Iraci LS Torres
Pharmacology Department, ICBS, UFRGS, Rua Sarmento Leite, 500 - Sala 237, 90.050-170, Porto Alegre, RS, Brazil
Tel +55 51 3308 3183
Fax +55 51 3308 3121
Email [email protected]
Abstract: Anxiety is one of the most prevalent and debilitating psychiatric conditions worldwide. Pharmaco- and psycho-therapies have been employed in the treatment of human anxiety to date. Yet, either alone or in combination, unsatisfactory patient outcomes are prevalent, resulting in a considerable number of people whose symptoms fail to respond to conventional therapies with symptoms remaining after intervention. The demand for new therapies has given birth to several noninvasive brain stimulation techniques. Transcranial direct current stimulation (tDCS) has arisen as a promising tool and has been proven to be safe and well tolerated for the treatment of many diseases, including chronic pain, depression, and anxiety. Here, reports of the use of tDCS in anxiety disorders in human patients were reviewed and summarized. A literature search was conducted in mid-2019, to identify clinical studies that evaluated the use of tDCS for the treatment of anxiety behavior. The PubMed, Web of Science, and Scielo and PsycInfo databases were explored using the following descriptors: “anxiety”, “anxious behavior”, “tDCS”, and “transcranial direct current stimulation”. Among the selected articles, considerable variability in the type of tDCS treatment applied in interventions was observed. Evidence shows that tDCS may be more effective when used in combination with drugs and cognitive behavioral therapies; however future large-scale clinical trials are recommended to better clarify the real effects of this intervention alone, or in combination with others.
Keywords: transcranial electrical stimulation, psychiatric disorder, anxious behavior, humans, clinical research
Introduction
Anxiety is one of the most prevalent and debilitating psychiatric conditions worldwide; the lifetime prevalence of anxiety disorders is around 17%.1,2 These disorders are usually classified based on the criteria defined by the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-V).3 According to the DSM-V, anxiety disorders include those that share features of excessive fear and anxiety and related behavioral disturbances. These disorders include separation anxiety disorder, selective mutism, specific phobia, social anxiety disorder (social phobia), panic disorder, agoraphobia, generalized anxiety disorder, substance/medication-induced anxiety disorder, and anxiety disorder due to another medical condition.3
The Global Burden of Disease study found that in 2010, anxiety disorders were the sixth leading cause of disability in terms of years of life lived with disability, in both high-income and low- and middle-income countries. These data account for 390 disability adjusted life years per 100,000 persons (95% confidence interval, 191–371), with the highest burden in women and those aged 15 to 34 years, but with no change over time and no identifiable differences in burdens across regions.4,5
Anxiety disorders are associated with a broad range of profound negative sequelae. The complexity of mechanisms involved in the pathophysiology of anxiety-related disorders is closely linked to a wide range of clinical conditions and individual functioning.6 Previous studies have reported that patients with some anxiety-related disorders can present an imbalance between the activity of the right and the left dorsolateral prefrontal cortex (DLPFC), with hypoactivity in the left side and hyperactivity in the right side.7,8 As suggested, the hypoactivity of the right DLPFC is associated with negative emotional judgment, while hyperactivity is linked to attentional modulation.7 Similar dysfunctional patterns were found in patients with major depressive disorder (MDD),9 despite this condition being categorized as a depressive disorder (DSM-V). Furthermore, state and trait anxiety might trigger the activation of different areas in the central nervous system (CNS), where high state anxiety is associated with elevated activation of the amygdala and superior temporal sulcus, and high trait anxiety is associated with reduced activation in the lateral PFC, and dorsal and rostral anterior cingulate cortex (ACC).10
The treatment options for anxiety-related disorders are based on pharmacological and cognitive behavioral therapy interventions. Considering the wide diversity of symptoms and interindividual variability, these conditions are currently undertreated. Pharmacological therapy comprises the prescription of different classes of drugs, such as tricyclic antidepressants, selective serotonin reuptake inhibitors, selective serotonin-norepinephrine reuptake inhibitors, pregabalin, buspirone, benzodiazepines, and others.11 All drugs have significant side effects, for example, nausea, restlessness, headache, fatigue, increased or decreased appetite, weight gain or loss, tremor, sweating, and others that can lead to low adherence to medication. Therefore, alternative and complementary methods that can improve quality of life, and reduce anxiety levels in patients diagnosed with anxiety-related disorders need to be explored.
Brain stimulation techniques have been used as an alternative tool to assess and treat anxiety-related disorders. As shown by diagnostic neuromodulatory techniques, the DLPFC is involved in threat processing.12 An imbalance between the right and left DLPFC, with hyperactivation of the right frontal areas, is involved in the processing of negative emotions and generation of anxiety.13 A recent study showed that inhibitory transcranial magnetic stimulation (TMS), with pulses applied over the left DLPFC, triggers a disengagement bias in highly anxious individuals and determines attentional avoidance in less anxious individuals,13 characterizing the baseline status as a predictive factor of response to treatment.
Along the same lines, transcranial electrical stimulation techniques, such as transcranial direct current stimulation (tDCS), have been applied for the treatment of various anxiety-related disorders, for example, generalized anxiety disorders,14 social anxiety disorders,15 anorexia nervosa,16 and others. tDCS is a simple and cheap technique using two electrodes applied to the scalp. The anode (positive) depolarizes the neuronal membrane threshold, while the cathode (negative) hyperpolarizes it.17,18 This technique has been used in clinical settings using a range of stimulations from 1 to 2 mA of current, with the electrodes applied over specific areas of the brain, according to the required outcome. For example, the suggested imbalance between DLPFC found in anxiety patients may be treated using bicephalic tDCS montage.19 Repeated tDCS sessions might have longer-lasting effects in comparison to a single session.20,21 Despite that the full tDCS mechanisms of action are still unclear, behavioral and psychological changes have been reported in clinical22,23 and preclinical studies,24,25 as well as alterations in cortico-spinal excitability parameters.26
tDCS is a well-tolerated method of neuromodulation that holds promise for the treatment of many diseases, and has been employed with therapeutic efficacy in patients with major depression (MD), chronic pain disorders, anxiety, and other conditions. Thus, non-invasive central neuromodulatory techniques can be considered as tools to treat or alleviate various anxiety symptoms. The use of off-label therapies, such as tDCS, tends to occur in the setting of diseases that are notoriously resistant to other treatment modalities. In this systematic literature review, we aimed to present the most recent available information regarding the therapeutic efficacy of tDCS for anxiety disorders in humans.
Methods
Search Strategy and Article Selection
A systematic literature review was conducted between June and September, 2019, and was performed by an electronic search of indexed articles in the PubMed, Web of Science, and SciELO and PsycINFO databases, using the following descriptors: “anxiety”, “anxious behavior”, “tDCS”, and “transcranial direct current stimulation”. Only articles describing empirical studies written in English were included. Pre-selected articles were independently assessed by two authors for the inclusion criteria, which was as follows: the article described a clinical experiment or case report; the article abstract was publicly available; the study used tDCS; and anxiety-related behavior was a primary study outcome. After excluding duplicates, both authors categorized articles for further analysis.
The process of selecting articles for inclusion in this review was performed in two stages. First, two independent reviewers screened the articles based on the title and selected only relevant articles. Second, the abstracts of relevant articles were assessed to verify that the inclusion criteria was met. After examining the titles and abstracts, a total of 11 articles were found to fulfill all the inclusion criteria. These articles were then fully examined and relevant information was extracted.
Results
Searches of the four databases using the defined descriptors resulted in a total of 217 articles. After excluding duplicates, there were 175 articles available for further analysis. Of these, 11 articles fulfilled all the inclusion criteria, and are described in Table 1.
 |
Table 1 Description of the Selected Studies |
The results highlight that there was high heterogeneity among the selected articles, mainly related to the samples, anxiety measures, tDCS stimulation patterns, and outcomes analyzed. The study sample was comprised of healthy individuals only in three articles; other studies included patients with various disorders, such as anorexia nervosa, generalized anxiety disorder, social anxiety disorder, major depressive disorder, or mathematics anxiety. Furthermore, only one selected study evaluated anxiety levels in patients during burn wound care.
Another interesting key observation was the difference in methodologies used for assessing anxiety parameters. Most studies employed questionnaires or scales, including the Anxiety Sensitivity Index (ASI) Scale, Hamilton Anxiety Rating Scale (HAM-A), State-Trait Anxiety Inventory (STAI) Scale, Generalized Anxiety Disorder 7-item (GAD-7) Scale, Beck Anxiety Inventory (BDI) Scale. As for the assessment of outcomes, different approaches were also taken, for example, attentional bias, arithmetic decisions, and Positive and Negative Affect Schedule (PANAS) scores were used for assessing the specific conditions of induced anxiety or anxiety-state frames.
The main aspects concerning the montage, region, and pattern of tDCS stimulation were that most studies used anodal stimulation over the left DLPFC and when bilateral stimulation was applied, the cathodal electrode was placed over the right DLPFC. In addition, different areas were stimulated: the sensory cortex, dorsomedial PFC, or ventrolateral PFC areas. Another interesting point was that the articles describe single and repeated sessions of tDCS that varied between 5, 10, 15, and 18 sessions. The intensity of current stimulation varied between 1 and 2 mA, being both safe for use in clinical settings.35–37
The benefits of tDCS (unimodal or bimodal) as outcomes were described in nine articles. tDCS reduced anxiety symptoms, approach behavior during conflict, attentional bias for threat, vigilance to threatening stimuli, and perceived extent of negative emotions, such as fear, anxiety, and sadness, and also improved reaction times on arithmetic decisions. However, a single session of anodal tDCS (1 mA) was not able to reduce anxiety in MDD patients. Furthermore, no significant improvement was observed in anxiety, mood, affectivity, or depression after 5 sessions of anodal tDCS in patients with generalized anxiety disorder.
Discussion
Overall, we reviewed 11 articles. In support of previous studies, nine of these articles showed that the effects of tDCS applied to the scalp are dependent on the montage and polarity of electrodes (anodal or cathodal), and the site of stimulation (right or left DLPFC, DMPFC, VLPFC, and others). In addition, tDCS was able to directly modulate anxiety levels in patients with some anxiety disorders,29,31,33 or change behavior during conflict or threat in healthy individuals.27,30
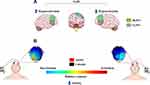
The articles selected for this review highlight the role of the DLPFC in anxiety behaviors, as well as the fact that the imbalance between the right and left DLPFC may contribute to some anxiety symptoms. This suggests that anodal stimulation over the left DLPFC and cathodal stimulation over the right DLPFC are more effective for the treatment of anxiety symptoms in humans (Figure 1). Moreover, studies using tDCS techniques might augment knowledge regarding diverse clinical conditions, as the activation or deactivation of CNS areas through weak electrical currents may also induce similar behaviors to those present in these conditions.
Neuropsychiatric conditions are difficult to treat since classical drug therapies are related to several side effects and poor clinical efficacy. In this context, neuromodulatory techniques, such as tDCS, have been studied to treat neurological diseases including depression, anxiety, epilepsy, drug addiction, and others. It is important to note that this technique is easy to apply, simple, and cheap. Advanced tDCS devices can contribute to the effectiveness of treatment for different conditions, when considering home-based applications.38
Different approaches were taken to assess the behavioral response in patients with different levels of anxiety or healthy individuals submitted to some anxiety situations. A case report study described one patient (female, 58 years old) with progressive GAD,33 where repeated cathodal tDCS stimulation over the right DLPFC decreased anxiety levels. After 1 month of treatment, this patient was asymptomatic. It is important to note that the authors point out that, theoretically, cathodal stimulation could decrease the activity of the right DLPFC, and thereby subsequently decrease the activity of other cortical and subcortical brain regions (such as the medial PFC, amygdala, and insula).39 In addition, the authors did not rule out the involvement of the left DLPFC, which might be modulated by deactivation of the right DLPFC after cathodal tDCS. Despite the fact this study is a case report, the benefits found in that patient using tDCS for GAD should encourage further controlled and randomized trials to verify the effect of tDCS on different types of anxiety.
Anodal tDCS over the left DLPFC reduced attentional bias for threat in female patients with social anxiety disorder using a crossover design.15 As suggested by Bishop and colleagues,40,41 the DLPFC exerts control over the amygdala. When control over the amygdala by the DLPFC fails, patients present higher attentional bias. Despite the small sample size, the authors found benefits from anodal tDCS treatment.
Movahed and colleagues31 compared the effectiveness between tDCS, sham-tDCS, and pharmacological treatment for reducing anxiety, depression, and worry in patients with GAD using a quasi-experimental design. This study showed that repeated cathodal tDCS over the right PFC decreased worry and depression, although changes in anxiety levels were not observed. Despite the benefits of tDCS for patients in this study, some important aspects of the methodology could have biased the results, for example, the small sample size, the non-random sex distribution, missing information concerning pharmacological therapy, and no clear data analysis.
A recent pilot double-blind, randomized sham-controlled trial,28 tested the effects of five sessions of anodal tDCS over the left DLPFC in 30 GAD patients. This study showed no improvement after tDCS in anxiety, mood symptoms of stress, affectivity, or depression. However, the authors observed beneficial effects of tDCS in the physical symptoms of patients.
It is important to note, despite that the studies using a single session of tDCS showed benefits, as measured by the behavioral assessment of healthy individuals, there was no similarity in the outcomes analyzed. This was because those studies used different methodologies, including site of stimulation, anxiety measures, and behavioral outcomes. Chrysikou and colleagues27 showed that anodal tDCS over the right DLPFC in healthy individuals decreased approach behavior during conflict, taking into account the anxiety baseline level (subclinical levels of anxiety) of the individuals under study. The results of this study corroborate previous studies,13,42 which showed that the right DLPFC is involved in anxiety symptoms. This suggests that cathodal stimulation over the right DLPFC may be a treatment option to improve anxiety symptoms in humans. Otherwise, this study suggests that anodal tDCS stimulation over the right DLPFC may secondarily affect different central structures (cortical and subcortical), impacting the outcomes measured.
In addition, Ironside and colleagues30 assessed the effect of tDCS on a battery of emotional processing measures sensitive to antidepressant action. DLPFC stimulation was carried out using two common (bimodal or unimodal) electrode montages and compared to a sham control. The authors found that tDCS bimontage over the DLPFC reduced vigilance to threatening stimuli. This significant reduction in fear vigilance is similar to that seen with anxiolytic treatments of the same cognitive paradigm.30 Changes in the processing of threatening information promoted by tDCS suggests a potential cognitive mechanism that can be related to treatment effects in clinical settings. Furthermore, a single session of anodal tDCS over the right VLPFC reduced negative emotions, such as fear, anxiety, and sadness, in healthy individuals.34 However, the authors highlighted that the nonfocal action of tDCS might additionally activate nearby areas, such as the DLPFC, and could not rule out the connection between adjacent areas in regulating emotion. In this context, it is possible to conclude that tDCS exhibits therapeutic effects in healthy individuals, modulating diverse aspects of emotion, vigilance, and conflict.
In a controlled randomized clinical trial, Hosseini Amiri and colleagues29 evaluated the effects of tDCS on pain anxiety in 60 patients with severe burns, immediately after wound dressing. Cathodal stimulation (20 min, 1 mA) over the sensory cortex reduced the mean pain anxiety score of 17.2% in the experimental tDCS group, compared to the sham stimulated group. According to the authors, despite promising results, future studies might determine whether repeated sessions of stimulation have better results over single interventions, and how long these effects last.
Focusing on the immediate impact of tDCS and its association with pre-stimulus brain activity (measured using EEG), Nishida and colleagues32 applied anodal tDCS at 1 mA in a single session of 20 min to the left DLPFC or DMPFC in 14 patients with MDD and 19 healthy controls. Regarding the left DLPFC stimulation site in patients with MDD, these findings show that the anxiety reduction effect of tDCS was related to higher baseline theta-band activity in the rostral anterior cingulate cortex (rACC). In contrast, the anxiety reduction was associated with higher baseline alpha activity in the precuneus in the healthy control group. For DMPFC stimulation, the anxiety reduction effect was associated with lower baseline alpha-band activity in the left inferior parietal lobule. In contrast, the anxiety reduction effect was associated with higher baseline alpha activity in the precuneus during DMPFC stimulation in healthy controls. The results of this study suggest that the association between pre-tDCS brain activity and the anxiety reduction effect of tDCS depends on the psychopathology (depressed or non-depressed), as well as the site of stimulation (DMPFC or left DLPFC), and that the tDCS response might be associated with baseline resting state electrophysiological neural activity.
In a placebo-controlled, double-blind, crossover experiment in 45 individuals with no history of psychiatric or neurological disorders screened for low and high mathematics anxiety, Sarkar and colleagues,19 using the case of mathematics anxiety in a sample of healthy individuals, showed that identical tDCS patterns exert opposite behavioral and physiological effects depending on individual trait levels. Bilateral tDCS was applied to the DLPFC (anode left, cathode right), which improved reaction times on simple arithmetic decisions, and decreased salivary cortisol concentrations in high mathematics anxiety individuals. In contrast, in low mathematics anxiety individuals, tDCS impaired reaction times and prevented a decrease in cortisol concentration compared with sham stimulated individuals. Both groups of individuals showed tDCS-induced side effects, clearly demonstrating that brain stimulation does not produce uniform benefits, even when applied in the same configuration during the same tasks, but may interact with traits to produce markedly opposite outcomes.
It is interesting to note that there is a limited number of published articles with similar methodologies or diagnostic criteria, according to two recent previous reviews. Vicario and colleagues (2019) examined all available research using repetitive transcranial magnetic stimulation (rTMS) and tDCS for the treatment of specific phobias, social anxiety disorder, panic disorder, agoraphobia, and generalized anxiety disorders. The search highlighted 26 studies: 12 of these were sham-controlled 15 were not. With regard to the latter sub-group of studies, nine were case reports, and six were open label studies.43 Also, Hampstead and colleagues (2016) reviewed the literature concerning disrupted neural circuitry in post-traumatic stress disorder (PTSD) and discussed the rationale for the commonly targeted prefrontal cortex (PFC), as it relates to PTSD. They then reviewed the few prior (case) studies that evaluated tDCS in patients with PTSD (one study) and other anxiety disorders (GAD, panic disorder, OCD) (four studies).44
In summary, it is difficult to draw a conclusion about the effectiveness of tDCS in anxiety frames based on the results of the articles selected for inclusion in the current review. These studies used different methodologies, small sample sizes, a broad range of anxiety disorders, non-standard tDCS montages, and analyzed diverse behavioral outcomes. However, it is interesting to note a recent increase in the number of studies interested in the use of tDCS for treating anxiety disorders, and some of them clearly show the benefits of tDCS for treating anxiety symptoms. As many authors suggested, it is impossible to rule out the benefits of tDCS being caused by activation of secondary areas of the CNS beyond the target areas, the size of the electrodes, or the in influence of interconnected areas such as the PFC and limbic areas.
Conclusion and Perspectives
The development of further high quality studies are encouraged in this field, with better experimental designs, using double-blind randomized trials, and including assessment of repeated sessions of tDCS (at least 10), as suggested by Brunoni and colleagues,45 and the long-term benefits of tDCS in GAD patients.
In conclusion, using tDCS in clinical research for anxiety has a short history compared to other interventions such as pharmaco- and psycho-therapies. Despite the low number of studies carried out thus far in this field, some promising results may lead to interesting future interventions. In addition, it is important to investigate additional or complementary therapies for anxiety disorders, as these conditions have a high prevalence in today’s society.
Acknowledgments
The authors of this study are supported by the Brazilian funding agencies National Council for Scientific and Technological Development (CNPq) and Brazilian Federal Agency for Support and Evaluation of Graduate Education (CAPES). The authors would also like to thank Bettega Costa Lopes for his outstanding contribution in the drawing of the Figure for this paper.
Disclosure
All authors declare no conflicts of interest.
References
1. Kessler RC, McGonagle KA, Zhao S, et al. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States. Arch Gen Psychiatry. 1994;51(1):8–19. doi:10.1001/archpsyc.1994.03950010008002
2. Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):593–602. doi:10.1001/archpsyc.62.6.593
3. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders.
4. Baxter AJ, Vos T, Scott KM, Ferrari AJ, Whiteford HA. The global burden of anxiety disorders in 2010. Psychol Med. 2014;44(11):2363–2374. doi:10.1017/S0033291713003243
5. Stein DJ, Scott KM, de Jonge P, Kessler RC. Epidemiology of anxiety disorders: from surveys to nosology and back. Dialogues Clin Neurosci. 2017;19(2):127–136.
6. Craske MG, Stein MB, Eley TC, et al. Anxiety disorders. Nat Rev Dis Primers. 2017;3:17024. doi:10.1038/nrdp.2017.24
7. Grimm S, Beck J, Schuepbach D, et al. Imbalance between left and right dorsolateral prefrontal cortex in major depression is linked to negative emotional judgment: an fMRI study in severe major depressive disorder. Biol Psychiatry. 2008;63(4):369–376. doi:10.1016/j.biopsych.2007.05.033
8. Nitschke JB, Heller W. Distinguishing neural substrates of heterogeneity among anxiety disorders. Int Rev Neurobiol. 2005;67(05):1–42.
9. Liu W, Mao Y, Wei D, et al. Structural asymmetry of dorsolateral prefrontal cortex correlates with depressive symptoms: evidence from healthy individuals and patients with major depressive disorder. Neurosci Bull. 2016;32(3):217–226. doi:10.1007/s12264-016-0025-x
10. Bishop SJ, Jenkins R, Lawrence AD. Neural processing of fearful faces: effects of anxiety are gated by perceptual capacity limitations. Cereb Cortex. 2007;17(7):1595–1603. doi:10.1093/cercor/bhl070
11. Bandelow B, Michaelis S, Wedekind D. Treatment of anxiety disorders. Dialogues Clin Neurosci. 2017;19(2):93–106.
12. Davidson RJ, Irwin W. The functional neuroanatomy of emotion and affective style. Trends Cogn Sci. 1999;3:11–21. doi:10.1016/S1364-6613(98)01265-0
13. Sagliano L, D’Olimpio F, Panico F, Gagliardi S, Trojano L. The role of the dorsolateral prefrontal cortex in early threat processing: a TMS study. Soc Cogn Affect Neurosci. 2016;11(12):1992–1998. doi:10.1093/scan/nsw105
14. Lin Y, Zhang C, Wang Y. A randomized controlled study of transcranial direct current stimulation in treatment of generalized anxiety disorder. Brain Stimul. 2019;12(2):403.
15. Heeren A, Billieux J, Philippot P, et al. Impact of transcranial direct current stimulation on attentional bias for threat: a proof-of-concept study among individuals with social anxiety disorder. Soc Cogn Affect Neurosci. 2017;12(2):251–260. doi:10.1093/scan/nsw119
16. Costanzo F, Menghini D, Maritato A, et al. New treatment perspectives in adolescents with anorexia nervosa: the efficacy of non-invasive brain-directed treatment. Front Behav Neurosci. 2018;12:133. doi:10.3389/fnbeh.2018.00133
17. Nitsche MA, Müller-Dahlhaus F, Paulu W, Ziemann U. The pharmacology of neuroplasticity induced by non-invasive brain stimulation: building models for the clinical use of CNS active drugs. J Physiol. 2012;590(Pt 19):4641–4662. doi:10.1113/jphysiol.2012.232975
18. Pelletier SJ, Cicchetti F. Cellular and molecular mechanisms of action of transcranial direct current stimulation: evidence from in vitro and in vivo models. Int J Neuropsychoph. 2917;18(2):pyu047. doi:10.1093/ijnp/pyu047
19. Sarkar A, Dowker A, Cohen Kadosh R. Cognitive enhancement or cognitive cost: trait-specific outcomes of brain stimulation in the case of mathematics anxiety. J Neurosci. 2014;34(50):16605–16610. doi:10.1523/JNEUROSCI.3129-14.2014
20. Nitsche MA, Fricke K, Henschke U, et al. Pharmacological modulation of cortical excitability shifts induced by transcranial direct current stimulation in humans. J Physiol. 2003a;553(Pt 1):293–301. doi:10.1113/jphysiol.2003.049916
21. Turski CA, Kessler-Jones A, Chow C, et al. Extended multiple-field high-definition transcranial direct current stimulation (HD-tDCS) is well tolerated and safe in healthy adults. Restor Neurol Neurosci. 2017;35(6):631–642. doi:10.3233/RNN-170757
22. Allenby C, Falcone M, Bernardo L, et al. Transcranial direct current brain stimulation decreases impulsivity in ADHD. Brain Stimul. 2018;11(5):974–981. doi:10.1016/j.brs.2018.04.016
23. Mendonca ME, Simis M, Grecco LC, Battistella LR, Baptista AF, Fregni F. Transcranial direct current stimulation combined with aerobic exercise to optimize analgesic responses in fibromyalgia: a randomized placebo-controlled clinical trial. Front Hum Neurosci. 2016;10:68. doi:10.3389/fnhum.2016.00068
24. Cioato SG, Medeiros LF, Marques Filho PR, et al. Long-lasting effect of transcranial direct current stimulation in the reversal of hyperalgesia and cytokine alterations induced by the neuropathic pain model. Brain Stimul. 2015;9(2):209–217. doi:10.1016/j.brs.2017.03.002
25. Yoon KJ, Lee YT, Chae SW, Park CR, Km DY. Effects of anodal transcranial direct current stimulation (tDCS) on behavioral and spatial memory during the early stage of traumatic brain injury in the rats. J Neurol Sci. 2016;362:314–320. doi:10.1016/j.jns.2016.02.005
26. Bastani A, Jaberzadeh S. a-tDCS differential modulation of corticospinal excitability: the effects of electrode size. Brain Stimul. 2013;6(6):932–937. doi:10.1016/j.brs.2013.04.005
27. Chrysikou EG, Gorey C, Aupperle RL. Anodal transcranial direct current stimulation over right dorsolateral prefrontal cortex alters decision making during approach-avoidance conflict. Soc Cogn Affect Neurosci. 2017;12(3):468–475. doi:10.1093/scan/nsw140
28. de Lima AL, Braga FMA, da Costa RMM, Gomes EP, Brunoni AR, Pegado R. Transcranial direct current stimulation for the treatment of generalized anxiety disorder: a randomized clinical trial. J Affect Disord. 2019;259:31–37. doi:10.1016/j.jad.2019.08.020
29. Hosseini AM, Tavousi SH, Mazlom SR, Manzari ZS. Effect of transcranial direct current stimulation on pain and anxiety during burn wound care. Burns. 2016;2(4):872–876. doi:10.1016/j.burns.2016.01.006
30. Ironside M, O’Shea J, Cowen PJ, Harmer CJ. Frontal cortex stimulation reduces vigilance to threat: implications for the treatment of depression and anxiety. Biol Psychiatry. 2016;79(10):823–830. doi:10.1016/j.biopsych.2015.06.012
31. Movahed FS, Goradel JA, Pouresmali A, Mowlaie M. Effectiveness of transcranial direct current stimulation on worry, anxiety, and depression in generalized anxiety disorder: a randomized, single-blind pharmacotherapy and sham-controlled clinical trial. Iran J Psychiatry Behav Sci. 2018;12(2):e11071.
32. Nishida K, Koshikawa Y, Morishima Y, et al. Pre-stimulus brain activity is associated with state-anxiety changes during single-session transcranial direct current stimulation. Front Hum Neurosci. 2019;13:266. doi:10.3389/fnhum.2019.00266
33. Shiozawa P, Leiva AP, Castro CD, et al. Transcranial direct current stimulation for generalized anxiety disorder: a case study. Biol Psychiatry. 2014;75(11):17–18. doi:10.1016/j.biopsych.2013.07.014
34. Vergallito A, Riva P, Pisoni A, Romero Lauro LJ. Modulation of negative emotions through anodal tDCS over the right ventrolateral prefrontal cortex. Neuropsychologia. 2018;119:128–135. doi:10.1016/j.neuropsychologia.2018.07.037
35. Iyer MB, Mattu U, Grafman J, et al. Safety and cognitive effect of frontal DC brain polarization in healthy individuals. Neurology. 2005;64(5):872–875. doi:10.1212/01.WNL.0000152986.07469.E9
36. Nitsche MA, Liebetanz D, Lang N, Antal A, Tergau F, Paulus W. Safety criteria for transcranial direct current stimulation (tDCS) in humans. Clin Neurophysiol. 2003b;114(11):2220–2222. doi:10.1016/S1388-2457(03)00235-9
37. Nitsche MA, Bikson M. Extending the parameter range for tDCS: safety and tolerability of 4 mA stimulation. Brain Stimul. 2017;10(3):541–542. doi:10.1016/j.brs.2017.03.002
38. Carvalho F, Brietzke AP, Gasparin A, et al. Home-based transcranial direct current stimulation device development: an updated protocol used at home in healthy subjects and fibromyalgia patients. J Vis Exp. 2018;137:57614.
39. Roy AK, Fudge JL, Kelly C, et al. Intrinsic functional connectivity of amygdala-based networks in adolescent generalized anxiety disorder. J Am Acad Child Adolesc Psychiatry. 2013;52(3):290–299. doi:10.1016/j.jaac.2012.12.010
40. Bishop S, Duncan J, Brett M, Lawrence AD. Prefrontal cortical function and anxiety: controlling attention to threat-related stimuli. Nat Neurosci. 2004;7(2):184–188. doi:10.1038/nn1173
41. Bishop SJ. Trait anxiety and impoverished prefrontal control of attention. Nat Neurosci. 2009;12(1):92–98. doi:10.1038/nn.2242
42. Kawashima C, Tanaka Y, Inoue A, et al. Hyperfunction of left lateral prefrontal cortex and automatic thoughts in social anxiety disorder: a near-infrared spectroscopy study. J Affect Disord. 2016;206:256–260. doi:10.1016/j.jad.2016.07.028
43. Vicario CM, Salehinejad MA, Felmingham K, et al. A systematic review on the therapeutic effectiveness of non-invasive brain stimulation for the treatment of anxiety disorders. Neurosci Biobehav Rev. 2019;96:219–231. doi:10.1016/j.neubiorev.2018.12.012
44. Hampstead BM, Briceño EM, Mascaro N, et al. Current status of transcranial direct current stimulation in posttraumatic stress and other anxiety disorders. Curr Behav Neurosci Rep. 2016;3(2):95–101. doi:10.1007/s40473-016-0070-9
45. Brunoni AR, Valiengo L, Baccaro A, et al. The sertraline vs. electrical current therapy for treating depression clinical study: results from a factorial, randomized, controlled trial. JAMA Psychiatry. 2013;70(4):383–391. doi:10.1001/2013.jamapsychiatry.32
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.