Back to Journals » Hepatic Medicine: Evidence and Research » Volume 6
Transarterial chemoembolization using iodized oil for unresectable hepatocellular carcinoma: perspective from multistep hepatocarcinogenesis
Authors Yoshimitsu K
Received 27 February 2014
Accepted for publication 29 April 2014
Published 3 July 2014 Volume 2014:6 Pages 89—94
DOI https://doi.org/10.2147/HMER.S31440
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Kengo Yoshimitsu
Department of Radiology, Faculty of Medicine, Fukuoka University, Fukuoka, Japan
Abstract: Transarterial chemoembolization (TACE) using iodized oil (Lipiodol®) (Lp-TACE) as a carrier of chemotherapeutic agents has been routinely performed to control hepatocellular carcinomas (HCC) in Japan, and its use has yielded fairly beneficial therapeutic results. Lipiodol is thought to pass through the tumor sinusoids of HCC and reach the outflow drainage areas, namely, the portal venous side of the tumor. By doing this, Lipiodol blocks not only the tumor's arterial inflow but also its portal venous outflow, providing sufficient ischemic effects. It is known that the inflow blood system, tumor sinusoids, and outflow blood system change drastically during the process of multistep hepatocarcinogenesis; thus, it is reasonable to postulate that the distribution of Lipiodol and the subsequent therapeutic effect of Lp-TACE may also change during that process. Arterial inflow to HCC is highest for moderately differentiated HCC (mHCC) and is relatively low in well or poorly differentiated HCC (wHCC and pHCC, respectively). It has been suggested that the metabolic state of wHCC and mHCC is aerobic, while that of pHCC is anaerobic. The tumor sinusoids in wHCC and mHCC are small in size and large in number, while those in pHCC are large in size and small in number. This finding results in a greater chance of tumor cell exposure to chemotherapeutic agents in the former and a lesser chance in the latter. The outflow tract, namely, the drainage system via the residual portal venous branches within the pseudocapsule, is more complete in mHCC and pHCC and less so in wHCC. Considering all of these components of HCC of different histological grades, Lp-TACE should have the greatest effect on mHCC and a relatively low effect on wHCC and pHCC. To achieve consistently high therapeutic results, it is important to consider these components, which affect the sensitivity of HCC to Lp-TACE, to maximize both the chemotherapeutic and ischemic effects of this therapy.
Keywords: liver cancer, multistep carcinogenesis, embolotherapy Lipiodol, histological grade
Introduction
Hepatocellular carcinomas (HCC) are histopathologically characterized by their abundant vascularity and the presence of a tumor plate/sinusoid system, and these structures and related hemodynamics have been shown to change dramatically during the process of multistep carcinogenesis.1–10
Transarterial chemoembolization (TACE) is an accepted treatment method for HCC; however, it is a palliative, rather than curative, therapeutic option.11,12 TACE usually involves chemotherapeutic agent injection followed by embolic material administration into the feeding arteries of the HCC.11,12 In Japan, TACE has been performed using iodized oil (Lipiodol®; Guerbet, Villepinte, France) (Lp-TACE) as a carrier of antitumor agents, and its use has achieved fairly good therapeutic results.11–15 The differences in the effects of Lp-TACE and TACE without Lipiodol are not fully understood, but are thought to be related to the half-liquid property of Lipiodol.11,12 It is thought that Lipiodol can pass through the tumor sinusoids of HCC and reach the outflow drainage areas, namely the portal venous side of the tumor or the areas radiologically appreciated as “corona-like enhancements” on computed tomography (CT) during hepatic arteriography (CTHA).14,15 Thus, Lipiodol may block not only the tumor’s arterial inflow but also its portal venous outflow.14,15
It is known that the inflow blood system, tumor sinusoids, and outflow blood system change dramatically during the process of multistep hepatocarcinogenesis; thus, it is reasonable that the distribution of Lipiodol and the subsequent therapeutic effects of Lp-TACE may also change during that process. In this review article, the presumed mechanism of Lp-TACE is presented, particularly in the context of the structural and hemodynamic changes of HCC during multistep hepatocarcinogenesis.
Hemodynamic and structural changes of HCC during multistep hepatocarcinogenesis
Inflow system
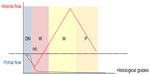
The hemodynamic changes in inflow for HCC during hepatocarcinogenesis have been well-documented1–7 and are briefly summarized in Figure 1. During the hepatocarcinogenic state of premalignant low-grade dysplastic nodules, the lesions are supplied by both hepatic arterial and portal venous blood flow in the same manner as normal liver parenchyma; however, when the lesions progress toward more malignant high-grade dysplastic nodules, their arterial supply diminishes, while their portal venous supply is unchanged. When the lesions become early HCC (eHCC), their portal venous flow diminishes in addition to their arterial flow. This causes the lesions to become hypoxic and leads to abundant angiogenesis or neovascularization. More strictly, it has been shown that neovascularization begins, to some extent, at the stage of dysplastic nodules,2 and becomes prominent at the eHCC stage, which corresponds histopathologically to well-differentiated HCC (wHCC). It has also been suggested that two different types of vessels are involved in this neovascularization process.16 As neovascularization increases, native arterial and portal venous supply further diminish, and eventually, disappear completely. At that time, the lesions are supplied exclusively by arterial flow via neovascularized vessels (tumor vessels), and the lesions correspond histopathologically to well to moderately differentiated HCC (mHCC). When HCC further dedifferentiates toward poorly differentiated HCC (pHCC), the arterial supply becomes diminished compared to mHCC.6,7 Thus, findings of HCC nodules on the arterial phase of dynamic CT or magnetic resonance imaging (MRI) may change in a triphasic fashion during the process of hepatocarcinogenesis as follows: no or little enhancement in dysplastic-to-early HCC nodules, subtle or weak enhancement in wHCC nodules, robust or prominent enhancement in mHCC nodules, and again subtle or weak enhancement in pHCC nodules (Figure 1). It has been reported that well, moderately, and poorly differentiated HCC comprise approximately 15%, 65%, and 20% of surgically resected lesions, respectively.7
Previous investigations have suggested that anaerobic metabolism (glycolysis) predominates in pHCC, while aerobic metabolism predominates in wHCC to mHCC,7 which may be the result of the decreased number of tumor vessels in pHCC. In other words, HCC address hypoxic stress by increasing the number of tumor vessels during the wHCC to mHCC transition and by shifting its metabolism from an aerobic to anaerobic state during the mHCC to pHCC transition.
Sinusoidal structure
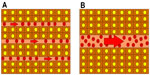
One of the histopathologic characteristics of HCC is the presence of tumor plates and tumor sinusoids, which imitate normal liver tissue. It has been well-documented that as the dedifferentiation of HCC progresses, the tumor plates tend to become thicker and more irregular; in other words, in wHCC, the tumor plates are thin and regular in shape, while in pHCC, they are thick and irregular (Figure 2).17,18 The tumor sinusoids are the vascular space between the tumor plates that convey the bloodstream and contrast medium, and their structure is affected in a sequential manner during multistep hepatocarcinogenesis.8 We have previously shown that the total area of the tumor sinusoids is constant irrespective of HCC histological grade; thus, the tumor sinusoids are smaller in size but larger in number during the wHCC state and relatively larger in size but smaller in number in the pHCC state. In other words, the individual tumor sinusoids become larger during the process of HCC dedifferentiation.8 This affects the appearance of HCC on the portal venous phase (PVP) of dynamic CT or MRI because the intratumoral flow may simply follow the Hagen–Poiseuille Law.8 Therefore, the resistance would be higher when there are a large number of small sinusoids and lower when there are a small number of large sinusoids, leading to slower flow within wHCC and faster flow within pHCC, respectively.8 Thus, wHCC tend to show persistent enhancement on PVP, whereas mHCC and pHCC tend to show so-called “washout” on PVP.8 A schematic presentation of this concept is shown in Figure 3.
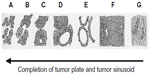  | Figure 2 Schematic presentation of architectural subtypes of hepatocellular carcinoma originally described by the Liver Cancer Study Group of Japan. |
Outflow system
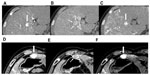
The HCC outflow system has also been shown to undergo significant sequential change during multistep hepatocarcinogenesis.3,9,10 In dysplastic or early HCC nodules, it is thought that hepatic vein-like vessels remain within the nodules, resembling normal liver tissue. Therefore, the intratumoral blood flow, which originates from the portal venous flow rather than the hepatic arterial flow as mentioned earlier, drains into the intratumoral venous system. In wHCC nodules, before formation of a pseudocapsule, the intratumoral venous structures diminish in number or are obliterated due to a gradually elevated intratumoral pressure secondary to increased cellular density. The intratumoral flow, which is mainly of hepatic arterial origin, outflows via the sinusoids into the surrounding nontumorous liver tissue, because in the absence of a fibrous capsule, the tumor sinusoids are in direct contact with the surrounding nontumorous sinusoids (trans-sinusoidal pathway or drainage). This transient blood influx into the nontumorous liver tissue surrounding the HCC nodules causes thin ring-like enhancement on single-level dynamic CTHA (SLCTHA) or the late phase of CTHA.3,9,10 In wHCC to mHCC nodules, which form pseudocapsules, the intratumoral venous structure completely disappears due to elevated intratumoral pressure.3,9,10 Because the pseudocapsule serves as a barrier to trans-sinusoidal drainage, the intratumoral blood flow, which is entirely of hepatic arterial origin, drains out of the tumors via small residual portal venous branches located within the pseudocapsule into the nontumoral liver tissue around the lesion, and subsequently, into the hepatic venous system.3,9,10 This transient blood influx into the nontumorous liver tissue surrounding the HCC nodules causes irregularly shaped, thick enhancement, known as “corona-like enhancement”, around the tumor on SLCTHA or the late phase of CTHA.3,9,10 In moderately to poorly differentiated HCC nodules, which have a disrupted or absent pseudocapsule, the drainage route of the intratumoral blood flow is a mixture of the trans-sinusoidal pathway and a partial “corona-like enhancement”. A schematic presentation of this concept is shown in Figure 4, and actual representative cases are shown in Figure 5.
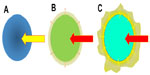  | Figure 4 Schematic presentation of the venous drainage of hepatocellular neoplasms during multistep hepatocarcinogenesis. |
Consideration of Lp-TACE based on structural and hemodynamic changes during multistep hepatocarcinogenesis
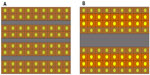
Considering the structural and hemodynamic changes of HCC during multistep hepatocarcinogenesis as mentioned above, it is reasonable that the therapeutic effects of Lp-TACE may be affected during this process. First, HCC nodules that are purely and abundantly supplied by arterial blood flow may be more susceptible to intra-arterially administered chemotherapeutic agents or embolic materials. In addition, lesions with anaerobic metabolism, which is characteristic of pHCC, should be resistant to ischemia caused by embolic therapy. mHCC, therefore, would be the most sensitive to TACE or Lp-TACE in this regard. Second, the size and number of HCC tumor sinusoids may influence the effects of chemotherapeutic agents. According to previous observations,14,15 a Lipiodol emulsion/suspension can pass through the tumor sinusoids and reach the portal aspect of the HCC. Assuming that Lipiodol emulsions/suspensions fill the sinusoidal space completely, the total area of HCC cells that are in direct contact with a Lipiodol emulsion/suspension is larger in HCC consisting of small tumor sinusoids than in those with larger tumor sinusoids, assuming the ratios of sinusoidal areas are the same (Figure 6). Therefore, tumor cells in wHCC or mHCC may have a greater chance of being exposed to chemotherapeutic agents than those in pHCC. Third, it is thought that the more established the outflow system is, the greater the embolic effect of TACE or Lp-TACE will be in terms of both arterial and portal venous side blockage. Thus, mHCC or pHCC may be more effectively treated by TACE or Lp-TACE than wHCC in this regard.
Considering all the above-mentioned factors, mHCC, which is the most commonly encountered histological grade of HCC in clinical practice, is the grade that is most effectively treated by TACE or Lp-TACE (Figure 7). Furthermore, some mHCC may be able to be controlled by simple ischemia caused by bland embolization without chemotherapeutic agents or simple chemo-lipiodol emulsion/suspension injection without embolic material administration. In contrast, for tumors that are more highly or poorly differentiated than typical mHCC, a well-balanced therapeutic approach may be required, particularly for pHCC; this approach provides sufficient chemotherapeutic and ischemic effects. In most cases of HCC nodules, some intralesional heterogeneity in terms of histological grade exists; thus, this balanced approach is necessary to achieve consistently good control of HCC by means of Lp-TACE.
In Japan, epirubicin is the most frequently used chemotherapeutic agent for Lp-TACE, but cisplatin19 and miriplatin20 are also used as second-line treatment or as alternative agents. Although there is no evidence to suggest which agent is the best suited for TACE at present, considering the repetitive nature of TACE procedures, it is important to use the agent that will not only provide the greatest antitumor effect but also minimize damage to nontumorous liver tissue to preserve liver function after TACE for any future procedures.21
Conclusion
From the perspective of the sequential changes to inflow hemodynamics, sinusoidal structures, and outflow hemodynamics (venous drainage) during multistep hepatocarcinogenesis, the therapeutic effect of Lp-TACE should be greatest for mHCC, which is the most common histological type encountered in clinical practice. To control pHCC, both robust chemotherapeutic and sufficient ischemic effects are necessary. Considering the histological heterogeneity within HCC nodules, the ideal Lp-TACE treatment would strike a good balance between chemotherapeutic and ischemic effects, while causing the least possible damage to nontumorous liver tissue.
Disclosure
KY has supervised the development of educational material for Dainippon Sumitomo Pharma Co, Ltd. The author reports no other conflicts of interest.
References
Matsui O, Kadoya M, Kameyama T, et al. Benign and malignant nodules in cirrhotic livers: distinction based on blood supply. Radiology. 1991;178(2):493–497. | |
Ueda K, Terada T, Nakanuma Y, Matsui O. Vascular supply in adenomatous hyperplasia of the liver and hepatocellular carcinoma: a morphometric study. Hum Pathol. 1992;23(6):619–626. | |
Matsui O, Kobayashi S, Sanada J, et al. Hepatocelluar nodules in liver cirrhosis: hemodynamic evaluation (angiography-assisted CT) with special reference to multi-step hepatocarcinogenesis. Abdom Imaging. 2011;36(3):264–272. | |
Hayashi M, Matsui O, Ueda K, et al. Correlation between the blood supply and grade of malignancy of hepatocellular nodules associated with liver cirrhosis: evaluation by CT during intraarterial injection of contrast medium. AJR Am J Roentgenol. 1999;172(4):969–976. | |
Tajima T, Honda H, Taguchi K, et al. Sequential hemodynamic change in hepatocellular carcinoma and dysplastic nodules: CT angiography and pathologic correlation. AJR Am J Roentgenol. 2002;178(4):885–897. | |
Asayama Y, Yoshimitsu K, Irie H, et al. Poorly versus moderately differentiated hepatocellular carcinoma: vascularity assessment by computed tomographic hepatic angiography in correlation with histologically counted number of unpaired arteries. J Comput Assist Tomogr. 2007;31(2):188–192. | |
Asayama Y, Yoshimitsu K, Nishihara Y, et al. Arterial blood supply of hepatocellular carcinoma and histologic grading: radiologic-pathologic correlation. AJR Am J Roentgenol. 2008;190(1):W28–W34. | |
Okamoto D, Yoshimitsu K, Nishie A, et al. Enhancement pattern analysis of hypervascular hepatocellular carcinoma on dynamic MR imaging with histopathological correlation: validity of portal phase imaging for predicting tumor grade. Eur J Radiol. 2012;81(6):1116–1121. | |
Ueda K, Matsui O, Kawamori Y, et al. Hypervascular hepatocellular carcinoma: evaluation of hemodynamics with dynamic CT during hepatic arteriography. Radiology. 1998;206(1):161–166. | |
Kitao A, Zen Y, Matsui O, Gabata T, Nakanuma Y. Hepatocarcinogenesis: multistep changes of drainage vessels at CT during arterial portography and hepatic arteriography–radiologic-pathologic correlation. Radiology. 2009;252(2):605–614. | |
Higashihara H, Okazaki M. Transcatheter arterial embolization of hepatocellular carcinoma: a Japanese experience. Hepatogastroenterology. 2002;49(43):72–78. | |
Matsui O, Miyayama S, Sanada J, et al. Interventional oncology: new options for interstitial treatments and intravascular approaches: superselective TACE using iodized oil for HCC: rationale, technique and outcome. J Hepatobiliary Pancreat Sci. 2010;17(4):407–409. | |
Ikeda M, Arai Y, Park SJ, et al; Japan Interventional Radiology in Oncology Study Group (JIVROSG); Korea Interventional Radiology in Oncology Study Group (KIVROSG). Prospective study of transcatheter arterial chemoembolization for unresectable hepatocellular carcinoma: an Asian cooperative study between Japan and Korea. J Vasc Intervent Radiol. 2013;24(4):490–500. | |
Terayama N, Matsui O, Gabata T, et al. Accumulation of iodized oil within the nonneoplastic liver adjacent to hepatocellular carcinoma via the drainage routes of the tumor after transcatheter arterial embolization. Cardiovasc Intervent Radiol. 2001;24(6):383–387. | |
Miyayama S, Matsui O, Yamashiro M, et al. Ultraselective transcatheter arterial chemoembolization with a 2-f tip microcatheter for small hepatocellular carcinomas: relationship between local tumor recurrence and visualization of the portal veins with iodized oil. J Vasc Intervent Radiol. 2007;18(3):365–376. | |
Ikeda K, Kobayashi M, Saitoh S, et al. Origin of neovascular structure in an early stage of hepatocellular carcinoma: study of alpha-smooth muscle actin immunohistochemistry in serial thin sections of surgically resected cancer. J Gastroenterol Hepatol. 2006; 21(1 Pt 1):183–190. | |
Okudaira M. II kansaibougan 2. soshiki bunrui [Hepatocellular carcinoma 2. Histological classification]. In: Okudaira M, Mizumoto R, Tanigawa H, editors. Toriatsukai kiyaku ni sotta shuyou kanbetsu shindan atlas kanzou. Tokyo: Bunkodo; 1991:22–27. Japanese. | |
The Liver Cancer Study Group of Japan. The General Rules for the Clinical and Pathological Study of Primary Liver Cancer. 5th ed. Tokyo: Kanahara; 2008:38–45. | |
Maeda N, Osuga K, Higashihara H, et al. Transarterial chemoembolization with cisplatin as second-line treatment for hepatocellular carcinoma unresponsive to chemoembolization with epirubicin-Lipiodol emulsion. Cardiovasc Intervent Radiol. 2012;35(1):82–89. | |
Kora S, Urakawa H, Mitsufuji T, Osame A, Higashihara H, Yoshimitsu K. Warming effect on miriplatin–lipiodol suspension as a chemotherapeutic agent for transarterial chemoembolization for hepatocellular carcinoma: preliminary clinical experience. Cardiovasc Intervent Radiol. 2013;36(4):1023–1029. | |
Iwazawa J, Hashimoto N, Ohue S, Muramoto O, Mitani T. Chemoembolization-induced arterial damage: evaluation of three different chemotherapeutic protocols using epirubicin and miriplatin. Hepatol Res. 2014;44(2):201–208. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.