Back to Journals » Cancer Management and Research » Volume 11
Trametes robiniophila Murr: a traditional Chinese medicine with potent anti-tumor effects
Authors Pan J, Yang C, Jiang Z, Huang J
Received 2 November 2018
Accepted for publication 14 January 2019
Published 14 February 2019 Volume 2019:11 Pages 1541—1549
DOI https://doi.org/10.2147/CMAR.S193174
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Ahmet Emre Eşkazan
Jun Pan,1,2 Chenghui Yang,1,2 Zhou Jiang,1,2 Jian Huang1,2
1Cancer Institute (Key Laboratory of Cancer Prevention & Intervention, National Ministry of Education; Key Laboratory of Tumor Microenvironment and Immune Therapy of Zhejiang Province), Second Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou 310009, P.R. China; 2Department of Surgical Oncology, Second Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou 310009, P.R. China
Abstract: Trametes robiniophila Murr also known as Huaier, one of the traditional Chinese medicines, has been shown an effective adjuvant of cancer therapy. Accumulating evidence suggests that the anti-cancer effects of Huaier can be briefly divided into two aspects: the direct effects on tumor cells and the indirect effects on immune cells. In vitro and in vivo experiment showed Huaier directly inhibited tumor cell proliferation, induced tumor cell death, prevented metastasis and interfered with angiogenesis via various signaling pathways. The immunomodulatory effect of Huaier is associated with enhancement of the number and function of CD4+ T cells and NK cells, regulation of the polarization and function of macrophages, and elevated secretion of immune stimulatory cytokines. In this review, the anti-cancer effects and combined treatments of Huaier with other anti-cancer therapies, and the underlying mechanisms are summarized and discussed.
Keywords: Huaier, cancer, anti-tumor therapy, traditional Chinese medicine, adjuvant
Introduction
Trametes robiniophila Murr, also called Huaier, a sandy beige mushroom, has been used as a traditional Chinese medicine (TCM) for over 1,600 years. The most common pharmaceutical preparations of Huaier include aqueous extracts and granules. The mainly active ingredient in Huaier is proteoglycan, which consists of polysaccharides, amino acids and water.1 Increasing evidence highlights that Huaier has a satisfactory clinical effect on nephrosis,2 colitis,3 tuberous sclerosis4 and cancers.5 In mesangial proliferative glomerulonephritis, Huaier reduces urinary protein excretion and relieves hyperplasia in mesangial cells as well as inhibits platelet-derived growth factor-BB-stimulated proliferation and DNA synthesis of mesangial cells in vitro.2 In ulcerative colitis, Huaier not only inhibited NLRP3 inflammasome activation-induced IL-1β secretion and caspase-1 cleavage but also promoted NLRP3 degradation through the autophagy lysosome pathway.3 Moreover, Huaier attenuated JAK2/STAT3 and MAPK signaling to inhibit the proliferation and metastasis in tuberous sclerosis complex.4
Previous experimental studies showed that Huaier could exert a potent anti-cancer effect on hepatocellular cancer (HCC),6,7 breast cancer,8,9 ovarian cancer,10 and so on. This was also verified in a clinical research of 53 HCC patients11 and a meta-analysis in gastrointestinal cancer.12 Several studies demonstrated that Huaier prolonged survival time of cancer patients and reduced the recurrence rate of HCC.11,13–15 Moreover, evaluations of serum hepatic and renal function parameters showed that Huaier almost had no cytotoxicity to normal liver and kidney.13,16 All these results show that Huaier is an effective adjuvant in therapy for cancers. In this review, the anti-tumor effects of Huaier and the underlying mechanisms are discussed.
The direct anti-tumor effects of Huaier and underlying mechanisms
Sustaining proliferative signaling, resisting cell death, inducing angiogenesis and activating invasion and metastasis are four of the hallmarks of cancer.17 In breast cancer cell line MDA-MB-231, Huaier regulates 387 genes including the genes that control cell proliferation, apoptosis, tumor metastasis and angiogenesis.18 Among the 387 genes, the top 5 of 226 up-regulated genes were MMP3, TCP11L2, IL24, CLEC2B and LIPH; while the top 5 of 161 down-regulated genes were MPP4, HIST1H2BM, ID1, F3 and SNORD14D. The direct anti-tumor effects of Huaier work through different mechanisms, including inhibition of cancer cell proliferation, induction of cancer death, inhibition of tumor-induced angiogenesis and suppression of cancer metastasis.
Inhibition of cancer cell proliferation
The cell cycle arrest
Uncontrolled cell proliferation results in tumorigenesis and tumor progression, thus cell cycle regulators are growing potent targets in cancer therapy. Among these regulators, cyclin-dependent kinases (CDKs) play a major role. INK4 and D-type cyclins regulate CDK4 and CDK6 to trigger cells in G0/G1 phase to re-enter and progress into S phase.19 Cyclin D1 is the dominant cyclin in G1 phase20 and can be regulated by Huaier.9 Huaier augmented the tamoxifen-induced inhibition of Akt/mTOR signaling, decreased the downstream element glycogen synthase kinase-3 and cyclin D1 expressions to induce the G0/G1 cell cycle arrest in breast cancer. In another study, Gao et al demonstrated that Huaier could induce G0/G1 arrest in both M7-TR (tamoxifen-resistant cells) and M7-FR (fulvestrant-resistant cells).21 The results showed that after treatment of Huaier, cyclin D1 and CDK4 were decreased significantly, but ataxia-telangiectasia mutation (ATM) and its downstream target replication protein A phosphorylation were increased. ATM was negatively correlated with tumorigenesis and progression, regulated directly by miR-203.22 Huaier significantly suppressed miR-203 and up-regulated ATM to inhibit the expression of cyclinD1 and CDK4, resulting in G0/G1 arrest.21 This was verified in other breast cancer cell lines MCF-7, MDA-MB-468 and MDA-MB-231.9,23–25 In addition to G0/G1 arrest in breast cancer, Huaier also induced cell cycle arrest with different mechanisms in other kinds of cancers. In HCC26 and lung cancer,27 Huaier repressed the expression of β-catenin and cyclin D1, resulting in remarkable increase of cells arrested in the S phase.26
In cervical cancer, Huaier aqueous extract treatment lead to, through activation of JNK/p38 signaling pathway, G2/M phase arrest.28 This effect of Huaier to induce a G2/M cell cycle arrest has also been proved in studies on gastric cancer cells,29 brosarcoma cells30 and melanoma cells.31 The mechanism may referred to the regulation of the expression of cyclin B1, which binds to Cdc2 to form the complex vital for G2 checkpoint.29
Therefore, Huaier can inhibit cancer cell proliferation by cell cycle arrest in G0/G1, S and G2/M phases in different types of cancers.
Enhancement of IκBα expression to inhibit NF-κB-mediated signaling pathway
NF-κB signaling pathway plays an essential role in the cell proliferation.32 Normally, NF-κB is kept as an inactive state, which is maintained by inhibitor of NF-κB (IκB).33 However in tumor microenvironment (TME), NF-κB can be aberrantly activated. After treated with Huaier, proliferation of breast cancer cells was significantly inhibited and mechanism analyses showed that the expression of IκBα was increased, while the expression of p65 and the up-stream c-Met decreased.23 These data demonstrated that Huaier could down-regulate the expression of c-Met, increase the expression of IκBα, inhibit the activation of NF-κB and finally control the abnormal proliferation of cancer cells.
Inhibition of NF-κB–estrogen receptor (ER) pathway
Steroid hormones regulate cell growth and differentiation by binding to steroid hormone receptors.34 ERs are essential members of these steroid hormone receptors. The mechanism of ER action involves forming homodimer and binding to estrogen response elements and other transcription factors, such as NF-κB.35 There are two subtypes of ERs (ERα and ERβ) exerting opposite effects. ERα mediates aberrant proliferation resulting in tumorigenesis and perturbation of ERα/ERβ ratio has been detected in several types of cancers, especially breast cancer.36 It was found that Huaier efficiently inhibited the expression of ERα and its downstream genes, abolished the estrogen-induced activation of NF-κB, thus inhibited the estrogen-stimulated proliferation.37
Inhibition of PI3K–Akt pathway
The PI3K–Akt signaling network is closely related to cancer cell proliferation, apoptosis, metastasis and the immune response and angiogenesis in TME.38 It was demonstrated that Huaier could act as a potent inhibitor of the PI3K–Akt signaling pathway.29,39 The proteins associated with this signaling pathway include PI3K, AKT1 and PTEN. The phosphorylation of these proteins was significantly down-regulated by Huaier in a dose-dependent manner,29 but the total Akt expression was not affected.39
Inhibition of Yes-associated protein 1 (YAP1) expression
YAP1 is the first to be activated and drives quiescent epithelial cells re-entry to the cell cycle.40 Shan et al found that Huaier had an inhibitory effect on YAP1 in HCC cells.16 The underlying mechanism may due to the Huaier-induced translocation of YAP1. After treated with Huaier, YAP1 locomoted from nucleus to the cytoplasm, phosphorylated, ubiquitinated and finally degraded, losing the ability to promote the proliferation of cancer cells.16
Suppression of cancer stem cells (CSCs)
CSCs play important roles in metastasis, inevitable recurrence and therapy resistance of cancer.41 It was found that Huaier had a great efficacy to inhibit spheroid formation potential of CSCs42,43 and could inhibit the viabilities, numbers and sizes of mammospheres in breast cancer.43 Besides the colony formation, Huaier can decrease the population of aldehyde dehydrogenase-positive cells42 and CD44+/CD24− cells, suppress the expression of stem cell marker OCT-4, NESTIN and NANOG.43
The Wnt signaling pathway and hedgehog pathway have been proved to regulate CSCs stemness and self-renewal.44 In a study on colorectal CSCs, Zhang et al found that the expression of total β-catenin was down-regulated dose-dependently and the expression of cyclin D1, one of the Wnt/β-catenin target genes, was also decreased after treated with Huaier. The down-regulation of Wnt/β-catenin signaling pathway resulted in the eradication of colorectal CSCs and ovarian cancer cells.10,42 However in breast cancer, Huaier exerted the inhibitory effect via inactivation of the hedgehog pathway. Among the five proteins (Ptch-1, Smo, Gil1, Gil2 and Gil3) vital in this pathway, Gil1 decreased significantly after Huaier treatment while others remained unchanged.43 Therefore, Huaier could inhibit the quantity and functions of CSCs via Wnt signaling pathway and hedgehog pathway.
Induction of cancer cell death
Stimulation of autophagy
Autophagy is a highly regulated multistep process to degrade long-lived proteins and damaged organelles in disease, especially cancer.45 The inhibition of mTOR46 and mTOR/S6K pathway47 activated cancer cell autophagy. It was found that Huaier-induced cytotoxicity is partially reacted on cell autophagy.9,48,49 After Huaier treatment, the expressions of phosphorylated mTOR and its downstream targets p70 ribosomal protein S6 kinase (p70S6K), S6 ribosomal protein and 4E-BP1 were decreased remarkably but the total level of mTOR was unaffected.48 In a study on hepatoma SK-HEP-1 cells, Yang et al indicated that Huaier could increase the expression of LC3, an autophagy marker, and induce the formation of autolysosome.49 The activation of autophagy induced by Huaier significantly inhibited the proliferation of cancer cells.
Induction of apoptosis
lncRNAs are RNAs that do not encode protein but have a length exceeding 200 nucleotides and play important roles in cancer.50 Wang et al found that Huaier could induce apoptosis via lncRNA-H19/miR-675-5p pathway.8 The H19 gene is one of the lncRNA dysregulated in many cancers. There are two conserved microRNAs (miR-675-3p and miR-675-5p) encoded by H19 exon1.51 Wang et al found that miR-675-5p was up-regulated in breast cancer. After treatment of Huaier, the expressions of H19 and miR-675-5p were significantly reduced. Furthermore, Huaier-induced apoptosis of breast cancer cells could be reversed by up-regulating miR-675-5p or over-expression of H19.8 miR-26b-5p was also found to function in Huaier-induced apoptosis.52
The mitochondrial pathway, which is regulated by Bcl-2 family, participates in regulating tumor cell apoptosis. Bax is a pro-apoptosis gene of the Bcl-2 family. Once activated, Bax will bind to Bcl-2 to inactivate the later to promote apoptosis.53 Treatment with Huaier activated the three MAPK pathways (ERK, JNK but mostly p38), enhanced the expression of Bax, but decreased the expressions of Bcl-2, resulting in the acceleration of apoptosis in cancer cells.7,16,25,27,54–56 Among the down-stream effectors, caspase-3 acts as the key apoptosis executors to kill cells, recruit macrophages and present an “eat me” signal.57 Researchers found that the apoptosis of breast cancer cells,25 melanoma cells31 and lung cancer cells27 increased significantly after Huaier treatment. Molecular mechanism analyses showed that increased cleavage caspase-9 and -3 expression but decreased pro-caspase-3 expression were found, indicating that Huaier-induced apoptosis was mainly mediated by caspase-3.25,31 A study on HCC showed that caspase-7 and its substrate PARP were also involved in the Huaier-induced cleavage.6
Inhibition of tumor-induced angiogenesis
Angiogenesis is a typical characteristic of tumor. Triggering the angiogenic switch is associated with the malignant progression of benign tumors.58 It had been observed in vivo that Huaier efficiently reduced the microvessel density in tumor.54,59,60 In vitro experiment demonstrated that Huaier could cause cell skeleton rearrangement in human umbilical vein endothelial cells (HUVECs), resulting in the distortion of vasculature architecture.59 The progression of angiogenesis can be regulated by numerous pro-angiogenic factors, including VEGF, TNF, matrix metalloproteinases (MMPs) and so on.58 Among these factors, VEGF, which can be induced via hypoxia inducible factor (HIF), acts as the master regulator.61 Several studies demonstrated that the expression of VEGF was significantly decreased after Huaier treatment.54,59,60,62 Wang et al determined the level of HIF in Huaier-treated HUVECs and found that Huaier failed to suppress the level of HIF.59 However, Li et al62 and Zou et al60 used the same detection method but found a totally different consequence in HCC cell SMMC-7721 that Huaier polysaccharide significantly inhibited the expression of HIF-1α. The possible causes that resulted in this discrepancy may involve the difference in cell type, the purification of Huaier and the different expression level between total HIF-1 and HIF-1α.
MMPs are also involved in the progression of tumor angiogenesis.63 It was found that Huaier-treated HCC patients had decreased concentration of serum MMP2, suggesting that the anti-angiogenic effect of Huaier is mediated, at least in part, by inhibition of MMP2 production.60
In the progression of angiogenesis, compositions of TME also play vital parts. Huaier also exhibited a inhibitory function on the angiogenesis function of tumor-associated macrophages (TAM).64 The mechanisms of this regulatory function of Huaier will be discussed in the following section of immunological regulation of Huaier.
Suppression of cancer metastasis
Degradation of the extracellular matrix (ECM) is an essential step for tumor cells to enter the circulation and invade into the peripheral tissues. MMPs play a crucial role in the degradation and remodeling of ECM.65 Therefore, MMPs serve as a “helper” to tumor metastasis. It was reported that administration of Huaier decreased the number of tumor colonies metastasized to the lung.13,60 Mechanism analyses showed the anti-metastasis effect of Huaier was performed in two ways. One is to directly react on tumor cells and the other is to affect TAM. Generally, Huaier decreased the expressions of MMP-2, MMP-9 and VEGF, helped to antagonize the degradation and remodeling of ECM.60,64,66
Another pivotal procedure related to cancer metastasis is the epithelial–mesenchymal transition (EMT). In maintaining the adherence between epithelial cells, E-cadherin plays the major effector and is considered a tumor-metastasis suppressor.67 However, N-cadherin, AEG-1 and TCF8/ZEB1 are the mesenchymal markers that promote the EMT process. Several studies demonstrated that Huaier could increase the expression of E-cadherin but decrease the N-cadherin, AEG-1 and TCF8/ZEB1,6,16,60,68–70 suggesting that the anti-metastasis effect of Huaier is mediated, at least in part, by suppression of EMT. Among the transcription factors involved in EMT, Huaier specifically down-regulated the expression of Twist1, while other factors remained unaffected.68 This suggests that the reverse of EMT induced by Huaier had to be carried out in a Twist-dependent manner.
Regulation of the immune function to support the anti-tumor effect
The TME consists of not only cancer cells but also immune cells, fibroblasts, blood vessels and other ECM.71 However, these immune cells penetrating in TME somehow have impaired and compromised functions. Therefore, the major goal of immunotherapy is to alleviate the tumor-induced immunological suppression. Huaier extract (mainly polysaccharide) has also been identified as a valid immunopotentiator, hereby exerting indirect anti-tumor effects.
Promotion on T cell proliferation and enhancement of secretion of immune-stimulating cytokines
After Huaier treatment, the number and functions of CD4+ T cells and the immune-stimulating cytokines IL-2 and IFN-γ in peripheral blood are enhanced. However, the immune-suppressive serum cytokine IL-10 is decreased.13 In TME, CD4+ T cells participate in the regulation by secreting different cytokines, whereas CD8+ T cells exist as a direct “killer” to cancer cells. Both in vitro and in vivo experiments showed Huaier treatment resulted in a promotion on T and B cell proliferation.13,14 Treatment of mice with Huaier induced an increase in CD4+ T cells in HCC microenvironment.13 Because CD4+ Th cells exist in various subtypes that may exhibit stimulatory or inhibitory effects on other immune cells and cancer cells within the TME,72 further studies on how does Huaier affect CD4+ T cells in TME need to be carried out.
Induction of “M2” to “M1” polarization of macrophages and enhancement of their anti-tumor functions
Macrophage is one of the indispensable components of innate immunity, and they play an essential part in TME. Generally, macrophages have been classified into classically activated macrophages (M1) or alternatively activated macrophages (M2), depending on several cell surface markers and cytokines produced, such as CD16, CD32, TNF, IL-6, Arg1 and CD206.73 M1 plays a vital role in promoting Th1 responses and anti-tumor functions. However, M2 exerts the anti-inflammatory and pro-tumor effects by expressing IL-10, Arg-1 and other immunosuppressive cytokines. M1/M2 polarization is mainly regulated by cytokines and TAM often exhibits a pro-tumor M2 phenotype.74 Polarization from “M1” to “M2” can be inhibited and reversed both quantitatively and functionally by Huaier. Li et al found that Huaier suppressed the motility and M2 markers, including CD206, Mrc-2, Arg-1 and IL-10, of TAM.64 Immunohistochemistry assay showed that the number of M2 in tumor was declined after Huaier treatment. However, the NO production and the M1 marker iNOS were increased.14,64 Huaier treatment rescued the repression of phagocytosis of macrophages derived from TME.14,64 Taken together, Huaier could regulate the polarization and functions of macrophages to antagonize tumor progression.
Besides the immune functions, macrophages also participate in tissue repair and angiogenesis. Huaier could exert an inhibitory effect on macrophage-induced formation of vascular structure through suppression of MMP2, MMP9 and VEGF expression.64
Enhancement of NK cell activity
NK cells are important effector cells of the innate immune system and play a role in immunological surveillance. Low NK cell activity in peripheral blood is associated with the increased risk of cancer.75 However, both the number and activity of NK cells were enhanced after Huaier treatment,13,39 emphasizing again the immune-modulating effect of Huaier in cancer therapy.
Application of Huaier in combined cancer therapy
Huaier combined with surgical therapy
Transcatheter arterial chemoembolization (TACE) combined with Huaier
TACE has become one of the most efficient treatments for unresectable HCC. Experiment on rabbit demonstrated that oral administration of Huaier after TACE treatment remarkably suppressed the proliferation and induced the apoptosis of HCC cells.54 Clinical trials on 62 patients with primary HCC indicated that combined treatment improved clinical efficacy. The 12-month overall survival in experimental group (treated with TACE, lobaplatin and Huaier) and control group (received TACE plus lobaplatin chemotherapy) was 93.5% and 80.6%, respectively. Moreover, the tumor objective response rates were higher in experimental group than control group (72.4% vs 64.3%).15
Liver transplantation combined with Huaier in anti-HCC therapy
A comparison between 53 HCC patients underwent liver transplantation with or without Huaier showed that Huaier treatment led to a higher predictive tumor-free survival rate (P=0.02 when compared with the control group). The 10-month predictive tumor recurrence rate was 17.9% in Huaier group but 60% in control group (P<0.05). Although the 30-month predictive tumor recurrence rate accelerated to 35.7% in Huaier group, it was still almost half of that in control group (64%, P<0.05).11
Huaier combined with chemotherapy
Rapamycin combined with Huaier
Hu et al found that the aberrant activation of mTOR signaling is necessary for Huaier to exert its anti-proliferative effect of tumor cells.76 Since deficiency in TSC1 or TSC2 caused activation of mTOR, TSC1- or TSC2-null mouse embryonic fibroblasts and ELT3 cells with TSC2 deficiency were used to determine the combined effects of Huaier (2, 4, 6, 8 mg/mL) and rapamycin (10 nM) on cell viability. They found that cell viability in the combined group was significantly lower than that in the rapamycin group (P<0.01). Mechanism analyses demonstrated that protein level of p-S6 (a marker of mTOR activity) was increased after Huaier treatment.76 These data suggest that Huaier could be used as a chemotherapy adjuvant to enhance the sensitivity of cancer cells with activated mTOR signaling pathway to rapamycin.
Tamoxifen combined with Huaier
Tamoxifen is widely used in treating ER+ breast cancer. The combination of Tamoxifen and Huaier significantly enhanced the autophagy, apoptosis, proliferation and metastasis of ER+ breast cancer cells. In the 40-day experiment on mice, the tumor volume in combined treatment group was 17.41±8.99 mm3, remarkably smaller than that in Huaier group (733.45±55.93 mm3), tamoxifen group (503.844±61.12 mm3) and control group (1127.40±100.07 mm3), suggesting the addition of Huaier did improve the treatment efficacy of Tamoxifen.9
Paclitaxel (PTX) combined with Huaier
Yang et al found Huaier and PTX combined treatments had a greater effect, in a dose-dependent manner, on the inhibition rate of breast cancer cell viability, compared to mono-therapy. In the xenograft model, the tumor volume was 158.1±25.2 mm3 in combined treatment group, while 367.3±22.1 mm3 in Huaier-treated group and 223.9±28.3 mm3 in PTX-treated group, respectively.23 The enhanced effects of combined treatment of Huaier and PTX were also proved by Chen et al.77
Huaier combined with radiotherapy
Huaier has also been found to alter the radiosensitization on breast cancer cells. The survival fraction at 2 Gy (SF2) was used to verify the regulatory effects of Huaier on the radiosensitivity on breast cancer. In MCF-7 cells, the SF2 was 28.55% ± 0.03% for cells pretreated Huaier (4 mg/mL), a significant reduction of ~47.65% compared to control group (P<0.01). The underlying mechanisms were due to the alteration on cell cycle-regulating proteins and the progression on homologous recombination.24
Huaier combined with dendritic cells and cytokine-induced killer cells (DC-CIK) immunotherapy
Compared with the mono-therapy, combined treatment of Huaier and DC-CIK exerted a restrained tumor growth effect in nude mice transplanted with HT-29 colon carcinoma cells.78 After 3-week treatment, tumor weight and size were significantly suppressed in combined treatment group when compared to two mono-therapy-treated groups and control group.
Taken together, Huaier can be used as an adjuvant to improve the therapeutic efficacy of surgical therapy, chemotherapy, radiotherapy and immunotherapy.
Conclusion
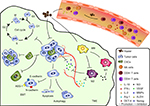
TCM has been widely used in clinical practice due to its cure without significant side effects. Huaier is one of the TCM and has been shown an effective adjuvant of cancer therapy. The anti-cancer effects of Huaier can be briefly divided into two aspects: the direct effects and the indirect effects (Figure 1). Huaier directly regulates the cell cycle, apoptosis of tumor cells, resulting in the repressed proliferation. Besides, it also directly inhibits tumor cell motility and secretion, suppresses tumor metastasis and angiogenesis. The indirect effect refers mainly to the immunomodulatory effect. As have discussed above, Huaier can enhance the number and function of CD4+ T cells, as well as NK cells, regulate the polarization and function of macrophages, increase secretion of immune stimulatory cytokines to antagonize the tumor progression (Table 1).
  | Table 1 Mechanisms of Huaier to exert anti-tumor effects Abbreviations: CSC, cancer stem cell; ECM, extracellular matrix; EMT, epithelial–mesenchymal transition. |
However, despite the aforementioned promising effects, there are also limitations. Firstly, although there are two forms of the drug (aqueous extract and granule) used in clinic, and it is generally accepted that the bioactive substance is proteoglycan, the detailed effective components of Huaier remain elusive. Secondly, although there is increasing studies focused on the direct effects on cancer cells, only a few studies explored the immunomodulatory effects of Huaier. It has been extensively demonstrated that immunosuppression exists in almost all TME. Detailed examination of the in vivo modulatory effects of Huaier on immune cells in the TME becomes urgently needed. Thirdly, although there are studies on the combined therapy of Huaier with surgical therapy, chemotherapy, radiotherapy and immunotherapy, most of them were carried out in animal cancer models. Only a very small numbers of patients were enrolled in clinical studies. Therefore, large-scale clinical studies are needed in future to verify the mechanisms and efficacy of Huaier in the combined therapy. With solution of these limitations, Huaier will be used in clinic more efficiently and safely.
Acknowledgment
This work was supported by National Natural Science Foundation of China, Grant No: G302302014, G302302013 and G3FW02302013.
Disclosure
The authors report no conflicts of interest in this work.
References
Guo Y, Cheng P, Chen Y. Isolation and analysis of the polysaccharide of Huaier mycelium. Chin J Biochem Pharm. 1993;63:56–59. | ||
Bai J, Geng W, Mei Y, et al. Effect of Huaier on the proliferation of mesangial cells in anti-Thy-1 nephritis. Cell Physiol Biochem. 2017;42(6):2441–2452. | ||
Wang L, Yu Z, Wei C, et al. Huaier aqueous extract protects against dextran sulfate sodium-induced experimental colitis in mice by inhibiting NLRP3 inflammasome activation. Oncotarget. 2017;8(20):32937–32945. | ||
Yang A, Fan H, Zhao Y, et al. Huaier aqueous extract inhibits proliferation and metastasis of tuberous sclerosis complex cell models through downregulation of JAK2/STAT3 and MAPK signaling pathways. Oncol Rep. 2016;36(3):1491–1498. | ||
Song X, Li Y, Zhang H, Yang Q. The anticancer effect of Huaier (review). Oncol Rep. 2015;34(1):12–21. | ||
Hu Z, Yang A, Su G, et al. Huaier restrains proliferative and invasive potential of human hepatoma SKHEP-1 cells partially through decreased lamin B1 and elevated NOV. Sci Rep. 2016;6:31298. | ||
Bao H, Liu P, Jiang K, et al. Huaier polysaccharide induces apoptosis in hepatocellular carcinoma cells through p38 MAPK. Oncol Lett. 2016;12(2):1058–1066. | ||
Wang J, Wang X, Chen T, Jiang L, Yang Q. Huaier extract inhibits breast cancer progression through a lncRNA-H19/miR-675-5p pathway. Cell Physiol Biochem. 2017;44(2):581–593. | ||
Qi W, Sun M, Kong X, et al. Huaier extract synergizes with tamoxifen to induce autophagy and apoptosis in ER-positive breast cancer cells. Oncotarget. 2016;7(18):26003–26015. | ||
Yan X, Lyu T, Jia N, et al. Huaier aqueous extract inhibits ovarian cancer cell motility via the AKT/GSK3β/β-catenin pathway. PLoS One. 2013;8(5):e63731. | ||
Lei JY, Yan LN, Zhu JQ, Wang WT. Hepatocellular carcinoma patients may benefit from postoperative Huaier aqueous extract after liver transplantation. Transplant Proc. 2015;47(10):2920–2924. | ||
Ma Y, Wang C, Zhang Q, et al. The effects of polysaccharides from Auricularia auricula (Huaier) in adjuvant anti-gastrointestinal cancer therapy: a systematic review and network meta-analysis. Pharmacol Res. 2018;132:80–89. | ||
Li C, Wu X, Zhang H, et al. A Huaier polysaccharide inhibits hepatocellular carcinoma growth and metastasis. Tumour Biol. 2015;36(3):1739–1745. | ||
Sun Y, Sun T, Wang F, et al. A polysaccharide from the fungi of Huaier exhibits anti-tumor potential and immunomodulatory effects. Carbohydr Polym. 2013;92(1):577–582. | ||
Zhao GS, Liu Y, Zhang Q, et al. Transarterial chemoembolization combined with Huaier granule for the treatment of primary hepatic carcinoma: safety and efficacy. Medicine (Baltimore). 2017;96(29):e7589. | ||
Shan L, Li Y, Jiang H, et al. Huaier restrains proliferative and migratory potential of hepatocellular carcinoma cells partially through decreased Yes-associated protein 1. J Cancer. 2017;8(19):4087–4097. | ||
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. | ||
Kong X, Ding X, Yang Q. Identification of multi-target effects of Huaier aqueous extract via microarray profiling in triple-negative breast cancer cells. Int J Oncol. 2015;46(5):2047–2056. | ||
Anders L, Ke N, Hydbring P, et al. A systematic screen for CDK4/6 substrates links FoxM1 phosphorylation to senescence suppression in cancer cells. Cancer Cell. 2011;20(5):620–634. | ||
Jeffrey PD, Tong L, Pavletich NP. Structural basis of inhibition of Cdk-cyclin complexes by INK4 inhibitors. Genes Dev. 2000;14(24):3115–3125. | ||
Gao S, Li X, Ding X, Jiang L, Yang Q. Huaier extract restrains the proliferative potential of endocrine-resistant breast cancer cells through increased ATM by suppressing miR-203. Sci Rep. 2017;7(1):7313. | ||
Zhou Y, Wan G, Spizzo R, et al. miR-203 induces oxaliplatin resistance in colorectal cancer cells by negatively regulating ATM kinase. Mol Oncol. 2014;8(1):83–92. | ||
Yang L, Song Z, Wang X, et al. Huaier extract enhances the treatment efficacy of paclitaxel in breast cancer cells via the NF-κB/IκBα pathway. Oncol Rep. 2017;38(6):3455–3464. | ||
Ding X, Yang Q, Kong X, et al. Radiosensitization effect of Huaier on breast cancer cells. Oncol Rep. 2016;35(5):2843–2850. | ||
Zhang N, Kong X, Yan S, Yuan C, Yang Q. Huaier aqueous extract inhibits proliferation of breast cancer cells by inducing apoptosis. Cancer Sci. 2010;101(11):2375–2383. | ||
Zhang C, Zhang J, Li X, et al. Huaier aqueous extract induces hepatocellular carcinoma cells arrest in S phase via JNK signaling pathway. Evid Based Complement Alternat Med. 2015;2015(2a):1–11. | ||
Chen Y, Wu H, Wang X, et al. Huaier granule extract inhibit the proliferation and metastasis of lung cancer cells through down-regulation of MTDH, JAK2/STAT3 and MAPK signaling pathways. Biomed Pharmacother. 2018;101:311–321. | ||
Yan L, Liu X, Yin A, et al. Huaier aqueous extract inhibits cervical cancer cell proliferation via JNK/p38 pathway. Int J Oncol. 2015;47(3):1054–1060. | ||
Xie HX, Xu ZY, Tang JN, et al. Effect of Huaier on the proliferation and apoptosis of human gastric cancer cells through modulation of the PI3K/Akt signaling pathway. Exp Ther Med. 2015;10(3):1212–1218. | ||
Cui Y, Meng H, Liu W, Wang H, Liu Q. Huaier aqueous extract induces apoptosis of human fibrosarcoma HT1080 cells through the mitochondrial pathway. Oncol Lett. 2015;9(4):1590–1596. | ||
Zhang F, Zhang Z, Liu Z. Effects of Huaier aqueous extract on proliferation and apoptosis in the melanoma cell line A875. Acta Histochem. 2013;115(7):705–711. | ||
Karin M. Nuclear factor-kappaB in cancer development and progression. Nature. 2006;441(7092):431–436. | ||
Hayden MS, Ghosh S. Shared principles in NF-kappaB signaling. Cell. 2008;132(3):344–362. | ||
Klinge CM. Estrogen receptor interaction with estrogen response elements. Nucleic Acids Res. 2001;29(14):2905–2919. | ||
Biswas DK, Singh S, Shi Q, Pardee AB, Iglehart JD. Crossroads of estrogen receptor and NF-kappaB signaling. Sci STKE. 2005;2005(288):pe27. | ||
Thomas C, Gustafsson JÅ. The different roles of ER subtypes in cancer biology and therapy. Nat Rev Cancer. 2011;11(8):597–608. | ||
Wang X, Zhang N, Huo Q, et al. Huaier aqueous extract suppresses human breast cancer cell proliferation through inhibition of estrogen receptor α signaling. Int J Oncol. 2013;43(1):321–328. | ||
Fruman DA, Rommel C. PI3K and cancer: lessons, challenges and opportunities. Nat Rev Drug Discov. 2014;13(2):140–156. | ||
Zheng J, Li C, Wu X, et al. Astrocyte elevated gene-1 (AEG-1) shRNA sensitizes Huaier polysaccharide (HP)-induced anti-metastatic potency via inactivating downstream P13K/Akt pathway as well as augmenting cell-mediated immune response. Tumour Biol. 2014;35(5):4219–4224. | ||
Benham-Pyle BW, Pruitt BL, Nelson WJ. Cell adhesion. mechanical strain induces E-cadherin-dependent Yap1 and β-catenin activation to drive cell cycle entry. Science. 2015;348(6238):1024–1027. | ||
Batlle E, Clevers H. Cancer stem cells revisited. Nat Med. 2017;23(10):1124–1134. | ||
Zhang T, Wang K, Zhang J, et al. Huaier aqueous extract inhibits colorectal cancer stem cell growth partially via downregulation of the Wnt/β-catenin pathway. Oncol Lett. 2013;5(4):1171–1176. | ||
Wang X, Zhang N, Huo Q, et al. Huaier aqueous extract inhibits stem-like characteristics of MCF7 breast cancer cells via inactivation of Hedgehog pathway. Tumour Biol. 2014;35(11):10805–10813. | ||
Vermeulen L, de Sousa E MeloF, van der Heijden M, et al. Wnt activity defines colon cancer stem cells and is regulated by the microenvironment. Nat Cell Biol. 2010;12(5):468–476. | ||
Levy JMM, Towers CG, Thorburn A. Targeting autophagy in cancer. Nat Rev Cancer. 2017;17(9):528–542. | ||
Saxton RA, Sabatini DM. mTOR signaling in growth, metabolism, and disease. Cell. 2017;168(6):960–976. | ||
Nicklin P, Bergman P, Zhang B, et al. Bidirectional transport of amino acids regulates mTOR and autophagy. Cell. 2009;136(3):521–534. | ||
Wang X, Qi W, Li Y, et al. Huaier extract induces autophagic cell death by inhibiting the mTOR/S6K pathway in breast cancer cells. PLoS One. 2015;10(7):e0131771. | ||
Yang AL, Xia TJ, Zhao YN, et al. [Huaier aqueous extract inhibits proliferation of human hepatoma SK-HEP-1 cells through up-regulation of autophagy]. Zhongguo Zhong Yao Za Zhi. 2018;43(3):591–595. | ||
Mendell JT. Targeting a long noncoding RNA in breast cancer. N Engl J Med. 2016;374(23):2287–2289. | ||
Dey BK, Pfeifer K, Dutta A. The H19 long noncoding RNA gives rise to microRNAs miR-675-3p and miR-675-5p to promote skeletal muscle differentiation and regeneration. Genes Dev. 2014;28(5):491–501. | ||
Wu T, Chen W, Liu S, et al. Huaier suppresses proliferation and induces apoptosis in human pulmonary cancer cells via upregulation of miR-26b-5p. FEBS Lett. 2014;588(12):2107–2114. | ||
Czabotar PE, Lessene G, Strasser A, Adams JM. Control of apoptosis by the Bcl-2 protein family: implications for physiology and therapy. Nat Rev Mol Cell Biol. 2014;15(1):49–63. | ||
Ren J, Zheng C, Feng G, et al. Inhibitory effect of extract of fungi of Huaier on hepatocellular carcinoma cells. J Huazhong Univ Sci Technolog Med Sci. 2009;29(2):198–201. | ||
Xu X, Wei Q, Wang K, et al. Anticancer effects of Huaier are associated with down-regulation of p53. Asian Pac J Cancer Prev. 2011;12(9):2251–2254. | ||
Luo Z, Hu X, Xiong H, et al. A polysaccharide from Huaier induced apoptosis in MCF-7 breast cancer cells via down-regulation of MTDH protein. Carbohydr Polym. 2016;151:1027–1033. | ||
Nagata S. Apoptosis and clearance of apoptotic cells. Annu Rev Immunol. 2018;36:489–517. | ||
De Palma M, Biziato D, Petrova TV. Microenvironmental regulation of tumour angiogenesis. Nat Rev Cancer. 2017;17(8):457–474. | ||
Wang X, Zhang N, Huo Q, Yang Q. Anti-angiogenic and antitumor activities of Huaier aqueous extract. Oncol Rep. 2012;28(4):1167–1175. | ||
Zou Y, Xiong H, Xiong H, et al. A polysaccharide from mushroom Huaier retards human hepatocellular carcinoma growth, angiogenesis, and metastasis in nude mice. Tumour Biol. 2015;36(4):2929–2936. | ||
Ferrara N, Adamis AP. Ten years of anti-vascular endothelial growth factor therapy. Nat Rev Drug Discov. 2016;15(6):385–403. | ||
Li C, Wu X, Zhang H, et al. A Huaier polysaccharide restrains hepatocellular carcinoma growth and metastasis by suppression angiogenesis. Int J Biol Macromol. 2015;75:115–120. | ||
Littlepage LE, Sternlicht MD, Rougier N, et al. Matrix metalloproteinases contribute distinct roles in neuroendocrine prostate carcinogenesis, metastasis, and angiogenesis progression. Cancer Res. 2010;70(6):2224–2234. | ||
Li Y, Qi W, Song X, et al. Huaier extract suppresses breast cancer via regulating tumor-associated macrophages. Sci Rep. 2016;6:20049. | ||
Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell. 2010;141(1):52–67. | ||
Liu Z, Tang Y, Zhou R, et al. Bi-directional solid fermentation products of Trametes robiniophila Murr with Radix Isatidis inhibit proliferation and metastasis of breast cancer cells. J Chin Med Assoc. 2018;81(6):520–530. | ||
Aiello NM, Brabletz T, Kang Y, et al. Upholding a role for EMT in pancreatic cancer metastasis. Nature. 2017;547(7661):E7–E8. | ||
Xu Z, Zheng G, Wang Y, et al. Aqueous Huaier extract suppresses gastric cancer metastasis and epithelial to mesenchymal transition by targeting twist. J Cancer. 2017;8(18):3876–3886. | ||
Zheng J, Li C, Wu X, et al. Huaier polysaccharides suppresses hepatocarcinoma MHCC97-H cell metastasis via inactivation of EMT and AEG-1 pathway. Int J Biol Macromol. 2014;64:106–110. | ||
Li C, Wu X, Zhang H, et al. A Huaier polysaccharide reduced metastasis of human hepatocellular carcinoma SMMC-7721 cells via modulating AUF-1 signaling pathway. Tumour Biol. 2015;36(8):6285–6293. | ||
Joyce JA, Fearon DT. T cell exclusion, immune privilege, and the tumor microenvironment. Science. 2015;348(6230):74–80. | ||
Becattini S, Latorre D, Mele F, et al. T cell immunity. Functional heterogeneity of human memory CD4+ T cell clones primed by pathogens or vaccines. Science. 2015;347(6220):400–406. | ||
Sica A, Erreni M, Allavena P, Porta C. Macrophage polarization in pathology. Cell Mol Life Sci. 2015;72(21):4111–4126. | ||
Gordon SR, Maute RL, Dulken BW, et al. PD-1 expression by tumour-associated macrophages inhibits phagocytosis and tumour immunity. Nature. 2017;545(7655):495–499. | ||
Imai K, Matsuyama S, Miyake S, Suga K, Nakachi K. Natural cytotoxic activity of peripheral-blood lymphocytes and cancer incidence: an 11-year follow-up study of a general population. Lancet. 2000;356(9244):1795–1799. | ||
Hu Z, Yang A, Fan H, et al. Huaier aqueous extract sensitizes cells to rapamycin and cisplatin through activating mTOR signaling. J Ethnopharmacol. 2016;186:143–150. | ||
Chen Y, Wang L, Liu H, et al. PET imaging on dynamic metabolic changes after combination therapy of paclitaxel and the traditional Chinese medicine in breast cancer-bearing mice. Mol Imaging Biol. 2018;20(2):309–317. | ||
Sun WW, Dou JX, Zhang L, et al. Killing effects of Huaier granule combined with DC-CIK on nude mice transplanted with colon carcinoma cell line. Oncotarget. 2017;8(28):46081–46089. |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.