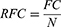Back to Journals » Infection and Drug Resistance » Volume 15
Traditional Medicine Practice and Its Role in the Management of Malaria in Jimma Town, Oromia, Ethiopia
Authors Million E, Mulugeta T , Umeta B
Received 1 December 2021
Accepted for publication 18 March 2022
Published 25 April 2022 Volume 2022:15 Pages 2187—2198
DOI https://doi.org/10.2147/IDR.S339782
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Suresh Antony
Eyob Million,1 Temesgen Mulugeta,2 Belachew Umeta3
1School of Pharmacy, Institute of Health, Jimma University, Jimma, Oromia, Ethiopia; 2Department of Clinical Pharmacy, School of Pharmacy, Jimma University, Jimma, Oromia, Ethiopia; 3Department of Pharmaceutical Sciences, School of Pharmacy, Jimma University, Jimma, Oromia, Ethiopia
Correspondence: Belachew Umeta, School of Pharmacy, Institute of Health, Jimma University, Box: 378, Jimma, Oromia, Ethiopia, Email [email protected]
Background: Traditional medicines have been used to treat malaria for thousands of years and are the source of the two main groups (artemisinin and quinine derivatives) of modern anti-malarial drugs.
Objective: To assess the preference, practice, and factors associated with the practice of traditional medicine for malaria management.
Methods: A community-based cross-sectional study was conducted among 403 residents of Jimma town, Southwest Ethiopia. The data were collected by interviewing selected households. A simple random sampling technique was used to select the households. The data were analyzed using SPSS version 26, and descriptive statistics were used for describing and summarizing the data. A multivariable logistic regression model was used to identify factors associated with the practice of traditional medicine. The medicinal plants were tabulated in excel 2010 with their parts used, route of administration and side effects reported. The relative frequency of citation (RFC) was calculated, and the commonly used traditional medicines were reported.
Results: More than half (64.1%) of the participants practiced traditional medicine. Age [AOR (95% CI) = 1.04 (1.01, 1.07)], family size [AOR (95% CI) = 1.78(1.42, 2.23)] and not having information on traditional medicine use for malaria [AOR (95% CI) = 0.95 (0.03, 0.31)] were factors associated with the practice of traditional medicine. However, only 10.6% of the participants preferred to use traditional medicine. Lepidium sativum (RFC= 0.016), Allium sativum (RFC= 0.014), and Zingiber officinale (RFC= 0.012) were the commonly used herbal products.
Conclusions and Recommendations: Even though the preference to use traditional medicine was low, the community practiced traditional medicine for malaria management. Age, family size and information about traditional medicine use for malaria management were associated with the practice of traditional medicine. Lepidium sativum, Sativum allium and Zingiber officinale were the commonly used traditional medicines. The stakeholders are advised to act accordingly.
Keywords: traditional medicine, malaria, complementary and alternative medicine, Jimma, preference, herbal medicine
Background
Traditional medicine is defined by the World Health Organization (WHO) as the sum of knowledge, skills, and practices based on theories, beliefs, and experiences indigenous to various cultures that are used to maintain health as well as to prevent, diagnose, improve, or treat physical and mental illnesses.1 The technique comprises plant, animal, and mineral-based medicines, massage, spiritual treatments, and other regional and culturally specific practices.2 Traditional medicines are still highly underutilized as a health resource. Traditional medicine can also give insights and creative techniques that may have a direct influence on public health and the economy.3 Traditional medicine serves the main healthcare requirements of around 65–80% of the world population.4 Traditional, complementary, and alternative medicine of demonstrated quality, safety, and efficacy provide many benefits, according to the World Health Organization (WHO).5
Traditional medicine and practitioners have a long history in Africa. And they are essential in delivering treatment to communities.6,7 The primary reasons for using TM were accessibility, cost, money, educational level, efficacy, and inaccessibility to modern health services.8 Ethiopian traditional medicinal practices represent the richness of Ethiopian cultures. The Ethiopian community utilized traditional medicine for disease prevention, treatment, and well-being promotion. Ethiopia’s health and drug regulations recognize the importance of traditional medicines in healthcare.9
Malaria is a risk to worldwide public health. In the year 2020, there were an estimated 241 million malaria cases worldwide, with around 627,000 deaths.
Malaria is one of the leading causes of illness and mortality in the country. Approximately 60% of the population lived in malaria-risk zones. Plasmodium falciparum and Plasmodium vivax are the most common parasites in Ethiopia, accounting for the vast majority of malaria cases.10
Five Plasmodium species are capable of infecting humans. The recognized parasites that infect humans include Plasmodium falciparum, Plasmodium vivax, Plasmodium ovale, Plasmodium malariae, and Plasmodium knowlesi.11 The rise of drug-resistant Plasmodium species has intensified the health and economic consequences of malaria. During the last two decades, research on alternative anti-malarial treatments has increased. Malaria is becoming more common, necessitating the identification of new therapeutic targets for both prevention and treatment. Plasmodium proteases, which play an important part in the parasite’s life cycle, could be potential therapeutic targets.12
Medicinal herbs have traditionally been utilized to create an effective anti-malarial drug. Quinines, triterpenes, sesquiterpenoids, quassinoids, limonoids, alkaloids, lignans, and coumarins have been identified.13 Traditional medicines have been used to treat malaria for thousands of years and are the source of the two main modern anti-malarial drugs.14 In 1971, Artemisia annua was isolated by Chinese scientists and confirmed to have anti-malarial potency, developed an effective extraction process, and The active ingredient identified in 1972.15–17
Terpenoidal, indole, bisindole, quinolone, and isoquinoline alkaloids were discovered to have promising antimalarial action.18
For the treatment of malaria, medicinal herbs were used traditionally. Proper documenting of traditional medicine and flora used in malaria prevention and treatment is critical not just for maintaining valuable indigenous knowledge and biodiversity. But also to provide opportunities for pharmaceutical companies and researchers to seek more viable alternatives. In Ethiopia, ethnobotanical and ethnopharmacological research on anti-malarial herbs is in the early stages.10 Furthermore, scientific literature on indigenous anti-malarial plants is exceedingly limited, underscoring the crucial need for careful compilation and synthesis., the current study aimed to assess the preference, practice, and factors associated with traditional medicine practice for the management of malaria.
Material and Methods
Setting and Period
The study was conducted in Jimma town. Jimma town is located 350 km southwest of Addis Ababa, the capital city of Ethiopia. The town lies in the climatic zone ideal for agriculture and human settlement.19 As per the Central Statistical Agency (CSA, 2007) report, the total population is 120,960, of whom 60,824 are males, and 32,191 households are present in the town.20 Jimma town has five public health centers, one medical center, and two general hospitals. The study was conducted from August-September 2021. The study area is reached in anti-malarial plants.13
Study Design and Population
A community-based cross-sectional study design was employed.
The study participants were selected households. Adults older than 18 years and who lived for at least six (6) months were included. However, Priority was given to the head of the household. Because they are expected to have more knowledge about traditional medicine used in the community than adults, and if they are unavailable during data collection, individual older than 18 years was included. Individuals who were unable to give the required information due to any reason (unwillingness, mental problem etc.) were excluded from the study.
Sample Size and Sampling Technique
The sample size was determined using the single population proportion formula for the cross-sectional study, n = Z2P (1−P)/d2. Where P = 50% (prevalence of traditional medicine practice for malaria prevention and treatment) and Z = 1.96 is the value under the standard normal table for the confidence level of 95%, the margin of error 5%, and nonresponse rate of 5%. Finally, 403 households were used as a sample size.
The list of all households was obtained from the Jimma Town Administration Office. The list is arranged in ascending order according to their registration number. Finally, a simple random sampling technique was used to select the required number of households using computer system. Trained graduating Pharmacy students collected the data.
Data Collection Tools
A pretested, structured questionnaire was adopted from a previously conducted study with modifications based on the local context.21,22 The questionnaire was prepared in English. The pretest was conducted on 5% of non-sampled households to check the reliability and clarity of the questionnaires.
The questionnaires consist of the following parts: Sociodemographic characteristics (age, sex, ethnicity, religion, marital status, occupation, educational level, family size and monthly income in Ethiopian birr).
The practice of traditional medicine (ever heard of traditional medicine before, current use of TM for the prevention and treatment of malaria, frequency of use, do you mix TM with modern medicine for the treatment of malaria, how do you rate the outcome of using traditional medicine for the prevention and treatment of malaria, source of information on the use of TM for the prevention and treatment of malaria, by whom traditional medicine was practiced), commonly used herbal medicines for the treatment of malaria (vernacular name, parts used, route of administration, and side effects of the medicinal product).
Preference: preference between traditional medicine, modern medicine and both traditional and modern medicine for the prevention and treatment of malaria, the reason for the preference of traditional medicine for the management of malaria.
Data Processing and Analysis
The data were entered and cleaned using Epi Info 3.1 software and exported to IBM SPSS Statistics for Windows, version 26.0 (IBM Corp., Armonk, N.Y., USA) for further analysis. Descriptive statistics were used for describing and summarizing the data. Bivariable logistic regression was performed to determine factors associated with the practice of traditional medicine. A variable with a p-value of less than 0.25 in binary logistic regression was eligible for multivariable logistic regression. P-value <0.05 was used to declare the significant association.
The medicinal plants were recorded in Microsoft excel 2010 and tabulated with their parts used, route of administration and side effects. The Relative Frequency of Citation (RFC was calculated using the following equation below.
Where FC is the number of respondents who mentioned the plant and N is the number of respondents who participated in the study. The results of the RFC and the top mostly used medicinal plants were presented in the radar diagram.
Ethical Consideration
Permission letter was obtained from Jimma University School of Pharmacy with reference number of pharm 697/2011. Additionally, permission letter was also obtained from Jimma town Administration office. Verbal informed consent was obtained from the participants after the purpose and methods of the study had been explained. Since the participants had only asked to describe traditional medicine practice they know, we used verbal informed consent.
Results
Sociodemographic Characteristics
The response rate of the study was 98.01 (395/403). The remaining questionnaires were excluded from the analysis because of incomplete and did not have the required information about traditional medicine practice. The participants were also unwilling to disclose whether they use TM or not. In addition, they were not willing to express their preference, and participants were reluctant to tell their herbal or non-herbal medicines they practice.
More than half (54.2%) of the participants were male. The age in years (mean ± SD) of the participants was 35.99 ± 11.99. Around 70% of them were married. 38% of the participants were followers of the Orthodox religion. More than half (56.2%) of the participants were of Oromo ethnicity, and 48.9% had an educational level of > 12 grade complete. Around 37.2% of participants were government employed. The monthly income in Ethiopian Birr (mean ± SD) and the family size (mean ± SD) of the participants were 7955.58 ± 3768.50 and 3.91 ± 1.42, respectively (Table 1).
 |
Table 1 Sociodemographic Characteristics of Participants |
Traditional Medicine Practice for the Management of Malaria
The majority, 91% of the participants, heard of traditional medicine used for malaria management. The majority of participants (64.1%) used traditional medicine for the management of malaria. Less than half (49%) of the participants practiced traditional medicine whenever they had malaria-like symptoms, and 68.4% experienced symptomatic relief after traditional medicine use. More than half (68.4%) of the participants did not mix prescription drugs with traditional medicines they practiced. Almost half of the participants responded the practice was practiced by the elderly (Table 2).
 |
Table 2 Traditional Medicine Practice for the Management of Malaria |
Preference of Community for the Management of Malaria
The majority (76.5%) of the participants preferred to use modern medicine. Cultural influence (83.3%) and therapeutic effectiveness of traditional medicine (54.8%) were the common reasons for the preference of traditional medicine (Table 3).
 |
Table 3 Preference of Community for the Management of Malaria |
Reported Route of Administration for the Management of Malaria
The oral route (82.86%) was the most used route of administration of traditional medicine, followed by the nasal route (14.29%) (Figure 1).
 |
Figure 1 Reported route of administration by the community for the management of malaria. |
Part of Plants Used for the Management of Malaria
Seed (40.0%), leaves (31.43%), rout (25.71%) and bulb (25.71%) was the commonly used parts of plants (Figure 2).
 |
Figure 2 Part of plants commonly used by the community for the prevention and treatment of malaria. |
Commonly Used Herbal Products for the Management of Malaria
Lepidium sativum (RFC= 0.015), Allium sativum (RFC= 0.013), Zingiber officinale (RFC= 0.01), Brassica nigra (RFC= 0.008) were the commonly used herbal products by the community for the management of malaria (Figure 3).
 |
Figure 3 Commonly Used Herbal Products by the Community for the Prevention and Treatment of Malaria. |
Reported Side Effects of Traditional Medicine Used for the Management of Malaria
Twenty-one herbal and non-herbal medicinal practices were reported. Gastritis, heartburn, and constipation were the common side effect reported by the participants (Table 4).
 |
Table 4 Reported Traditional Medicines Practice and Side Effects |
Determinants of Traditional Medicine Practice for the Management of Malaria
The practice of traditional medicine was associated with monthly income [(AOR: 95% CI): 1.00 (0.999, 1.000)], age [(AOR: 95% CI): 1.04 (1.01, 1.07)] and participants who heard of traditional medicine use for the management of malaria practice more than their respective friend who did not have information about traditional medicine use for malaria (Table 5).
 |
Table 5 Factors Associated with Traditional Medicine Use for the Management of Malaria |
Discussion
Traditional medicines (TM) have been utilized for thousands of years to treat malaria and are the basis of current antimalarial drugs. Over 1200 plant species from 160 families are used to treat malaria and fever. Traditional herbal remedies are utilized for malaria management by one-fifth of patients in endemic areas.23 Herbs and herbal extracts are used in malaria therapy in several regions of the world because they are inexpensive and widely available, and there is evidence that they are effective.24
Majority 91.1% of the respondents heard traditional medicine used for the management of malaria. An earlier study from the same area reported, 54.4% of the households had adequate had information.25 The difference might be related to the difference in the year of data collection.
Traditional medicine was practiced by 64.1%. A similar result was reported from Mali, where traditional medicines were utilized by 50% of children with severe malaria.26 However, studies conducted in different regions of Ethiopia and Indonesia (Northwest, Southern, and Southwest) reported that 22.2%, 8%, 43.0%, and 18.77% were used medicinal plants for malaria-like symptoms (fever and flu-like illness, including shaking chills, headache, muscle aches, and tiredness), respectively.27–30 The increased use of TMs for malaria may be attributable to the diversity of indigenous anti-malarial medicinal plants, the geographical variance in malaria distribution,13 and the community inability to buy contemporary medicine.
Less than half (49.0%) of the community used traditional medicine when they had malaria-like symptoms. However, in a study by Suswardan et al, 75.2% of the respondents who used TMs for malaria had malaria-like symptoms in the last month.30
For almost half (49.8%) of the participants, the medicines were delivered by the elderly. Around 25% of the participants had the medicines from traditional medicine practitioners. A similar finding was reported in Ethiopia, in which family members (13.5%) provided traditional medicines more than traditional healers (8.5%).25 More than two-thirds (68.4%) of the participants experienced only symptom relief after traditional medicine use. A comparable finding was reported in which 69.0% of the children with malaria who used herbal medicines got a cure.27
Regarding the preference of the community, 10.6% and 12.9% of the respondents preferred to use traditional medicine alone and both TMs and modern medicine, respectively. The reasons for preferring traditional medicine were cultural influence (83.3%), therapeutic effectiveness (54.8%), and affordability issues (50.0%). A comparable finding was reported from the Southern part of Ethiopia, where 27.9% of the households went to traditional healers, and 13.2% used homemade remedies when they were ill. The main reasons for this preference were effectiveness, low cost, and ease of availability. The discrepancy between traditional medicine preference (10.6%) and practice (64.1%) might be due to cultural influence and affordability to the use of modern medicine. Malaria was the top-treated illness with herbal remedies in households and by traditional healers.31
Lepidium sativum, Allium sativum, Zingiber officinale, and Brassica nigra were the herbal products used for malaria. The commonly used route of administration for the management of malaria was the oral and nasal route. Gastritis, heartburn, headache, constipation and diarrhea were the reported side effects of traditional medicines used for malaria management.
Traditional medicine practice for malaria was linked to monthly income, ethnicity, the number of households, and information on malaria management using traditional medicine. Several studies have linked the use of traditional medicine for malaria to factors such as accessibility, cheap cost, ethnicity, lack of knowledge about contemporary treatment, and the idea that traditional medicine is superior.25,29,30
Limitation of the Study
The study did not test (in vitro and in vivo) the efficacy, safety and quality of the medicinal plants reported. Therefore, it is not recommended to practice by the community.
Conclusions and Recommendations
For the treatment of malaria, the majority of the participants used traditional medicine. Traditional medicine was preferred by only one-tenth of the participants. Cultural influence, therapeutic effectiveness, price, and religious influence were reasons for traditional medicine preference. Age, family size, and information on traditional medicine for management of malaria were factors associated with the practice.
Weakness and Strength of the Study
The study includes large sample size and reported the non-herbal products used by the community.
Abbreviations
AOR, adjusted odd ratio; CI, confidence interval; COR, crude odd ratio; CSA, Central Statistical Agency; RFC, relative frequency of citation; SD, standard deviation; TM, traditional medicine; WHO, World Health Organization.
Data Sharing Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Ethical Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and permission letter was obtained from Jimma University School of Pharmacy with reference number Phar 697/2011. Additionally, permission letter was also obtained from Jimma town Administration office. Verbal informed consent was obtained from the participants after the purpose and methods of the study had been explained. Since the participants were only asked to describe traditional medicine practice they know, we used verbal informed consent.
Disclosure
The authors disclosed that they have no conflicts of interest for this work.
References
1. World Health Organization. Traditional medicine; 2008 [July 3, 2012].
2. Chali BU, Hasho A, Koricha NB, Mukudai Y. Preference and practice of traditional medicine and associated factors in Jimma town, Southwest Ethiopia. Evid Based Complement Altern Med. 2021;2021. doi:10.1155/2021/9962892
3. Graz B, Kitua AY, Malebo HM. To what extent can traditional medicine contribute a complementary or alternative solution to malaria control programmes? Malar J. 2011;10:S6. doi:10.1186/1475-2875-10-S1-S6
4. Pan Y, Litscher G, Chan K, Yu ZL, Chen HQ, Ko KM. Traditional medicines in the world: where to go next? Evid Based Complement Altern Med. 2014;2014:4. doi:10.1155/2014/739895
5. World Health Organization. WHO Traditional Medicine Strategy: 2014–2023. World Health Organization; 2013.
6. Ezekwesili-Ofili JO, Okaka AN. Herbal Medicines in African Traditional Medicine, Herbal Medicine. Philip F. Builders. Vol. 10. Intech Open; 2019.
7. Ozioma EO, Chinwe OA. Herbal medicines in African traditional medicine. Herbal Med. 2019;30(10):191–214.
8. Tizazu D, Workineh Y, Ayalew Y. Parental traditional medicine use for children and associated factors in North Mecha district, North West Ethiopia. Pediatric Health Med Ther. 2020;11:505–512. doi:10.2147/PHMT.S275249
9. Kassaye KD, Amberbir A, Getachew B, Mussema Y. A historical overview of traditional medicine practices and policy in Ethiopia. Ethiop J Health Dev. 2006;20(2):127–134.
10. Esayas E, Tufa A, Massebo F, et al. Malaria epidemiology and stratification of incidence in the malaria elimination setting in Harari Region, Eastern Ethiopia. Infect Dis Poverty. 2020;9:160. doi:10.1186/s40249-020-00773-5
11. Aschale Y, Mengist A, Bitew A, Kassie B, Talie A. Prevalence of malaria and associated risk factors among asymptomatic migrant laborers in West Armachiho District, Northwest Ethiopia. Res Rep Trop Med. 2018;9:95–101. doi:10.2147/RRTM.S165260
12. Coppi A, Cabinian M, Mirelman D, Sinnis P. Antimalarial activity of allicin, a biologically active compound from garlic cloves. Antimicrob Agents Chemother. 2006;50(5):1731–1737. doi:10.1128/AAC.50.5.1731-1737.2006
13. Alebie G, Urga B, Worku A. Systematic review on traditional medicinal plants used for the treatment of malaria in Ethiopia: trends and perspectives. Malar J. 2017;16:307. doi:10.1186/s12936-017-1953-2
14. Suleman S, Beyene tufa T, Kebebe D, et al. Treatment of malaria and related symptoms using traditional herbal medicine in Ethiopia. J Ethnopharmacol. 2017;213(2018):262–279. doi:10.1016/j.jep.2017.10.034
15. Klayman DL. Qinghaosu (Artemisinin): an antimalarial drug from China. Science. 1985;228:1049–1055. doi:10.1126/science.3887571
16. Zamiska N, McKay B. Global health, China’s price in malaria clash. Wall St J. 2007;2007:A1–A14.
17. Hien TT, White NJ. Qinghaosu. Lancet. 1993;341:603–608. doi:10.1016/0140-6736(93)90362-K
18. Uzor PF. Alkaloids from plants with antimalarial activity: a review of recent studies. Evid Based Complement Altern Med. 2020;2020:1–17. doi:10.1155/2020/8749083
19. Alemu A, Abebe G, Tsegaye W, Golassa L. Climatic variables and malaria transmission dynamics in Jimma town, South West Ethiopia. Parasit Vectors. 2011;4:30. doi:10.1186/1756-3305-4-30
20. Central Statistical Authority of Ethiopia. Jimma Zone Population Survey. Addis Ababa, Ethiopia: Central Statistical Authority (CSA) of Ethiopia; 2007.
21. Kebede A. Ethiopia National Malaria Indicator Survey 2015. Ethiopia: Ethiopian Public Health Institute; 2016.
22. Linna Suswardany D, Sibbritt DW, Supardi S, Pardosi JF, Chang S, Adams J. Across-sectional analysis of traditional medicine use for malaria alongside free antimalarial drugs treatment amongst adults in high-risk malaria endemic provinces of Indonesia. Prevalence of traditional medicine use for malaria in malaria endemic provinces across Indonesia. PLoS One. 2017;12(3). doi:10.1371/journal.pone.0173522
23. Willcox ML, Bodeker G. Traditional herbal medicines for malaria. Br Med J. 2004;329(7475):1156–1159. doi:10.1136/bmj.329.7475.1156
24. Okello D, Kang Y. Exploring antimalarial herbal plants across communities in Uganda based on electronic data. Evid Based Complement Altern Med. 2019;2019:1–27. doi:10.1155/2019/3057180
25. Karunamoorthi K, Tsehaye E. Ethnomedicinal knowledge, belief and self-reported practice of local inhabitants on traditional antimalarial plants and phytotherapy. J Ethnopharmacol. 2012;141(1):143–150. doi:10.1016/j.jep.2012.02.012
26. Diallo D, Graz B, Falquet J, et al. Malaria treatment in remote areas of Mali: use of modern and traditional medicines, patient outcome. Trans R Soc Trop Med Hyg. 2006;100(6):515–520. doi:10.1016/j.trstmh.2005.08.003
27. Gurmu AE, Kisi T, Shibru H, Graz B, Willcox M. Treatments used for malaria in young Ethiopian children: a retrospective study. Malar J. 2018;17(1):1–8. doi:10.1186/s12936-018-2605-x
28. Deressa W, Ali A, Enqusellassie F. Self-treatment of malaria in rural communities, Butajira, southern Ethiopia. Bull World Health Organ. 2003;81:261–268.
29. Adera TD. Beliefs and traditional treatment of malaria in Kishe settlement area, southwest Ethiopia. Ethiop Med J. 2003;41(1):25–34.
30. Suswardany DL, Sibbritt DW, Supardi S, Pardosi JF, Chang S, Adams J. A cross-sectional analysis of traditional medicine use for malaria alongside free antimalarial drugs treatment amongst adults in high-risk malaria endemic provinces of Indonesia. PLoS One. 2017;12(3):e0173522.
31. Paulos B, Fenta TG, Bisrat D, Asres K. Health seeking behavior and use of medicinal plants among the Hamer ethnic group, South Omo zone, southwestern Ethiopia. J Ethnobiol Ethnomed. 2016;12(1):1–3. doi:10.1186/s13002-016-0107-x
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.