Back to Journals » Infection and Drug Resistance » Volume 15
Tracking the Outbreak of Carbapenem-Resistant Klebsiella pneumoniae in an Emergency Intensive Care Unit by Whole Genome Sequencing
Authors Li L, Wang R, Qiao D, Zhou M, Jin P
Received 19 August 2022
Accepted for publication 19 October 2022
Published 27 October 2022 Volume 2022:15 Pages 6215—6224
DOI https://doi.org/10.2147/IDR.S386385
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Professor Suresh Antony
Li Li,1 Renying Wang,2 Dan Qiao,1 Min Zhou,1 Peipei Jin1
1Department of Clinical Laboratory, Ruijin Hospital, Shanghai Jiaotong University, School of Medicine, Shanghai, People’s Republic of China; 2Department of Emergency, Ruijin Hospital, Shanghai Jiaotong University, School of Medicine, Shanghai, People’s Republic of China
Correspondence: Peipei Jin, Department of Clinical Laboratory, Ruijin Hospital, Shanghai Jiaotong University, School of Medicine, 999 Xiwang Road, Shanghai, 201801, People’s Republic of China, Tel +86-21-67888999, Fax +86-21-64333548, Email [email protected]
Purpose: The spread of carbapenem-resistant Klebsiella pneumoniae (CRKP) has become a great threat to human health, especially in the intensive care unit. The aim of this study was to identify the origin and transmission route of a CRKP outbreak in an emergency intensive care unit (EICU), so as to provide prevention and control strategies for CRKP outbreak.
Methods: Between Mar and Jun 2018, 10 CRKP isolates from 5 patients in the EICU ward of Shanghai Ruijin hospital north were collected. Modified carbapenem inactivation method (mCIM) and whole-genome sequencing (WGS) were performed on all 10 CRKP isolates. By integrating the genomic and epidemiological data of our isolates and 9 CRKP isolates from an outbreak in another hospital, a putative transmission map was constructed.
Results: All 10 outbreak strains were carbapenemase positive in mCIM and belonged to the sequence type 11 (ST11) clone, harbored a set of resistance genes and virulence genes. The phylogenetic tree of CRKP isolates based on two outbreaks revealed that the initial isolate A1 in our EICU ward belonged to one branch of isolates in another hospital, this introductive isolate evolved and caused a subsequent outbreak in our EICU.
Conclusion: Integration of genomic and epidemiological data can yield a clear transmission map of CRKP outbreak. Monitoring the rapid evolution of CRKP at the early stage of outbreak, CRKP monitoring after patients are discharged, active surveillance of newly admitted patients, environmental hygiene and efficient antibiotic treatment may be the key to prevent and control of CRKP outbreak.
Keywords: carbapenem-resistant Klebsiella pneumoniae, whole-genome sequencing, transmission map
Introduction
Klebsiella pneumoniae (K. pneumoniae), often commensally colonizes in the upper respiratory tract, gut, and skin of human can cause invasive infections in the lower respiratory tract, urinary tract and blood in immunosuppressed individuals.1 As the multiclass antimicrobial resistance (AMR) of K. pneumoniae is widespread in hospitals, especially in the intensive care unit, it becomes a great threat to public health.2 Although carbapenem antibiotics can be used for the treatment of drug-resistant bacteria, K. pneumoniae has acquired carbapenemase that can inactive carbapenems, thus conferring carbapenem-resistant, other mechanisms including porin loss, efflux pump, and the production of extended spectrum beta-lactamases or AmpC beta-lactamases also led to carbapenem-resistant.1 Carbapenem-resistant K. pneumoniae (CRKP) is a great challenge for clinicians, as it can cause invasive infections, with few therapeutic options and high mortality.3
K. pneumoniae carbapenemase (KPC) was first discovered in the USA in 1996,4 this plasmid-mediated carbapenemase gene was transferred horizontally worldwide and then isolated in China in 2014.5 With the help of some special mobile elements (Tn1721 transposons and IncFII-like plasmids),6 blaKPC-producing K. pneumoniae spread wildly in China and formed a dominant ST11 clone of blaKPC-producing K. pneumoniae.7,8 In 2016, the prevalence of CRKP in different provinces of China ranged from 0.9% to 23.6%, with an average incidence rate of 8.7%.9 Nosocomial outbreaks of CRKP were reported in different cities of China,10–16 community disseminations were also observed.17,18 CRKP strains constitute the majority of carbapenem-resistant Enterobacteriaceae (CRE) in clinical settings, which has become a major public health problem. However, the transmission map within or between different institutions was not well depicted, making it difficult to develop efficient strategies for prevention and control of CRKP outbreak.
Traditional typing methods, such as pulsed-field gel electrophoresis (PFGE) and multi-locus sequence typing (MLST) can characterize the epidemiological linkage of outbreak, but they only target a small portion of the bacterial genome, limiting the resolution and, thus, our understanding of the molecular epidemiology of pathogens. Whole-genome sequencing (WGS) based SNP-calling (short-read or long-read sequencing) provides enough resolution for outbreak tracking. Long-read sequencing can provide information of mobile genetic elements (MGEs), but its high error rates pose challenges for SNP-calling. Although short-read sequencing cannot provide information of MGEs, it is relatively straightforward and can yield highly accurate results, making it a powerful tool for outbreak tracking.
In the present study, the WGS data (short-read sequencing) of CRKP strains in our EICU department and another hospital were integrated with epidemiological data, a putative transmission map was constructed and some prevention and control strategies were proposed.
Materials and Methods
Patients and Isolates
In late Mar and Jun 2018, 5 cases of infection due to carbapenem-resistant K. pneumoniae in the EICU ward of Shanghai Ruijin hospital north (Shanghai, China) were identified. As in the medical institutions more than 3 cases of infection with similar clinical symptoms and suspected common infection source in a short time can be determined as suspected outbreak of hospital infection, therefore we initiated a retrospective outbreak investigation. Isolates from 5 patients were collected. The sample types consisted of blood, urine, deep vein catheters and sputum. Clinical data including the patient’s age, gender, diagnosis, outcome, time of admission, time of discharge, and length of hospital stay were extracted from the patient administration system.
Species identification and antimicrobial susceptibility testing were performed with the MALDI-TOF MS spectrometry (bioMérieux, France) and VITEK-2 compact system (bioMérieux, France), the susceptibility of the strain to imipenem, meropenem and ertapenem was checked by K-B paper diffusion method (Oxoid, UK). Modified carbapenem inactivation method (mCIM) was used for phenotypic detection of carbapenemase. If the test isolate produces a carbapenemase, the meropenem in the disk will be hydrolyzed and there will be no inhibition or limited growth inhibition of the meropenem-susceptible E. coli ATCC® 25922. The zone diameter of 6–15mm or presence of pinpoint colonies within a 16–18mm zone is considered to be carbapenemase positive.19 Antibiotic susceptibility and minimum inhibitory concentrations (MIC) were determined according to the Clinical and Laboratory Standards Institute (CLSI)-M100 2021 guidelines.
DNA Extraction and Quality Control
Genomic DNA was extracted using Wizard® Genomic DNA Purification Kit (Promega) according to manufacturer’s protocol. Purified genomic DNA was quantified by TBS-380 fluorometer (Turner BioSystems Inc., Sunnyvale, CA). High-quality DNA (OD260/280=1.8~2.0, >1μg) was used for further research.
Whole Genome Sequencing
Total DNA was extracted from 10 CRKP isolates and sequenced with next-generation sequencing technology (Illumina Hiseq×10 with 2×150bp pair-end reads). Raw sequence data were pre-processed as follows: (1) removing adaptor sequence. (2) removing the bases containing non-A, G, C and T at the 5’ end. (3) removing the ends of reads with low sequencing quality (quality score <Q20). (4) removing the reads with the proportion of N-base removal reached 10%. (5) removing reads shorter than 25bp after the above trim. The Q20 of raw reads were about 97% and Q30 of raw reads were about 93%. The Q20 of clean reads were above 98% and the Q30 of clean reads were above 95%. Clean data were de-novo assembled with SOAPdenovo2. Draft genome sequences were compared with the Genome Taxonomy Database (GTDB), the constructed phylogenetic tree based on the difference of 31 housekeeping genes (dnaG, frr, infC, nusA, pgk, pyrG, rplA, rplB, rplC, rplD, rplE, rplF, rplK, rplL, rplM, rplN, rplP, rplS, rplT, rpmA, rpoB, rpsB, rpsC, rpsE, rpsI, rpsJ, rpsK, rpsM, rpsS, smpB, tsf) indicated that K. pneumoniae JM45, complete genome (CP006656.1) was the closest strain to our isolates and could be used as a reference genome.
Multi-Locus Sequence Typing
Seven housekeeping genes of K. pneumoniae (gapA, mdh, tonB, infB, pgi, rpoB and phoE) were used to characterize the CRKP isolates by MLST. Data of 10 CRKP isolates in our study were analyzed on the online tool of Majorbio Cloud Platform (https://cloud.majorbio.com/page/tools/).
Identification of Antimicrobial-Resistant (AMR) Genes and Virulence Factor (VF) Genes
The AMR genes of 10 CRKP isolates were identified by ResFinder tools V4.1.0 in the ResFinder (https://cge.cbs.dtu.dk/services/ResFinder/) database. The VF genes of 10 CRKP isolates were identified by Diamond in the virulence factor database (VFDB) (http://www.mgc.ac.cn/VFs/).
In silico Detection of Plasmid Replicons
The clean short sequencing reads of CRKP strains were assembled into contigs by SOAPdenovo2, these assembled contigs were uploaded to Center for Genomic Epidemiology (http://www.genomicepidemiology.org/) and analyzed by PlasmidFinder 2.1 (threshold for minimum % identity, 80%; minimum % coverage, 60%).
Single Nucleotide Polymorphisms (SNPs) Identification and Phylogenetic Tree
To detect SNPs between isolates of our study and another hospital, the genome data of 9 isolates downloaded from the Short Read Archive (SRR9600321 to SRR9600329, sample ID kp-s1 to kp-s9) and the clean data of 10 isolates in our study were mapped to the JM45 reference genome with CLC Genomics Workbench 21.0.5. High-quality SNPs were identified, SNP matrix across samples and SNP phylogenetic tree were constructed by Compare Variants Across Samples Workflow V0.3 in microbial genomics module plugin of CLC Genomics Workbench. The required significance level for low frequency variant calls was set as 0.01. Variants with a frequency below 20% were removed and variants with an average base quality below 20% were also removed. Neighbor-Joining Algorithm was used for the construction of SNP tree.
Results
Outbreak Description
Between March and June 2018, 5 cases of infection due to carbapenem-resistant K. pneumoniae occurred in the EICU ward of Shanghai Ruijin hospital north. Epidemiological data showed that a 59-year-old man was patient zero (indicated as patient A), he had been transferred to the EICU from another hospital, with diabetes and hepatoma. The first CRKP strain was obtained from the mid-stream urine upon his admission and was named as A1. Within the following days of patient A’s hospitalization, the CRKP strain was detected in his urine 8 times but was not found in his sputum and blood samples. During his stay in the EICU, infection spread to other four patients.
Patient B’s bed was adjacent to patient A’s bed. Before patient A’s admission, patient B had been subjected to multiple sputum and urine cultures, including perianal screening, and no CRKP was detected. However, 10 days after patient A’s admission, CRKP strains (B1, B2, B3, B4) were found in patient B’s sputum, blood, deep vein catheters and urine successively.
Despite implementing control measures which included bedside isolation, contact precautions and hand hygiene, another 5 CRKP isolates (C1, D1, D2, D3, E1) were detected in 3 patients within the next 45 days, giving an average of one new K. pneumoniae infection case emerging every 15 days. Finally, two cases died due to CRKP blood infection and another 3 cases improved and were discharged. The 5 patients’ clinical characteristics are summarized in Table 1 and the timelines of patients’ admission, discharge, sample taking and outcomes are shown in Figure 1, hospital time overlaps were found among these patients.
 |
Table 1 Clinical Characteristics of Patients in the EICU Outbreak |
Antimicrobial Susceptibility Testing and MLST
As shown in Figure 2A, all suspected outbreak strains showed multiple drug classes resistance, particularly against carbapenems, all 10 strains were resistant to cefazolin, ceftriaxone, ceftazidime, cefepime, cefotetan, ampicillin-sulbactam, piperacillin-tazobactam, aztreonam, imipenem, ertapenem, meropenem, gentamicin, amikacin, tobramycin, ciprofloxacin, levofloxacin, trimethoprim-sulfamethoxazole and fosfomycin, but still susceptible to tigecycline and polymyxin B. All the 10 CRKP isolates were positive to mCIM and WGS produced draft genomes showed that they were blaKPC-2 producing strains. MLST analysis showed that all the strains in this outbreak belonged to ST11 K. pneumoniae strains.
Genetic Determinants of Resistance and Virulence
The next-generation sequencing analysis was performed on the 10 CRKP strains. Almost all suspected CRKP outbreak strains harbored multiple genes encoding resistance to β-lactams (blaTEM-1B, blaSHV-182, blaCTX-M-65, blaLAP-2, blaKPC-2), aminoglycosides (rmtB, aadA2b, aadA1, aph(6)-Id), fluoroquinolones (qnrS1, OqxB, OqxA), sulfamethoxazoles (sul1, sul2, sul3, dfrA12), fosfomycins (fosA3, fosA), macrolides (mef(B)) and tetracyclines (tet(A)). However, the antimicrobial-resistant gene character of A1 was a little different from the other 9 strains (Figure 2B).
Moreover, the 10 CRKP outbreak strains also harbored multiple virulence genes. Gene coding for type I fimbriae (fim), type III fimbriae (mrk), efflux pump (acrAB), regulation factors (rcsAB) and the siderophore genes (enterobactin, salmochelin, yersiniabactin, iutA) were detected in all strains and almost all strains shared the same virulence factors. However, type IV pili biosynthesis associated genes (pilURV), aerobactin siderophore genes (iucABCD) and T6SS genes associated secretion system (vipA/tssB) were not detected in strain A1, but were detected in the other 9 strains (Figure 2C).
Plasmid of Isolates
Nine different plasmids [ColRNAI, IncFIB(K), IncFII(pHN7A8), IncR, IncFIB(pKPHS1), IncFII(pKP91), Col(pHAD28), IncFII(pCRY) and IncFII(K)] were identified by PlasmidFinder, with the first four being present in the 10 CRKP strains. IncFIB(pKPHS1), IncFII(pKP91) and Col(pHAD28) were only identified in strain A1, IncFII(pCRY) was not identified in strain A1 but in other 9 strains, IncFII(K) was identified in some strains (Supplement Table 1).
WGS-Based SNPs, Phylogenetic Tree and Putative Transmission Map
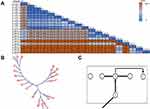
After integrating the WGS data of our strains and strains from another hospital, high-quality SNPs (frequency and base quality all above 20%) between each pair of strains were detected in all strains. Taking strain A1 as a reference, the different SNPs revealed that these strains can be divided into 3 clades. Clade 1 comprised strain A1 in our EICU and strains kp-s1, kp-s2, kp-s3, kp-s4 and kp-s6 in another hospital, whereas strains kp-s5, kp-s7, kp-s8 and kp-s9 in another hospital formed clade 2. Clade 3 consisted of 9 strains in our EICU (B1, B2, B3, B4, C1, D1, D2, D3, E1). The internal members of each clade had high homology, the differences in the internal strains of clade 1, clade 2 and clade 3 were in the range of 1–68, 3–41 and 0–8 SNPs, respectively. In contrast, 922–959 SNPs differed between clades 1 and 2, 366–379 SNPs were found between clades 1 and 3, and there were 961–984 SNPs differences between clades 2 and 3 (Figure 3A). Based on the SNPs differences, the phylogenetic tree of 19 CRKP strains was established, and this phylogenetic tree showed that the initial strain A1 in our EICU also belonged to one branch of another hospital (Figure 3B).
Focused on clade 3 composed of 9 strains in our EICU, only 8 SNPs’ differences were found among them, indicating that they had the same source. It should be noted that there were only 6 beds in our EICU, and one bed had not been occupied by the patient during outbreak period. Before patient A was admitted, CRKP was not detected in all samples of other patients, including drug-resistant bacteria screening samples. Combined with the temporal and spatial epidemiological data in Figures 1 and 3C, patient B was at the center of the timeline and the center of the ward, strains from patient B were also in the center of clade 3 in the phylogenetic tree. Based on epidemiological and SNPs’ differences data, the strains from patient B might come from patient A and spread to others directly (Figure 3C). Meanwhile, although patient E had no direct connections with patient B in time and space, the SNPs matrix in Figure 3A shows that strains from patient B and patient E had the highest homology, SNPs between them were almost 0, indicating the strain source of patient E was also patient B, not the other 3 patients.
Discussion
The spread of CRKP brought great challenges to public health,20–22 understanding the molecular characteristics of CRKP strains can help outbreak control. Traditional typing methods such as PFGE and MLST cannot accurately track the spread of CRKP strains due to insufficient resolution. In recent years, WGS-based SNP-calling has been used in CRKP outbreak tracking,23 some studies13,16,24 used it to depict the CRKP transmission map within hospitals. However, the CRKP transmission map between institutions still masked, making it hard for the prevention and control of CRKP outbreak. In this study, the genomic and epidemical data of two CRKP outbreaks were integrated by SNP-calling and epidemiological investigation, a hypermutable strain linked two outbreaks was identified and some outbreak control strategies were proposed.
Epidemiological data showed that before patient A’s admission, no CRKP was detected in our EICU. The first CRKP strain A1 was detected from the mid-stream urine upon patient A’s admission, then 9 CRKP strains were successively cultured from different specimen types of the other four patients, therefore it can be speculated that patient A was the initial patient causing this outbreak. But the SNPs analysis based on WGS revealed that there were about 360 SNPs’ differences between A1 and the other 9 outbreak strains. There might be several possibilities for this difference, first, patient A was not the index patient, second, patient A carried various K. pneumoniae mutants and A1 was not the real index strain, third, strain A1 was hypermutable. To clarify the real reason, relevant factors were analyzed, on the one hand, our EICU is a relatively independent environment with only 6 beds, doctors and nurses were also relatively independent, only caring for EICU patients. It was almost impossible that CRKP strains come from patients or staff outside the EICU. On the other hand, we had performed bacterial culture on sputum, blood and perianal specimens of patient A, and no CRKP was detected. Therefore, it can be concluded that A1 from patient A was the index strain and it was a hypermutable strain.
During the hospitalization of patient A, CRKP strain was detected in his urine 9 times, as the urinary tract is filled with various stress factors, such as mechanical shear stress, limitation of nutrients, iron and oxygen, host immune responses and antimicrobial therapy,25 these might be the reasons for A1 to become a hypermutable strain. Some studies reported that when spread into a new environment, the intraspecies shift may happen in CRKP to adapt to the environmental changes.26 CRKP may acquire enhanced virulence and this hypervirulent CRKP may cause severe and untreatable invasive infections in hospitals.27,28 In our study, plasmid analysis showed that compared with A1, other 9 strains lost three plasmids [IncFIB(pKPHS1), IncFII(pKP91), Col(pHAD28)], obtained one plasmid IncFII(pCRY) while some of them obtained IncFII(K), these differences might be caused by environmental selection pressure or plasmid incompatibility. The loss and acquisition of plasmids may cause the other 9 strains to carry more resistance genes and virulence genes such as aadA2b, aadA1, OqxB, OqxA, sul1, sul3, dfrA12, fosA, mef(B), tet(A), pilURV, iucABCD and vipA/tssB, these genes may make the outbreak strain more adaptable to environment and more harmful to patients. Although these outbreak strains in our study were only virulent, not hypervirulent (harboring mediated genes related to host cell adhesion (fim, mrk), iron-transport-related genes (aerobactin, ent, fep, iro, ybt, irp), efflux pump (acrAB) and regulation factors related genes (rcsAB), not harboring capsular polysaccharide production gene rmpA and wabG), they still led to the death of 2 patients because of blood infection. Based on these data, we proposed that in the early stage of the outbreak, rapid evolution of CRKP should be monitored and the emergence of hypervirulent CRKP should be alerted.
SNP-based phylogenetics analysis showed that the initial strain A1 in our EICU ward belonged to one branch of strains in another hospital, epidemiological data showed that since two hospitals were located in the adjacent area of the same city, patient A had visited two hospitals successively. All these data showed that the outbreak of our EICU in Mar 2018 may originate from the outbreak of another hospital in Apr 2017. Zimmerman et al found that carbapenem-resistant Enterobacteriaceae (CRE) patients from a previous hospitalization may carry CRE for almost one year and they may readmit to the hospital because of the same strain infection.29 In our study, patient A had visited many hospitals, in this process, he may carry colonized CRKP strain and then led to the outbreak of our EICU one year later after the outbreak of another hospital. Based on these data, we proposed that CRKP patients should be monitored for at least one year after they are discharged, for those with immune insufficiency, the monitoring time could be longer.
A recent CRKP transmission dynamics model30 showed that the admission of CRKP colonized patients, whether they have symptoms or not, impacted the endemic transmission in health-care units significantly. Spyridopoulou et al found that screening patients at admission can reduce the spread of pathogens within the hospital.31 In our study, CRKP was identified in the patient A’s urine on the day of his admission, proper measures including bedside isolation, contact precautions and hand hygiene had been taken and the outbreak was limited to 5 patients within 3 months. Our data showed that active surveillance of newly admitted patients attained maximum containment of CRKP infection in EICU.
Based on epidemiological and SNPs differences data, the strains from patient B came from patient A and spread to patient C, patient D and patient E. It is worth noting that, patients B and E did not have an overlap in time and space, but the genomic data of their strains were identical, which proves that the transmission between them may be indirect and may be mediated by devices or environment. Some studies showed that hospital devices such as ventilators and monitors, hospital environment including beds, beddings, saline stands and relevant objects in the immediate vicinity of the patients may also take part in the transmission of CRKP in an outbreak.32–34 Unfortunately, no CRKP strain was detected in samples of our equipment and environment, but the equipment or environmental pollution cannot be ruled out. These data showed that during the control of an outbreak, in addition to bedside isolation, contact precautions and hand hygiene, the environment and equipment hygiene are also nonnegligible.
In addition, in our study, all CRKP strains were sensitive to tigecycline and polymyxin B, and the antimicrobial resistance phenotype was consistent with the resistance genes based on WGS, and proper antibiotics were given to all patients and the outbreak ended in a relatively short period. A CRKP transmission dynamics model by WGS has also proved that antibiotic treatment efficacy was also an important factor for CRKP control.30 These data showed that effective antibiotic use may reduce the duration time of CRKP infection and colonization in EICU patients, which was important to control outbreak.
There were several limitations in our study. First, our study was only a retrospective study so it cannot provide real-time assistance to CRKP dissemination control. Second, the WGS data in our study were based on short-read sequencing, although we have analyzed the presence of plasmids, the specific information of MGEs was still unclear. Thus, experiments such as conjugation and transformation tests were needed to further explore the dissemination mechanisms of CRKP. Finally, CRKP strain was detected in patient A’s urine 9 times, although they showed the same characters in antimicrobial susceptibility testing and mCIM, they may have different WGS characters, future studies are needed to further analyze the WGS differences of these isolates.
Conclusion
By integrating the genomic and epidemiological data of two CRKP outbreaks, we tracked the source and the route of CRKP transmission in our EICU. Through analysis of the key links in the transmission, we proposed that monitoring the rapid evolution of CRKP at the early stage of outbreak, CRKP monitoring after patients are discharged, active surveillance of newly admitted patients, environmental hygiene and efficient antibiotic treatment may be the key for prevention and control of CRKP outbreak.
Data Sharing Statement
The WGS data of 10 isolates in our EICU were deposited in GenBank with BioProject accession number PRJNA847729. All scripts and data are available from the corresponding authors upon request.
Ethical Approval
This study was reviewed and approved by the Ethics Committee of Ruijin Hospital (full name: Ruijin Hospital Ethics Committee, Shanghai JiaoTong University School of Medicine) (ethics approval number: 2018015-1). Informed consent from patients was not required by the Ethics Committee, because there was no contact with patients and all data were deidentified. This study was conducted in accordance with the Declaration of Helsinki.
Acknowledgments
We thank researchers in Huashan hospital for sequencing their isolates and sharing their data online. This study is funded by the funding project of Shanghai Ruijin hospital north area (2018ZY06). All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this study.
References
1. Chang SL, Dela Cruz CS, Zhang D, Zhang D. Clinical epidemiology, risk factors, and control strategies of Klebsiella pneumoniae infection. Front Microbiol. 2021;12:750662. doi:10.3389/fmicb.2021.750662
2. de Oliveira Santos JV, da Costa Junior SD, de Fatima Ramos Dos Santos Medeiros SM, et al. Panorama of bacterial infections caused by epidemic resistant strains. Curr Microbiol. 2022;79(6):175. doi:10.1007/s00284-022-02875-9
3. Munoz-Price LS, Poirel L, Bonomo RA, et al. Clinical epidemiology of the global expansion of Klebsiella pneumoniae carbapenemases. Lancet Infect Dis. 2013;13(9):785–796. doi:10.1016/S1473-3099(13)70190-7
4. Yigit H, Queenan AM, Anderson GJ, et al. Novel carbapenem-hydrolyzing beta-lactamase, KPC-1, from a carbapenem-resistant strain of Klebsiella pneumoniae. Antimicrob Agents Chemother. 2001;45(4):1151–1161. doi:10.1128/AAC.45.4.1151-1161.2001
5. Wei ZQ, Du XX, Yu YS, Shen P, Chen YG, Li LJ. Plasmid-mediated KPC-2 in a Klebsiella pneumoniae isolate from China. Antimicrob Agents Chemother. 2007;51(2):763–765. doi:10.1128/AAC.01053-06
6. Fu P, Tang Y, Li G, Yu L, Wang Y, Jiang X. Pandemic spread of blaKPC-2 among Klebsiella pneumoniae ST11 in China is associated with horizontal transfer mediated by IncFII-like plasmids. Int J Antimicrob Agents. 2019;54(2):117–124. doi:10.1016/j.ijantimicag.2019.03.014
7. Qi Y, Wei Z, Ji S, Du X, Shen P, Yu Y. ST11, the dominant clone of KPC-producing Klebsiella pneumoniae in China. J Antimicrob Chemother. 2011;66(2):307–312. doi:10.1093/jac/dkq431
8. Zhang R, Liu L, Zhou H, et al. Nationwide surveillance of clinical carbapenem-resistant enterobacteriaceae (CRE) strains in China. EBioMedicine. 2017;19:98–106. doi:10.1016/j.ebiom.2017.04.032
9. Hu F, Zhu D, Wang F, Wang M. Current status and trends of antibacterial resistance in China. Clin Infect Dis. 2018;67(suppl_2):S128–S134. doi:10.1093/cid/ciy657
10. Chi X, Hu G, Xu H, et al. Genomic analysis of a KPC-2-producing Klebsiella pneumoniae ST11 outbreak from a teaching hospital in Shandong Province, China. Infect Drug Resist. 2019;12:2961–2969. doi:10.2147/IDR.S221788
11. Zhang M, Li J, Lu Y, et al. Expanding of ST11 carbapenemase-producing Klebsiella pneumoniae subclones in a Chinese Hospital, Shenzhen, China. Infect Drug Resist. 2021;14:1415–1422. doi:10.2147/IDR.S299478
12. Sui W, Zhou H, Du P, et al. Whole genome sequence revealed the fine transmission map of carbapenem-resistant Klebsiella pneumonia isolates within a nosocomial outbreak. Antimicrob Resist Infect Control. 2018;7(1):70. doi:10.1186/s13756-018-0363-8
13. Chen C, Zhang Y, Yu SL, et al. Tracking carbapenem-producing Klebsiella pneumoniae outbreak in an intensive care unit by whole genome sequencing. Front Cell Infect Microbiol. 2019;9:281. doi:10.3389/fcimb.2019.00281
14. Yang Y, Chen J, Lin D, Xu X, Cheng J, Sun C. Prevalence and drug resistance characteristics of carbapenem-resistant Enterobacteriaceae in Hangzhou, China. Front Med. 2018;12(2):182–188. doi:10.1007/s11684-017-0529-4
15. Liu J, Yu J, Chen F, et al. Emergence and establishment of KPC-2-producing ST11 Klebsiella pneumoniae in a general hospital in Shanghai, China. Eur J Clin Microbiol Infect Dis. 2018;37(2):293–299. doi:10.1007/s10096-017-3131-4
16. Jiang Y, Wei Z, Wang Y, Hua X, Feng Y, Yu Y. Tracking a hospital outbreak of KPC-producing ST11 Klebsiella pneumoniae with whole genome sequencing. Clin Microbiol Infect. 2015;21(11):1001–1007. doi:10.1016/j.cmi.2015.07.001
17. Hu H, Mao J, Chen Y, et al. Clinical and microbiological characteristics of community-onset carbapenem-resistant enterobacteriaceae isolates. Infect Drug Resist. 2020;13:3131–3143. doi:10.2147/IDR.S260804
18. Qiu Y, Lin D, Xu Y, et al. Invasive Klebsiella pneumoniae infections in community-settings and healthcare settings. Infect Drug Resist. 2021;14:2647–2656. doi:10.2147/IDR.S315871
19. Sfeir MM, Satlin MJ, Fauntleroy KA, Jenkins SG, Westblade LF. Blood-modified carbapenem inactivation method: a phenotypic method for detecting carbapenemase-producing enterobacteriaceae directly from positive blood culture broths. J Clin Microbiol. 2020;58(2). doi:10.1128/JCM.01377-19
20. Agyeman AA, Bergen PJ, Rao GG, Nation RL, Landersdorfer CB. A systematic review and meta-analysis of treatment outcomes following antibiotic therapy among patients with carbapenem-resistant Klebsiella pneumoniae infections. Int J Antimicrob Agents. 2020;55(1):105833. doi:10.1016/j.ijantimicag.2019.10.014
21. Liao W, Liu Y, Zhang W. Virulence evolution, molecular mechanisms of resistance and prevalence of ST11 carbapenem-resistant Klebsiella pneumoniae in China: a review over the last 10 years. J Glob Antimicrob Resist. 2020;23:174–180. doi:10.1016/j.jgar.2020.09.004
22. Liu Y, Zhang X, Cai L, Zong Z. Enhanced survival of ST-11 carbapenem-resistant Klebsiella pneumoniae in the intensive care unit. Infect Control Hosp Epidemiol. 2020;41(6):740–742. doi:10.1017/ice.2020.68
23. Simar SR, Hanson BM, Arias CA. Techniques in bacterial strain typing: past, present, and future. Curr Opin Infect Dis. 2021;34(4):339–345. doi:10.1097/QCO.0000000000000743
24. Snitkin ES, Zelazny AM, Thomas PJ, et al. Tracking a hospital outbreak of carbapenem-resistant Klebsiella pneumoniae with whole-genome sequencing. Sci Transl Med. 2012;4(148):148ra116. doi:10.1126/scitranslmed.3004129
25. Tielen P, Rosin N, Meyer AK, et al. Regulatory and metabolic networks for the adaptation of Pseudomonas aeruginosa biofilms to urinary tract-like conditions. PLoS One. 2013;8(8):e71845. doi:10.1371/journal.pone.0071845
26. Zhao D, Shi Q, Hu D, et al. The emergence of novel sequence type strains reveals an evolutionary process of intraspecies clone shifting in ICU-spreading carbapenem-resistant Klebsiella pneumoniae. Front Microbiol. 2021;12:691406. doi:10.3389/fmicb.2021.691406
27. Chen L, Kreiswirth BN. Convergence of carbapenem-resistance and hypervirulence in Klebsiella pneumoniae. Lancet Infect Dis. 2018;18(1):2–3. doi:10.1016/S1473-3099(17)30517-0
28. Gu D, Dong N, Zheng Z, et al. A fatal outbreak of ST11 carbapenem-resistant hypervirulent Klebsiella pneumoniae in a Chinese hospital: a molecular epidemiological study. Lancet Infect Dis. 2018;18(1):37–46. doi:10.1016/S1473-3099(17)30489-9
29. Zimmerman FS, Assous MV, Bdolah-Abram T, Lachish T, Yinnon AM, Wiener-Well Y. Duration of carriage of carbapenem-resistant Enterobacteriaceae following hospital discharge. Am J Infect Control. 2013;41(3):190–194. doi:10.1016/j.ajic.2012.09.020
30. Changruenngam S, Modchang C, Bicout DJ. Modelling of the transmission dynamics of carbapenem-resistant Klebsiella pneumoniae in hospitals and design of control strategies. Sci Rep. 2022;12(1):3805. doi:10.1038/s41598-022-07728-w
31. Spyridopoulou K, Psichogiou M, Sypsa V, et al. Containing Carbapenemase-producing Klebsiella pneumoniae in an endemic setting. Antimicrob Resist Infect Control. 2020;9(1):102. doi:10.1186/s13756-020-00766-x
32. Sharma S, Banerjee T, Kumar A, Yadav G, Basu S. Extensive outbreak of colistin resistant, carbapenemase (blaOXA-48, blaNDM) producing Klebsiella pneumoniae in a large tertiary care hospital, India. Antimicrob Resist Infect Control. 2022;11(1):1. doi:10.1186/s13756-021-01048-w
33. Shao C, Jin Y, Wang W, Jiang M, Zhao S. An outbreak of carbapenem-resistant Klebsiella pneumoniae of K57 capsular serotype in an emergency intensive care unit of a teaching hospital in China. Front Public Health. 2021;9:724212. doi:10.3389/fpubh.2021.724212
34. Wei L, Wu L, Wen H, et al. Spread of carbapenem-resistant Klebsiella pneumoniae in an intensive care unit: a whole-genome sequence-based prospective observational study. Microbiol Spectr. 2021;9(1):e0005821. doi:10.1128/Spectrum.00058-21
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.