Back to Journals » International Journal of Nanomedicine » Volume 15
Toxicological and Anti-Rheumatic Potential of Trachyspermum ammi Derived Biogenic Selenium Nanoparticles in Arthritic Balb/c Mice
Authors Qamar N, John P , Bhatti A
Received 25 December 2019
Accepted for publication 23 February 2020
Published 15 May 2020 Volume 2020:15 Pages 3497—3509
DOI https://doi.org/10.2147/IJN.S243718
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Anderson Oliveira Lobo
Naila Qamar, Peter John, Attya Bhatti
Department of Healthcare Biotechnology, Atta-ur-Rahman School of Applied Biosciences, National University of Sciences and Technology (NUST), Islamabad, Pakistan
Correspondence: Peter John
Department of Healthcare Biotechnology, Atta-ur-Rahman School of Applied Biosciences, National University of Sciences and Technology (NUST), Sector H-12, Islamabad, Pakistan
Tel +92 51-90856151
Email [email protected]
Purpose: The existing treatment modalities for rheumatoid arthritis are less effective and safe, therefore it is essential to develop new treatments that particularly target the inflamed joints with decreased off-target side-effects. The current study proposes a nanoparticle-based therapeutic approach to target the anti-oxidant defense system of arthritic Balb/c mice.
Methods: Biogenic selenium nanoparticles (SeNPs) were synthesized by using Trachyspermum ammi seed extract and were evaluated for their toxicological, as well as their therapeutic potential in collagen-induced arthritic mice.
Results: The tested doses of SeNPs had no significant toxic effects on liver, kidney, spleen, and serum biochemical parameters in comparison to healthy mice. The SeNPs treatment reduced the disease severity, as demonstrated by decreased paw edema along with reduced lymphocytic cellular infiltration in the histopathological findings. SeNPs also revealed dose-independent improvement in the redox state of inflamed synovium by significantly improving the activity of antioxidant enzymes in comparison to the arthritic controls.
Conclusion: It is therefore concluded that nano-selenium in combination with TAE extract showed enhanced therapeutic efficacy as compared to their individual effects.
Keywords: rheumatoid arthritis, collagen-induced arthritis, selenium nanoparticles, Trachyspermum ammi
Introduction
Rheumatoid arthritis (RA) is an autoimmune inflammatory disease characterized by synovial hyperplasia leading to the erosion of bones and cartilage, resulting in joint dysfunction and increased mortality rates.1,3 RA affects about 0.5–1% of the world’s population4,7 and its prevalence is slightly higher in Western countries as compared to Asian populations. In Pakistan the prevalence of RA is greater in northern areas in comparison to other regions.5 RA is more common in females than males, with a ratio of 3:1.2,8 The current treatment of RA with NSAIDs and DMARDs produces symptomatic relief and is restricted in use because of the off-targeted adverse effects.3
Selenium is a cofactor of enzymes thioredoxin and glutathione peroxidase, which are the key components of the antioxidant defense system of the cells.9 Selenium is an important trace element of the diet essential for growth and health conservation of humans, and its deficiency causes severe health effects, i.e., Keshan Disease, and it may also lead to increased risk of cancer and cardiac diseases. In recent studies, selenium has had remarkable attention due to its central role in selenoenzymes activation for defense against oxidative stress generated by reactive nitrogen species (RNS) and reactive oxygen species (ROS).7,10 It is reported that ROS damage the activity of the anti-oxidant defense system leading to oxidative stress as well as to lipid peroxidation, prominent clinical signs of arthritis.3 The production and application of SeNPs evolved remarkable attention due to the number of advantages, such as biocompatibility, chemical stability, and low toxicity.10 More attention should be paid to biological methods to synthesize SeNPs alternate to physical and chemical methods, as green synthesis is more safe, economical, and eco-friendly.11
Trachyspermum ammi, commonly known as “Ajwain”, is a member of the family Apiaceae.12,13 Phytochemical analysis revealed that T. ammi contains various constituents such as glycosides, carbohydrates, phenolic compounds, saponins, protein, fiber, fat, volatile oil (i.e., γ-terpinene, thymol, α- and β-pipene, and para-cymene), and minerals such as phosphorus, calcium, nicotinic acid, and iron. Due to the presence of thymol, a biologically active compound, T. ammi exhibits anti-inflammatory, anti-oxidant, immunomodulatory,12 anti-microbial, anti-filarial, anthelmintic,14 and gastro-protective activities.12 In spite of its extensive use in traditional medicine for the treatment of inflammatory and pain conditions, very limited data has been reported about its anti-inflammatory and anti-oxidant properties.3
Various studies have been conducted to find out the anti-rheumatic and anti-oxidant potential of T. ammi seed extract and SeNPs in arthritic models individually,3,15 but their combined efficacy has not yet been evaluated, to the best of our knowledge. Therefore, the present study aims to combine the anti-inflammatory and anti-oxidant potential of T. ammi seed extract and nano-Se by utilizing T. ammi derived SeNPs to treat RA. This approach would not only target the characteristic inflammatory pathways of RA, but also assist in restoring selenoproteins dependent oxidative balance of the body.
Methods
Preparation of Trachyspermum ammi Seed Extract
The seeds purchased from the local market of Haripur were verified with accession number 38452 as Trachyspermum ammi seeds by the Plant Genetic Research Institute of the National Agricultural Research Center (NARC), Islamabad, Pakistan. The extract of T. ammi seeds was prepared by soaking 12 g powdered seeds in 200 mL deionized water and kept at 40°C in a shaking incubator overnight in a dark environment at 2,000 rpm. After 24 hours the mixture was centrifuged at 6,000 rpm for 20 minutes at 4°C and then filtered by using whattsman filter paper with a 0.45 μm pore size.
Biosynthesis of SeNPs by Using T. ammi Seed Extract
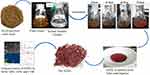
For the preparation of nanoparticle reaction mixture, 50 mL of T. ammi seed extract was added drop wise to 100 mL of Na2SeO3 (10 mM) solution and the mixture was then placed in a shaking incubator at 2,000 rpm for 72 hours at 40°C under dark conditions. The initial confirmation of SeNPs biosynthesis was carried out by noticing the color change of the reaction mixture from yellowish brown to brick red. After biogenic synthesis, the SeNPs were purified by centrifugation of reaction mixture at 7,500 rpm for 30 minutes. The supernatant was discarded and the red pellet at the bottom of the tube was washed twice with deionized water for 20 minutes at 6,000 rpm. The purified hard pellet was collected in a china dish and allowed to dry at 37°C in an incubator overnight (Figure 1).
Characterization of SeNPs
The characteristic analysis of these nanoparticles was performed by using multiple techniques. UV-Visible Spectroscopy of the T. ammi seed extract and SeNPs mixture was performed by using a spectrophotometer (A & E Lab, Guangzhou, Guangdong, China). Fourier Transform Infrared-Spectroscopy (FTIR) was performed to identify the functional groups present in the wavelength range of 500–4,500 cm−1 by using a FTIR spectrophotometer (Perklin-Elmer Spectrum-109 100 FTIR Spectrophotometer, Waltham, MA, USA). The crystallinity of SeNPs was determined by X-ray Diffractometry (XRD) by using an X-ray Diffractometer (D8 ADVANCE BRUKER, AXS, Germany) operated at 40 KV with constant current of 30 mA and the source of radiation was Cu K alpha over the scanning range of Bragg angle 10–80°. A Scanning Electron Microscope (SEM) (TESCAN VEGA3 tungsten thermionic emission, model: 51 – ADD0007 sensor 51-1385-046, Kohoutovice, Czech Republic) was used to determine the shape and size of the nanoparticles by coating the sample slide with gold for mounting, and the images were obtained with the accelerating voltage of 29 KV. Energy Dispersive X-ray spectroscopy (EDX) analysis was performed to determine the elemental composition of these nanoparticles by using an EDX instrument (Oxford X-act, Tubney Woods Abingdon, Oxfordshire, UK) attached with SEM at the accelerating voltage of 20 KeV.
Animal Acquisition and Acclimatization
Female Balb/c mice of 8–12 weeks age, weighing 30–35 grams were obtained from Animal House facility ASAB. All mice included in the experimental groups were housed at the Laboratory of Animal House, ASAB. To support the circadian cycles a daylight-to-darkness ratio of 2:1 was maintained. All the mice were fed with standard feed and UV-radiation treated water throughout the experiment.
Toxicity Evaluation of Biogenic SeNPs
To check their biosafety profile, the toxicity of these SeNPs was evaluated in 25 mice, comprised of five groups (one healthy control and four experimental groups). The experimental groups were given SeNPs in their feed at doses of 2.5 mg/kg, 5 mg/kg, 10 mg/kg, and 20mg/kg for 14 consecutive days, and the control group was given normal feed throughout this period. The weight of each mouse was recorded on day 0, 7, and 14 to observe any change in weight. After 14 days of treatment followed by 24-hours fasting the mice were anesthetized by chloroform to collect the blood through transthoracic cardiocentesis and serum was separated. The liver function tests [Alanine amino-transferase (ALT), Alkaline Phosphatase (ALP), Total Bilirubin] and Renal function tests (Urea and Creatinine) were performed by using Roche Diagnostic kits (Mannheim, Germany) on the serum samples of mice. For histopathological studies, liver, kidney, and spleen specimens were collected and observed for any change at cellular level due to the SeNPs administration. The spleen indexing was done as previously defined.16,17
Collagen Type-II Arthritic Model Construction
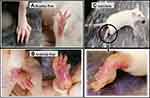
Complete Freund’s adjuvant (Sigma-Aldrich, St. Louis, MO, USA) and Bovine Type-II Collagen (Worthington Biochemical corporation, Lakewood, NJ, USA) was used for induction of RA. Type-II collagen was dissolved in acetic acid (0.1 M) prepared in Hartmann solution (0.89%) and was mixed in Complete Freund’s adjuvant at the ratio of 1:1 followed by vortex for 2–3 minutes to form an immunization mixture. Then BSA (1 mg/mL) dissolved in Hartmann solution (0.89%) was added to the immunization mixture at a ratio of 2:1. The prepared mixture (0.2 mL) was injected in the sub-dermal region of the tail cautiously without puncturing the dorsal or ventral coccygeal veins on day 0, 7, and 14. On day 21 and 28 the booster doses of Complete Freund’s adjuvant were administered in the hind paw of mice without puncturing any vein. The degree of inflammation in the paw of the mice was recorded with the help of (GmBH) vernier caliper before and after each dose in the presence of an observer who had no information on the groups. The quantification of arthritic severity was done by arthritic clinical scores from 0–4 (Table 1).18 Only grade 3 and 4 scoring mice (Figures 2 and 3) were included in the therapeutic evaluation.
 |
Table 1 Grading Criteria for Arthritis Induction |
Therapeutic Evaluation of Biogenic SeNPs in Arthritic Mice
The evaluation of therapeutic efficacy of SeNPs began one week after the second booster dose. Thirty mice comprised of five groups; one positive (arthritic) and one negative control (healthy), standard drug Leflunomide (10 mg/kg), and two doses (5 and 10 mg/kg) of SeNPs that were proved to be non-toxic by safety study were administered to arthritic mice through feed for 14 consecutive days. The control groups were given normal feed throughout this period. The weight and hind paw volumes (edema) of each mice was recorded on day 0, 7, and 14 of the treatment. After the completion of treatment, the samples (blood, spleen, and hind paws) were collected by the aforementioned procedure following the 24 hours fasting. Clinical chemistry analysis of serum, spleen indexing, and histopathological analysis of paw tissues were performed. The in-vivo free radical scavenging activity of SeNPs in a serum sample of treated mice was evaluated through DPPH (DPPH 95% Alfa Aesar materials, Ward Hill, MA, USA) assay19 with slight modifications. The serum sample (25 μL) was mixed with 0.1 mM DPPH (975 μL) solution and incubated for 30 minutes at 37°C. The absorbance was measured at 517 nm by using a spectrophotometer (A & E Lab, Guangzhou, 180 Guangdong, China) and a change in the color of DPPH from violet to yellow indicated their radical scavenging activity.
Catalase activity was evaluated20 in the liver tissues to further evaluate the anti-oxidant potential of biogenic SeNPs. The lysate was prepared by homogenization of 0.5 g of liver tissue in 2.5 mL of 0.01 M cold phosphate buffer (pH 7.0). The homogenization mixture was then centrifuged at 8,000 g for 10 minutes at 4°C and the supernatant was used for the catalase estimation. A total of 1.5 mL reaction mixture was prepared by adding 1 mL of phosphate buffer (pH 7.0), 0.4 mL of H2O2, and 0.1 mL of the tissue lysate, and incubated at room temperature for 1 minute. Then 2 mL of dichromate-acetic acid reagent (1:3 of 5% potassium dichromate and glacial acetic acid) was added to the mixture to stop the reaction and incubated for 10 minutes at 37°C. The absorbance was read at the wavelength of 620 nm by using a spectrophotometer (A & E Lab).
Statistical Analysis
The statistical analysis was done by using IBM SPSS version 21 and GraphPad Prism 5 and results were reported as mean±SD. The variance analysis was done by applying one-way and two-way ANOVA, followed by Turkey’s test and Bonferroni’s test. The variation was considered statistically significant at P<0.05.
Results and Discussion
Biosynthesis and Characterization of SeNPs
The present study was focused on the biosynthesis and characterization of SeNPs prior to further experimentations. The initial confirmation of nanoparticle synthesis was done by the color change of the reaction mixture from yellowish brown to brick red (Figure 1) within 72 hours of reaction, the characteristic feature of SeNPs as previously described.21 It was further confirmed by UV-visible spectra due to the maximum absorption peak of SeNPs at 275 nm22 (Figure 4B) that was not presented by the spectra of seed extract (Figure 4A). The FTIR was performed to identify the functional groups present in the Trachyspermum ammi plant extract and its derived SeNPs. The FTIR spectral bands of plant extract at 3,430 cm−1, 1,631.88 cm−1, and 1,046 cm−1 shifted to 3,416.68 cm−1, 1,630.55 cm−1, and 1,051.63 cm−1 in the spectra of SeNPs, indicating the involvement of O-H, N-H, and C-F groups in the biosynthesis of SeNPs (Figure 5), and was in agreement with the spectra previously reported.21 The crystallinity of SeNPs was confirmed by XRD spectra with the diffraction peaks at 2-theta values of 23°, 30°, 41°, 43°, 45°, 51°, 55°, 61°, and 65° that are indexed (101), (110), (102), (111), (201), (103), (210) planes of selenium (Figure 6). The obtained XRD spectra of biogenic SeNPS was in agreement with the JCPDS File No. 00-042-1425 and similar types of spectra have also been reported.21,,23 The SEM analysis determined the spherical to irregular morphology of SeNPs and their average particle size measured from SEM image, which turned out to be 43.28 nm (Figure 7). EDX spectra confirms the presence of Se in the sample by its specific peaks at 1.37, 11.22, and 12.49 KeV (Figure 8) as discussed in previous studies,24,25 while the separate peaks of oxygen in the spectrum confirms that nanoparticles are not present in its oxide form, they are pure selenium nanoparticles.
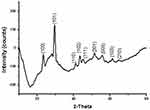 |
Figure 6 X-Ray diffraction pattern of T. ammi derived biogenic SeNPs. |
 |
Figure 7 Scanning electron microscope images of T.ammi derived biogenic SeNPs at 2 μm, 5 μm, and 500 nm representing spherical to irregular morphology of SeNPs. |
 |
Figure 8 Graph of energy dispersive X-ray spectroscopy representing the number of counts on the y-axis and energy on the x-axis showing elemental composition of TAE derived biogenic SeNPs. |
Clinical Chemistry Parameters of Healthy Mice after SeNPs Administration
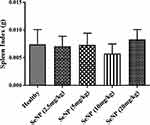
The toxicological potential of nanoparticles having smaller size can be determined by their accumulation in various tissues because larger size particles are efficiently cleared by macrophages.26 In the present study the in-vivo toxicity of SeNPs was assessed by a method previously described.27 The body weight (Figure 9) and spleen indices (Figure 10) of all the treated groups were found to be normal as compared to the healthy controls. According to the literature, the liver is the main organ in animals effected by selenium toxicity, damaging its physical integrity, and this is measured by elevated levels of liver function associated enzymes such as ALT, AST, ALP,27 and serum bilirubin.28 Similarly for kidney dysfunction RFTs including serum urea and creatinine was performed.29 Hence, in this study LFTs and RFTs were performed and no significant differences were found in serum biochemical parameters in SeNPs-administered healthy mice and our results are consistent with previous studies.29,30 In the present study the level of urea was found to be significantly higher only in the group receiving 10 mg/kg SeNPs (Table 2) which may be due to altered membrane penetrability prompted by SeNPs.31
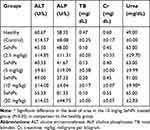 |
Table 2 Serum Biochemical Parameters of SeNPs-Administered Healthy Mice (Toxicity Evaluation) |
 |
Figure 9 Weight record of SeNPs-administered healthy mice at day 0, 7, and 14 of toxicity evaluation. |
Histopathological Analysis of SeNPs Administered Healthy Mice
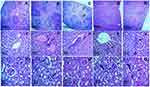
To evaluate the toxicity of biogenic SeNPs at tissue level the histopathological analysis of the liver, kidney, and spleen was done and no significant signs of toxicity were observed in the spleen (Figure 11A–E). However, in the liver tissue occasional mononuclear lymphocytic cellular infiltration was observed (Figure 11F–J), as previously reported,29 but mild cellular influx was found in the glomerular surroundings at a dose rate of 10 and 20 mg/kg (Figure 11K–O) that is in line with high serum urea level.
Paw Edema and Swelling of SeNP Treated Mice
Due to anti-oxidant potential, nano-selenium can be used as anti-oxidant and chemo-therapeutic agents with a low risk of toxicity.26,32 At present, the anti-oxidant and anti-arthritic activity of TAE mediated SeNPs was evaluated by administration of two doses of SeNPs (5 and 10 mg/kg) along with the 10 mg/kg dose of standard drug Leflunomide to CIA mice through feed. The reduction in the inflammation and paw edema was evident of its anti-arthritic activity throughout the treatment period (Figure 12) which is in correlation with the observation of Umar et al.3 After completion of treatment the reduction in the paw edema of the Leflunomide treated group was considerably higher (P<0.001) than the groups receiving SeNPs (P<0.05) (Figure 13). The weight of arthritic group started to decrease after one week, while other experimental groups restored the weight towards normal (Figure 14).
Clinical Chemistry Parameters of SeNPs Treated Mice
To assess the effect of biogenic SeNPs on the liver and kidney functioning, LFTs and RFTs were performed. All the parameters were found to be normal, while the level of ALP was found to be significantly higher only in the arthritic mice (Table 3), which is correlated with the disease severity in RA17 and was restored to its normal level among the SeNPs treated groups.
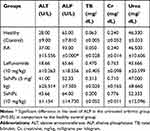 |
Table 3 Serum Biochemical Parameters of SeNPs and Leflunomide Treated Groups in Comparison to Untreated Arthritic Mice (Therapeutic Evaluation) |
Estimation of Anti-Oxidant Activity of SeNPs in Treated Mice
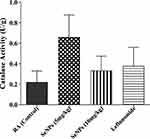
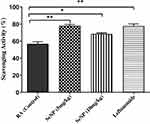
The catalase part of anti-oxidant defense system of the body responsible for catalyzing the reduction of hydrogen peroxides and its reduced level leads to increased production and accumulation of free radicals that alter the biological activities of cells.28,33 Administration of these biogenic SeNPs increased the activities of catalase in the liver to some extent in the present study (Figure 15) but a significant increase was observed in the case of TAE extract as reported by Umar et al.3 The free radical scavenging activity of these SeNPs was also evaluated to assess their in-vivo anti-oxidant potential, and the groups receiving 5 mg/kg SeNPs showed higher anti-oxidant activity than the 10 mg/kg SeNPs (Figure 16), similar to the results of Leflunomide drug.
Histopathological Analysis of SeNPs Treated Mice Paw
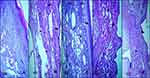
Histopathological examination was carried out to analyze the damage in the bone-surrounding areas of CIA paw tissue (Figure 17B) in comparison to healthy controls (Figure 17A). The microphotographic data also showed a positive effect of SeNPs treatment on reducing the arthritic severity by relieving the cellular infiltration and synovial hyperplasia along with restoration of normal bone morphology (Figure 17D and E) that is similar to the results of a study previously conducted by Malhotra et al,15 whereas the standard drug Leflunomide restored normal bone morphology but did not reduce the cellular influx (Figure 17C). Hence a lower dose (5 mg/kg) of biogenic SeNPs turns out to be more effective in treating RA by significantly enhancing the level of anti-oxidants and alleviating the joint damage in collagen-induced arthritis.
Conclusion
In the current study we have developed Trachyspermum ammi derived biogenic SeNPs. The findings of our study confirmed that these biogenic SeNPs exhibit anti-oxidant, anti-rheumatic and immunomodulatory properties. However further investigation is needed to elucidate the effect of these biogenic SeNPs in the mechanism of action on different enzymes and genes involved in inflammation and oxidative stress in RA.
Abbreviations
SeNPs, Selenium nanoparticles; RA, Rheumatoid Arthritis; NIH, National Institute of Health; CIA, Collagen Induced Arthritis; LFTs, Liver Function Tests; RFTs, Renal Function Tests; ALT, Alanine amino-transferase5; ALP, Alkaline Phosphatase; tb, Total Bilirubin; Cr, Creatinine; U/L, unit per liter; mg/dl, milligram per deciliter; SEM, Scanning Electron Microscopy; FTIR, Fourier Transform Infrared spectroscopy; DPPH, 2, 2-Diphenyl-1-Picrylhydrazyl; TAE, Trachyspermum ammi extract; XRD, X-Ray Diffractometer; UV, Ultra Violet.
Ethical Statement
The protocols and procedures used in this study were approved by the Institutional Review Board (IRB) Atta-ur-Rahman School of Applied Biosciences (ASAB). All the experimentation was carried out in accordance to the National Institute of Health’s (NIH) guidelines and recommendations for animal related research and analysis.
Author Contributions
All authors contributed to data analysis, drafting, or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors declare no conflict of interest.
References
1. Chandrasekar R, Chandrasekar S. Natural herbal treatment for rheumatoid arthritis-a review. Int J Pharm Sci Res. 2017;8(2):368.
2. Nogueira E, Gomes AC, Preto A, Cavaco-Paulo A. Folate-targeted nanoparticles for rheumatoid arthritis therapy. Nanomedicine. 2016;12(4):1113–1126. doi:10.1016/j.nano.2015.12.365
3. Umar S, Asif M, Sajad M, et al. Anti-inflammatory and antioxidant activity of Trachyspermum ammi seeds in collagen induced arthritis in rats. Int J Drug Dev Res. 2012;4:210–219.
4. Guo Q, Wang Y, Xu D, Nossent J, Pavlos NJ, Xu J. Rheumatoid arthritis: pathological mechanisms and modern pharmacologic therapies. Bone Res. 2018;6:1–14.
5. Alam SM, Kidwai AA, Jafri SR, et al. Epidemiology of rheumatoid arthritis in a tertiary care unit, Karachi, Pakistan. J Pak Med Assoc. 2011;13:87.
6. Firestein GS. Evolving concepts of rheumatoid arthritis. Nature. 2003;423(6937):356. doi:10.1038/nature01661
7. Dolati S, Sadreddini S, Rostamzadeh D, et al. Utilization of nanoparticle technology in rheumatoid arthritis treatment. Biomed Pharmacother. 2016;80:30–41.
8. Doan T, Massarotti E. Rheumatoid arthritis: an overview of new and emerging therapies. J Clin Pharmacol. 2005;45(7):751–762. doi:10.1177/0091270005277938
9. Wadhwani SA, Gorain M, Banerjee P, et al. Green synthesis of selenium nanoparticles using Acinetobacter sp. SW30: optimization, characterization and its anticancer activity in breast cancer cells. Int J Nanomedicine. 2017;12:6841. doi:10.2147/IJN.S139212
10. Skalickova S, Milosavljevic V, Cihalova K, Horky P, Richtera L, Adam V. Selenium nanoparticles as a nutritional supplement. Nutrition. 2017;33:83–90. doi:10.1016/j.nut.2016.05.001
11. Warda Majeed MZ, Bhatti A, John P. Therapeutic potential of selenium nanoparticles. J Nanomed Nanotechnol. 2018;9:1.
12. Bajpai VK, Agrawal P. Studies on phytochemicals, antioxidant, free radical scavenging and lipid peroxidation inhibitory effects of Trachyspermum ammi seeds. Indian J Pharm Educ Res. 2015;49:58–65. doi:10.5530/ijper.49.1.8
13. Saleem U, Riaz S, Ahmad B, Saleem M. Pharmacological screening of Trachyspermum ammi for antihyperlipidemic activity in Triton X-100 induced hyperlipidemia rat model. Pharmacognosy Res. 2017;9(Suppl 1):S34. doi:10.4103/pr.pr_37_17
14. Bairwa R, Rajawat BS, Sodha B. Trachyspermum ammi. Pharmacogn Rev. 2012;6(11):56. doi:10.4103/0973-7847.95871
15. Malhotra S, Welling M, Mantri S, Desai K. In vitro and in vivo antioxidant, cytotoxic, and anti‐chronic inflammatory arthritic effect of selenium nanoparticles. J Biomed Mater Res B Appl Biomater. 2016;104(5):993–1003. doi:10.1002/jbm.b.33448
16. Liu Y, Zhang L, Wu Y, et al. Therapeutic effects of TACI-Ig on collagen-induced arthritis by regulating T and B lymphocytes function in DBA/1 mice. Eur J Pharmacol. 2011;654(3):304–314.
17. Chandrakar BL, Harish CS, Chandrakar KS. Activity of serum alkaline phosphatase in rheumatoid arthritis for diagnosis and management. Int J Med Res Prof. 2017;3(3):281–284.
18. Tong T, Zhao W, Wu Y-Q, et al. Chicken type II collagen induced immune balance of main subtype of helper T cells in mesenteric lymph node lymphocytes in rats with collagen-induced arthritis. Inflamm Res. 2010;59(5):369–377.
19. Jang D-Y, Kim S-J, Jeong J-H, et al. Protective effects of sodium selenite and selenium nanoparticles against experimental colon carcinogenesis in mice. J Prev Vet Med. 2016;40(3):101–108.
20. Sinha AK. Colorimetric assay of catalase. Anal Biochem. 1972;47(2):389–394. doi:10.1016/0003-2697(72)90132-7
21. Jiang F, Cai W, Tan G. Facile synthesis and optical properties of small selenium nanocrystals and nanorods. Nanoscale Res Lett. 2017;12(1):401. doi:10.1186/s11671-017-2165-y
22. Anu K, Singaravelu G, Murugan K, Benelli G. Green-synthesis of selenium nanoparticles using garlic cloves (Allium sativum): biophysical characterization and cytotoxicity on vero cells. J Cluster Sci 2017;28(1):551–563. doi:10.1007/s10876-016-1123-7
23. Fresneda MAR, Martín JD, Bolívar JG, et al. Green synthesis and biotransformation of amorphous Se nanospheres to trigonal 1D Se nanostructures: impact on Se mobility within the concept of radioactive waste disposal. Environ Sci Nano. 2018;5(9):2103–2116.
24. Cremonini E, Zonaro E, Donini M, et al. Biogenic selenium nanoparticles: characterization, antimicrobial activity and effects on human dendritic cells and fibroblasts. Microb Biotechnol. 2016;9(6):758–771. doi:10.1111/1751-7915.12374
25. Sharma G, Sharma A, Bhavesh R, et al. Biomolecule-mediated synthesis of selenium nanoparticles using dried Vitis vinifera (raisin) extract. Molecules. 2014;19(3):2761–2770.
26. Kapur M, Soni K, Kohli K. Green synthesis of selenium nanoparticles from broccoli, characterization, application and toxicity. Adv Tech Biol Med. 2017;5:198. doi:10.4172/2379-1764.1000198
27. Shakibaie M, Shahverdi AR, Faramarzi MA, Hassanzadeh GR, Rahimi HR, Sabzevari O. Acute and subacute toxicity of novel biogenic selenium nanoparticles in mice. Pharm Biol. 2013;51(1):58–63. doi:10.3109/13880209.2012.710241
28. Nurrochmad A, Rahman Hakim A, Margono SA, Yuniarti N. Evaluation of hepatoprotective and antioxidant activity of hexagamavunon-1 against carbon tetrachloride-induced hepatic injury in rats. Int J Pharm Pharm Sci. 2010;2:3.
29. Keyhani A, Mahmoudvand H, Shakibaie M, et al. Histopathological and toxicological study of selenium nanoparticles in BALB/C Mice. Entomol Appl Sci Lett. 2018;5(1):31–35.
30. Hadrup N, Loeschner K, Skov K, et al. Effects of 14-day oral low dose selenium nanoparticles and selenite in rat—as determined by metabolite pattern determination. PeerJ. 2016;4:e2601.
31. Jagatheesh K, Arumugam V, Elangovan N, PavanKumar PJIJCPS. Evaluation of the anti‑tumor and antioxidant activity of Amorphophallus Paeonifolius on DMBA induced mammary carcinoma. Int J Pharm Chem Biol Sci. 2010;1(2):40–50.
32. Kojouri GA, Sadeghian S, Mohebbi A, Dezfouli M. The effects of oral consumption of selenium nanoparticles on chemotactic and respiratory burst activities of neutrophils in comparison with sodium selenite in sheep. Biol Trace Elem Res. 2012;146(2):160–166.
33. Sajeeth C, Manna P, Manavalan R. Antioxidant activity of polyherbal formulation on streptozotocin induced diabetes in experimental animals. Der Pharmacia Sinica. 2011;2(2):220–226.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.