Back to Journals » Journal of Pain Research » Volume 12
Toward more focused multimodal and multidisciplinary approaches for pain management in Parkinson’s disease
Authors Cuomo A, Crispo A, Truini A, Natoli S , Zanetti O , Barone P, Cascella M
Received 21 March 2019
Accepted for publication 2 July 2019
Published 22 July 2019 Volume 2019:12 Pages 2201—2209
DOI https://doi.org/10.2147/JPR.S209616
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Michael A Ueberall
Arturo Cuomo,1 Anna Crispo,2 Andrea Truini,3 Silvia Natoli,4 Orazio Zanetti,5 Paolo Barone,6 Marco Cascella1
1Division of Anesthesia and Pain Medicine, Istituto Nazionale Tumori, IRCCS Fondazione G. Pascale, Naples, Italy; 2S.C. Epidemiologia e Biostatistica, Istituto Nazionale Tumori, IRCCS Fondazione G. Pascale, Naples, Italy; 3Department of Human Neuroscience, University Sapienza, Rome, Italy; 4Department of Clinical Science and Translational Medicine, Tor Vergata University of Rome, Rome, Italy; 5U.O. Alzheimer, IRCCS Centro San Giovanni di Dio-Fatebenefratelli, Brescia, Italy; 6Center for Neurodegenerative Disease-CEMAND, University of Salerno, Fisciano, Italy
Abstract: In Parkinson’s disease (PD), pain represents a significant issue in terms of prevalence, clinical features, and treatment. Painful manifestations not strictly related to the disease are often amplified by the motor dysfunction. On the other hand, typical pain problems may specifically concern this vulnerable population. In turn, pain may have a deep impact on patients’ health-related quality of life. However, pain treatment in PD remains an unmet need as only about half of patients with pain use analgesics and pain is often managed by simply increasing doses of PD medications. In this complex scenario, pain treatments should follow multimodal approaches through a careful combination of pharmacological agents with non-pharmacological strategies, depending on the type of pain and the clinical context. A multidisciplinary approach involving medical specialists from different disciplines could be a winning strategy to address the issue. This work is aimed to provide practical suggestions useful for different types of clinicians and care professionals for pain management in this vulnerable population.
Keywords: Parkinson disease, pain management, pain assessment, opioids, acetaminophen, non-steroidal anti-inflammatory agents
Introduction
Parkinson’s disease (PD) is a neurodegenerative disorder associated with substantial morbidity and mortality and featuring progressive disabling motor and cognitive impairment. It has been well recognized that among the clinical features of the disease, pain represents a non-motor symptom (NMS) of great importance.1 There is convergent evidence that pain affects a high proportion of PD patients and painful manifestations may impair their daily activities and health-related quality of life (HRQoL). Again, it is important to note that pain and other NMSs may affect HRQoL with a greater impact than the motor symptoms (MSs).2 In the last decade, the perception of this problem has increased because of the alarming data on pain prevalence in this population, which was estimated to be up to 85%.3 Because following Alzheimer’s disease PD represents the second most common neurodegenerative disorder with a prevalence of approximately 1% of the population above 60 years,4 Parkinson-related pain (PRP) is an issue of social relevance that requires high health care costs. Furthermore, serious clinical implications have been highlighted as pain seems to increase as parkinsonian symptoms worsen,5 whereas painful manifestations could also anticipate the full expression of MSs of the disease.6 Despite these data, treatment of PRP remains an unmet need because it has been found that compared to the general population PD patients receive less prescription of analgesics.7 Furthermore, only about half of the patients with pain use analgesics,5 and pain is often managed by simply increasing doses of PD medications. In turn, this huge clinical and social problem requires the identification of effective solutions.
The topic seems to be particularly complex due to difficulties in pain assessment, the multiplicity of clinical features, and the issue concerning the choice of the more appropriate therapeutic strategy. In the lack of guidelines for PRP treatment, the choice of the more congruous analgesic agent and the fair of potential interference with antiparkinsonian agents must be carefully addressed. Moreover, the issue of opioids use in this non-cancer setting, non-pharmacological approaches as well as the role of the general practitioner (GP) and other health professionals such as neurologists, pain therapists, physical therapists, are multiple aspects of a significant topic that must be better elucidated.
This manuscript was written by a multidisciplinary team for different purposes. An overview on methods used for pain assessment in this vulnerable population is offered. Epidemiological data from the literature are also discussed, underlying limitations in study designs. Another objective was to provide practical suggestions useful for different types of clinicians and care professionals involved in therapeutic paths of PD patients suffering from pain.
The issue of pain assessment
Usually, clinical assessment predominantly deals with the quality and severity of pain. However, in patients with PD the clinical assessment should necessarily evaluate a wide range of categories such as those exploring the disabling effects of painful manifestations as well as their impact on psychological well-being. For instance, mood evaluation of patients is crucial, and depression and anxiety require specific treatment options, because in PD patients there is a strong relationship between depression, chronic pain, and worsening of HRQoL.8 Moreover, the symptom pain is underreported and often not routinely assessed in clinical consultations.9 Concerning the pain assessment tool/strategies used in previous investigations on the topic, very different methodologies such as the Brief Pain Inventory (BPI) and the Pain-O-Meter have been adopted.3,10–12 However, these tools are not specific for PD and have been developed with the purpose of measuring pain in different clinical settings. The NonMotor Symptom Questionnaire (NMS-Quest) is a 30-item tool focused on 30 NMSs associated with PD, including pain. The NMS-Quest is a yes/no styled questionnaire useful to detect the presence, or not, of NMSs, although not able to perform their characterization.13 The recent King’s PD Pain Scale (KPPS) represents the first pain tool designed for PD patients and is aimed at assessing the presence of pain in clinical situations frequently relevant in this setting.14 It is easy to administer and approximately requires 10–15 mins to be completed. The scale encompasses 14 questions which are scored on severity and frequency of pain. In addition, seven domains provide information on different types of pain. More recently, it has been proposed a patient-completed tool which could be complementary to the KPPS. The King’s College PD Pain Questionnaire (KPPQ) is a 14-item questionnaire composed of “yes” or “no” questions designed for assessing whether, or not, a specific kind of pain is present. In particular, each question of the KPPQ corresponds to one of the items of the KPPS.15 According to Martinez-Martin et al,15 the KPPQ could be used as a screening tool when the patient answered “yes” to the pain-related item – “Unexplained pains (not due to known conditions such as arthritis)” – in the NMS-Quest. In addition, KPPS can be helpful especially to better discriminate and characterize pain.
Epidemiology of pain in PD
While there is a convergent evidence that pain affects a high proportion of PD patients with consequences on daily activities and interference on HRQoL,2,8,15 few studies evaluated the frequency of pain according to epidemiological quality criteria. Broen et al systematically reviewed the prevalence of pain in PD literature from 1996 to 2011 using a modified Quality Assessment of Diagnostic Accuracy Studiestool,3 an instrument developed for judging prevalence studies on their methodological quality.16 The authors found 18 articles, of which only 8 met the methodological criteria. A part from the Broen’s investigation, other studies have been conducted for evaluating pain prevalence in PD (Table 1).5,10–13,17–20 Pain prevalence was estimated to be between 40% and 85%, with variability due to differences in 1) study designs, 1) pain assessment methodologies, 3) type of pain explored, and 4) stages of the disease.
 |
Table 1 Study results pain I Parkinson’s disease |
With regard to the study design, major criticisms are related to the small number of patients,11,12 the use of normative data for country rather than control subjects,10 the lack of information about the modality of consecutive patients’ recruitment (7 studies over 10). With regard to the assessment tool, very different methodologies have been applied, ranging from validated questionnaire for PD,14 general screening pain questionnaire such the BPI to the simple positive answer to one item of the Health-Related Quality of Life scale SF-36. One study explored only the shoulder pain,12 two articles focused on back pain while the majority of paper evaluated the occurrence of all pain in PD patients.
Clinical features
Although PD patients may report different types of pain,2,21 it is difficult to refer to a precise clinical classification. These patients, indeed, can experience multiple types of pain with different features. The Ford’s approach recognizes five pain categories, including musculoskeletal, radicular/neuropathic, dystonic, central (or primary) pain, and akathisia.22 While the first four types are well-recognized PRP categories, akathisia, which is the subjective inner restlessness and feeling to need to move, may be not necessarily associated with pain and often resembles characteristics of musculoskeletal pain. A special issue concerns the Legs Syndrome/Willis-Ekbom Disease (RLS/WED), a common sleep-related movement disorder that can be associated with PD. There is doubt if it represents an early manifestation (and a risk factor) for the disease or the consequence of the dopaminergic therapy.23 Nevertheless, the PD’s therapy falls out of the scope of this manuscript.
Musculoskeletal pain is the most frequently reported type of pain.22 It ranges from 10.6% to 69.8% and is usually described as aching and cramping in muscles or articular/periarticular locations in different body regions.24,25 Concerning its pathophysiology, musculoskeletal pain is the consequence of parkinsonian rigidity, immobility, and postural abnormalities. Moreover, the motor impairment may exacerbate joint pain from arthritis or other disorders. Among musculoskeletal painful conditions, shoulder pain of variable severity has been reported in patients with PD.12 It is an under-recognized pre-motor PD symptom which exposes the patient to surgical procedures that often result in poor improvements, while it should be an important warning for the disease.
The second most frequent type of PRP is the radicular/neuropathic pain which ranges from 3.2% to 30%.26 The radicular involvement is mostly induced by the abnormal posture and altered spine curvature in PD which cause compression and irritation of the sensory root, or dorsal root ganglion of a spinal nerve. Nerve impairment in the course of PD is a phenomenon of paramount importance which must be better investigated. Probably, it can be recognized as subclinical finding. Tremor and rigidity, indeed, impair neuronal transmission and contribute to entrapment neuropathy. In a recent study, Yardimci et al.27 demonstrated, by using electrodiagnostic criteria, that median and ulnar nerve demyelination may occur prior to the development of severe deformities in the extremities. Regardless the cause, neuropathic pain is generally described as shooting or burning, radiates directly along the course of a spinal nerve root and may be associated with sensory and/or motor dysfunction. Central pain (ranging from 2.1% to 36%) is probably a direct consequence of the disease.26,28 According to Kosek et al,29 it may be explained as a type of “nociplastic pain”, it is probably caused by changes in cerebral activation, connectivity, or structures. This type of PRP is often described by patients as diffuse aching, burning, or cramping. It has generally spontaneous onset and can be intermittent with periods of exacerbation or persistent in nature. Again, different parts of the body can be affected and it is often combined with autonomous symptoms. Non-uncommon pain descriptions are from facial, abdominal, or even genital locations.
A high prevalence of neuropathy is described in patients long-lasting treated with oral levodopa (30%), or by oral levodopa/carbidopa intestinal gel (LCIG) infusion (42%) which is useful in advanced stages of PD when symptoms are not adequately controlled by optimized oral medication regimen.30 This iatrogenic condition, which represents a potential cause of levodopa withdrawal, shows the features of a prevalently sensory, mild axonal polyneuropathy expressed as distal numbness or tingling, and associated to weakness, mostly in the lower extremities; pain can be included among the clinical aspects of the neuropathy. The pathogenesis is complex and encompasses a combination of factors, including vitamin deficiency (low serum B12) and inflammatory damage. Duration of exposure to levodopa, and high-homocysteine and methylmalonic acid levels have been indicated as causes of the neuropathy.31
Finally, a specific PRP is the dystonic pain. It ranges from 4.7% to 22.3% and is associated with the sustained movements that lead to abnormal postures and deformities.25,32
Multimodal approaches for pain management
The treatment strategies for PRP require a deep knowledge of the mechanisms responsible for pain experiences in individual patients. For instance, changes in the central pathways involved in sensory processing reduce pain thresholds in PD.33 On the other hand, early degeneration of peripheral nociceptive afferent fibers may occur.22 Furthermore, studies have confirmed the existence of pathways other than those secondary to rigidity, tremor, or any other motor manifestations of the disease.23,34 Again, chronic PRP is closely associated with co-symptoms of stress, as well as other forms of chronic and/or acute pain. Taken together, these complex pathophysiological processes are the simple explanation of how pain in PD is so difficult to treat.
Because pain in PD patients has a multifactorial etiology,15 and is a dynamic process, pain treatments should follow multimodal approaches through a combination of pharmacological agents with non-pharmacological strategies. These dynamical approaches should be based on pain intensity and the complexity of symptoms, pain pathophysiology, and presence of comorbidities.34 Pharmacological agents and the non-pharmacological methods foreseen and coded in pain therapy are determined on the basis of therapeutic needs.
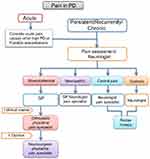
In this scenario, therapeutic approaches may be widely different depending on the type of pain and the clinical context. To simplify the whole pathway, we propose a simple flowchart which combines assessment and therapeutic options (Figure 1). The first step concerns the distinction between acute and persistent/recurrent chronic pain. After the first assessment has been carried out, the therapeutic path is addressed according to the type of pain and through the involvement of different professional figures.
In PD, acute pain crises have generally a muscular or musculoskeletal cause such as low back pain (LBP) or neck pain, or arise from exacerbation of chronic diseases (eg, gonarthrosis). Yet, it is mandatory to consider acute pain causes other than PD.
In case of persistent/recurrent chronic pain, because pain assessment can be performed within the NMSs evaluation, the first pain assessment is generally made by the neurologist. Although the GP may be able to assess different types of pain, the neurologic evaluation seems to be more appropriate for assessing specific PRP manifestations, such as dystonic pain or central pain. Once the features of pain have been characterized, the patient route is defined according to the flowchart, possibly involving a second step which often requires the expertise of other specialists, such as pain therapists, orthopedic/physiatrists, or neurosurgeons. In case of musculoskeletal pain, after the neurological evaluation, the patient can be referred to the GP. Sometimes, it is necessary to obtain a second opinion from specialists (eg, orthopedic, physiatrist, pain specialist) or a rheumatological consultation. The therapeutic approaches may require pharmacological and non-pharmacological treatment, such as physical or occupational therapy, or orthopedic surgery and subsequent rehabilitation.
Although neuropathic pain can be usually managed by the GP, it is often severe in nature and requires the expertise of a neurologist or a pain therapist. Sometimes, it is necessary to follow a dedicated diagnostic path and, in turn, to refer to a neurosurgeon for possible surgical approaches. Concerning pharmacological therapy, although several medications have been demonstrated in multiple clinical trials to ameliorate the painful manifestations,35 these agents have not been specifically tested for treating PRP. Thus, for addressing PD-related neuropathic pain, it is possible to refer to guidelines published by international and regional professional associations which recommend (strong evidence) three drug classes for first-line therapy, regardless from the cause of the painful condition. These medications include tricyclic antidepressants such as amitriptyline; the serotonin-norepinephrine reuptake inhibitors (SNRIs) such as duloxetine; and the calcium channel alpha-2-delta ligands gabapentin and pregabalin.36–38 Tramadol, a weak opioid and an SNRI, is recommended by most guidelines as second-line treatment, although it has been shown that the repeated intake of this drug may alter the dopaminergic pathway inducing tremor (tramadol-induced parkinsonism) in elderly.39 Finally, strong opioids, anti-epileptic agents other than gabapentinoids, and cannabinoids may be used as third- and fourth-line treatment. Topical preparations of capsaicin and lidocaine are recommended for localized neuropathic pain.40 In patients affected by levodopa-induced neuropathy and identified to have cobalamin deficiency, the therapeutic approach is based on cobalamin supplementation (eg, monthly intramuscular injections of 1000 µg of vitamin B12).30
Dystonic pain is due to excess muscle contractions. It may resolve with stretching the involved joint but usually this type of pain requires the re-evaluation of PD therapy, to reduce “off” fluctuations or episodes of medication-induced dystonia. Furthermore, consultations from pain specialist and physical therapist may be requested for evaluating potential pharmacological or physical therapies. Subcutaneous apomorphine or LCIG can represent a useful strategy for advanced PD patients with severe motor fluctuations and hyper-/dyskinesia.41
In drug-naïve PD patients, central PRP can be successfully managed by initiating levodopa therapy. Subsequently, during the wearing-off periods and after the beginning of dopaminergic therapy, it may be very difficult to treat because the optimization of the PD therapy may not solve the problem. Medications used to manage neuropathic pain and analgesics for moderate to severe pain must be considered.
Pharmacological considerations
Medications for mild to moderate pain include NSAIDs or acetaminophen. Although the effects of these drugs for pain relief in PD are not negligible, they are not indicated in subgroups of PRP types (eg, neuropathic pain). Acetaminophen is an analgesic with a central activity, acting through multiple mechanisms involving endogenous cannabinoid and serotoninergic pathways. This drug can also be used for long-term therapy, because of its advantageous profile and the balance between efficacy and safety. In patients with normal hepatic activity, in fact, the hepatotoxic dosages are much higher than the therapeutic dosages. Finally, there are no specific contraindications in PD and this drug does not interfere with antiparkinson medications. NSAIDs can be prescribed according to indications and contraindications followed in non-PD patients as their use was not found to be associated with risk of PD worsening.42
In a minority of patients, pain is severe and intractable.15 Although opioids may represent an effective strategy for the treatment of moderate to severe pain, their use in chronic non-cancer pain is controversial and should be managed under the supervision of a pain therapist. Furthermore, it is often required the consultation from a neurologist to evaluate potential interferences with antiparkinson medications. For instance, methadone, tramadol, and meperidine must be avoided in patients receiving selegiline, rasagiline, or safinamide.43 Based on their potency, analgesic opioids are subdivided into weak opioids (codeine and tramadol, both combined or not with acetaminophen) and strong opioids (morphine, oxycodone, alone or in association with acetaminophen or naloxone, buprenorphine, fentanyl, hydromorphone, tapentadol) (Table 2). The weak opioids are prodrugs that must be converted by the enzymatic system of cytochromes; thus, their efficacy could be limited by enzymatic modifications or pharmacological interactions.
 |
Table 2 Selected analgesic opioid drugs |
The association of opioids with acetaminophen is clinically advantageous as it does not simply add the analgesic effects of the drugs, but gives a strengthened effect connected to the synergic and complementary action of the medications. Morphine is, even today, the opioid of reference in oncological-related pain. While there are no clinical studies on the antalgic use of morphine in PD, it has been proved that it may be able to elevate dopamine levels in the mesolimbic dopamine system,44 and can decrease levodopa-induced dyskinesia;45 thus, its use is not contraindicated in these patients and it could also bring a clinical benefit. When administrated for oral use morphine can be prescribed in slow-release capsule form for the treatment of chronic pain, or in rapid-release capsule form for the control of the dose, at the beginning of therapy or for the treatment of foreseeable acute pain crises.
The association oxycodone/naloxone, in a fixed 2/1 proportion, is widely used in the therapy of chronic pain because it combines the analgesic activity of oxycodone with the antagonist activity of naloxone on the µ opioid receptors (MORs) at the intestinal level for preventing opioid-induced constipation without diminishing the central analgesic activity. Recently, Trenkwalder et al (PANDA study)46 demonstrated the potential efficacy of the combination of oxycodone with the opioid antagonist naloxone on PRP. Although the primary endpoint (ie, average 24-hr pain scores at 4 months) was not significant, this research was the first randomized controlled trial conducted to investigate the pharmacological (opioid) treatment of PRP. However, a post-hoc analysis with the KPPS tool demonstrated significant improvement in only the subgroups of patients with severe musculoskeletal pain. Of note, there were no differences between treatment groups for other types of PRP.47 Another investigation showed that low-dose oxycodone/naloxone (5/2.5 mg twice daily) for a period of 8 weeks induced a significant pain relief without the occurrence of serious side effects.48
Fentanyl is an opioid 100 times more powerful than morphine and it is widely used transdermal for the treatment of chronic pain. A clinical report indicated that in a patient with PD it induced bradykinesia and rigidity postoperatively after deep brain stimulation.49 Tapentadol is a molecule characterized by a dual mechanism by acting on the MORs and through inhibition of the reuptake of noradrenaline, thereby, increasing endogenous modulating activity.50 Despite its potential efficacy, there are no data on PRP.
Minimally invasive and invasive treatments
Botulinum neurotoxin acts by blocking the release of acetylcholine at the neuromuscular junction and is recognized as a safe and effective treatment for spasticity resulting from different conditions, such as stroke, multiple sclerosis, and spinal cord injury, tremor, and dystonia.51 It can be helpful in the treatment of both dystonic and central pain, especially in combination with rehabilitation procedures.
The efficacy of some invasive approaches has been evaluated for the treatment of PRP. Oshima et al26 investigated on the application of chronic subthalamic nucleus stimulation (SNS) in patients with advanced PD and found that musculoskeletal pain and dystonic pain responded well to this intervention with long-term pain relief (1 year). On the contrary, in patients with peripheral neuropathic pain they observed a worsening of the PRP. Probably, musculoskeletal pain and dystonic pain responded to the treatment because in both the pathophysiological mechanism involves an increase in muscle tone. More recently, Jung et al52 proved that SNS was effective in approximately all the patients with dystonic pain. On the contrary, results on musculoskeletal pain and LBP were less positive. Prior to these studies, other neurosurgical attempts were the unilateral pallidotomy by which Honey et al53 obtained good results and the unilateral/bilateral pallidal stimulation. This latter, when bilateral, induced significant improvement in pain control.54 Furthermore, it has been proposed the use of a spinal cord stimulator to relieve intractable neuropathic pain.55 Our suggestion is to limit these invasive approaches to selected cases after a careful multidisciplinary case-by-case analysis (Table 3).
 |
Table 3 Practical suggestions for managing pain in Parkinson’s disease |
Conclusion
A multimodal approach obtained by using different methods or medications seems to be the more appropriate strategy for managing pain in PD patients. This strategy, indeed, could be helpful for both reducing side effects and minimizing interference with antiparkinson medications. On the other hand, due to the complexity of the clinical scenario in which the symptom pain is included, and peculiar features of pain problems in this vulnerable population, pain management often requires a multidisciplinary approach involving different care professionals. This perspective could offer an improvement in PRP management, from the diagnosis to treatment. Moreover, results from multidimensional and multidisciplinary approach in different field of pain medicine should encourage clinical investigations in the context of PD. For instance, further investigations should be conducted: 1) by combining pharmacological with non-pharmacological treatments; 2) on the use of opioids in moderate to severe pain; 3) on the interference of pain medications on antiparkinsonian drugs; 4) on the use of agents against neuropathic pain in PD patients; and 5) on different subgroups of PRP.
Disclosure
Professor Andrea Truini reports receiving honoraria for speaking at symposia or research financial supports from Alpha-Sigma, Angelini, Epitech, FB Health, and Pfizer. Dr Silvia Natoli reports grants from Grunenthal Italia srl, outside the submitted work. Professor Paolo Barone reports personal fees from UCB, Lundbeck, Zambon, Chiesi, and grants and personal fees from Biogen, outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Skogar O, Lokk J. Pain management in patients with Parkinson’s disease: challenges and solutions. J Multidiscip Healthc. 2016;9:469–479. doi:10.2147/JMDH.S105857
2. Barone P, Antonini A, Colosimo C, et al.; PRIAMO study group. The PRIAMO study: a multicenter assessment of nonmotor symptoms and their impact on quality of life in Parkinson’s disease. Mov Disord. 2009;24(11):1641–1649. doi:10.1002/mds.22643
3. Broen MP, Braaksma MM, Patijn J, Weber WE. Prevalence of pain in Parkinson’s disease: a systematic review using the modified QUADAS tool. Mov Disord. 2012;27:480–484. doi:10.1002/mds.24054
4. Tysnes OB, Storstein A. Epidemiology of Parkinson’s disease. J Neural Transm (Vienna). 2017;124(8):901–905. doi:10.1007/s00702-017-1686-y
5. Nègre-Pagès L, Regragui W, Bouhassira D, Grandjean H, Rascol O; DoPaMiP Study Group. Chronic pain in Parkinson’s disease: the cross-sectional French DoPaMiP survey. Mov Disord. 2008;23(10):1361–1369. doi:10.1002/mds.22142
6. Sophie M, Ford B. Management of pain in Parkinson’s disease. CNS Drugs. 2012;26(11):937–948. doi:10.1007/s40263-012-0005-2
7. Chaudhuri KR, Odin P, Antonini A, Martinez-Martin P. Parkinson’s disease: the non-motor issues. Parkinsonism Relat Disord. 2011;17:717–723. doi:10.1016/j.parkreldis.2011.02.018
8. Rana AQ, Qureshi ARM, Kachhvi HB, Rana MA, Chou KL. Increased likelihood of anxiety and poor sleep quality in Parkinson’s disease patients with pain. J Neurol Sci. 2016;369:212–215. doi:10.1016/j.jns.2016.07.064
9. Chaudhuri KR, Prieto-Jurcynska C, Naidu Y, et al. The nondeclaration of nonmotor symptoms of Parkinson’s disease to health care professionals: an international study using the nonmotor symptoms questionnaire. Mov Disord. 2010;25:704–709. doi:10.1002/mds.22868
10. Beiske AG, Loge JH, Rønningen A, Svensson E. Pain in Parkinson’s disease: prevalence and characteristics. Pain. 2009;141(1–2):173–177. doi:10.1016/j.pain.2008.12.004
11. Quittenbaum BH, Grahn B. Quality of life and pain in Parkinson’s disease: a controlled cross-sectional study. Parkinsonism Relat Disord. 2004;10(3):129–136. doi:10.1016/j.parkreldis.2003.12.001
12. Madden MB, Hall DA. Shoulder pain in Parkinson’s disease: a case-control study. Mov Disord. 2010;25(8):1105–1106. doi:10.1002/mds.23048
13. Chaudhuri KR, Martinez-Martin P, Schapira AH, et al. International multicenter pilot study of the first comprehensive self-completed nonmotor symptoms questionnaire for Parkinson’s disease: the NMSQuest study. Mov Disord. 2006;21(7):916–923. doi:10.1002/mds.20844
14. Chaudhuri KR, Rizos A, Trenkwalder C, et al.; EUROPAR and the IPMDS Non Motor PD Study Group. King’s Parkinson’s disease pain scale, the first scale for pain in PD: an international validation. Mov Disord. 2015;30(12):1623–1631. doi:10.1002/mds.26270
15. Martinez-Martin P, Rizos AM, Wetmore J, et al.; EUROPAR and MDS Non-motor PD Study Group. First comprehensive tool for screening pain in Parkinson’s disease: the King’s Parkinson’s disease pain questionnaire. Eur J Neurol. 2018;25(10):1255–1261. doi:10.1111/ene.13691
16. Etchepare F, Rozenberg S, Mirault T, et al. Back problems in Parkinson’s disease: an underestimated problem. Joint Bone Spine. 2006;73(3):298–302. doi:10.1016/j.jbspin.2005.05.006
17. Broetz D, Eichner M, Gasser T, Weller M, Steinbach JP. Radicular and nonradicular back pain in Parkinson’s disease: a controlled study. Mov Disord. 2007;22(6):853–856. doi:10.1002/mds.21439
18. Defazio G, Berardelli A, Fabbrini G, et al. Pain as a nonmotor symptom of Parkinson disease: evidence from a case-control study. Arch Neurol. 2008;65(9):1191–1194. doi:10.1001/archneurol.2008.2
19. Ehrt U, Larsen JP, Aarsland D. Pain and its relationship to depression in Parkinson disease. Am J Geriatr Psychiatry. 2009;17(4):269–275.
20. Kubo S, Hamada S, Maeda T, et al. A Japanese multicenter survey characterizing pain in Parkinson’s disease. J Neurol Sci. 2016;365:162–166. doi:10.1016/j.jns.2016.04.015
21. Whiting PF, Weswood ME, Rutjes AW, Reitsma JB, Bossuyt PN, Kleijnen J. Evaluation of QUADAS, a tool for the quality assessment of diagnostic accuracy studies. BMC Med Res Methodol. 2006;6:9. doi:10.1186/1471-2288-6-9
22. Defazio G, Tinazzi M, Berardelli A. How pain arises in Parkinson’s disease? Eur J Neurol. 2013;20(12):1517–1523. doi:10.1111/ene.12260
23. Ford B. Pain in Parkinson’s disease. Mov Disord. 2010;25:S98–S103. doi:10.1002/mds.22716
24. Ferini-Strambi L, Carli G, Casoni F, Galbiati A. Restless legs syndrome and Parkinson disease: a causal relationship between the two disorders? Front Neurol. 2018;9:551. doi:10.3389/fneur.2018.00551
25. Lin XJ, Yu N, Lin XG, et al. A clinical survey of pain in Parkinson’s disease in Eastern China. Int Psychogeriatr. 2016;28(2):283–289. doi:10.1017/S1041610215001659
26. Oshima H, Katayama Y, Morishita T, et al. Subthalamic nucleus stimulation for attenuation of pain related to Parkinson disease. J Neurosurg. 2012;116(1):99–106. doi:10.3171/2011.7.JNS11158
27. Yardimci N, Cemeroglu O, Ozturk E, et al. Median and ulnar neuropathy assessment in Parkinson’s disease regarding symptom severity and asymmetry. Parkinsons Dis. 2016;2016:4958068. doi:10.1155/2016/4958068
28. Tinazzi M, Del Vesco C, Fincati E, et al. Pain and motor complication in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2009;77:822–825. doi:10.1136/jnnp.2005.079053
29. Kosek E, Cohen M, Baron R, et al. Do we need a third mechanistic descriptor for chronic pain states? Pain. 2016;157:1382–1386. doi:10.1097/j.pain.0000000000000473
30. Romagnolo A, Merola A, Artusi CA, Rizzone MG, Zibetti M, Lopiano L. Levodopa-induced neuropathy: a systematic review. Mov Disord Clin Pract. 2018;6(2):96–103. doi:10.1002/mdc3.12688
31. Uncini A, Eleopra R, Onofrj M. Polyneuropathy associated with duodenal infusion of levodopa in Parkinson’s disease: features, pathogenesis and management. J Neurol Neurosurg Psychiatry. 2015;86(5):490–495. doi:10.1136/jnnp-2014-308586
32. Valkovic P, Minar M, Singliarova H, et al. Pain in Parkinson’s disease: a cross-sectional study of its prevalence, types, and relationship to depression and quality of life. PLoS One. 2015;10(8):e0136541. doi:10.1371/journal.pone.0136541
33. Schestatsky P, Kumru H, Valls-Solé J, et al. Neurophysiologic study of central pain in patients with Parkinson disease. Neurology. 2007;69(23):2162–2169. doi:10.1212/01.wnl.0000295669.12443.d3
34. Cuomo A, Bimonte S, Forte CA, Botti G, Cascella M. Multimodal approaches and tailored therapies for pain management: the trolley analgesic model. J Pain Res. 2019;12:711–714. doi:10.2147/JPR.S178910
35. Markman JD, Jensen TS, Semel D, et al. Effects of pregabalin in patients with neuropathic pain previously treated with gabapentin: a pooled analysis of parallel-group, randomized, placebo-controlled clinical trials. Pain Pract. 2017;17:718–728. doi:10.1111/papr.12550
36. Finnerup NB, Attal N, Haroutounian S, et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol. 2015;14:162–173. doi:10.1016/S1474-4422(14)70251-0
37. Cruccu G, Sommer C, Anand P, et al. EFNS guidelines on neuropathic pain assessment: revised 2009. Eur J Neurol. 2010;17:1010–1018. doi:10.1111/j.1468-1331.2010.02969.x
38. National Institute for Health and Care Excellence (NICE). Neuropathic pain: the pharmacological management of neuropathic pain in adults in non-specialist settings. 2013 [
39. Singh R. Tramadol-induced parkinsonism: a case report of a 75-year-old woman. J Basic Clin Physiol Pharmacol. 2018;30:275–278. doi:10.1515/jbcpp-2018-0113
40. Cruccu G, Truini A. A review of neuropathic pain: from guidelines to clinical practice. Pain Ther. 2017;6(Suppl 1):35–42. doi:10.1007/s40122-017-0087-0
41. Lowin J, Sail K, Baj R, et al. The cost-effectiveness of levodopa/carbidopa intestinal gel compared to standard care in advanced Parkinson’s disease. J Med Econ. 2017;20(11):1207–1215. doi:10.1080/13696998.2017.1379411
42. Ren L, Yi J, Yang J, Li P, Cheng X, Mao P. Nonsteroidal anti-inflammatory drugs use and risk of Parkinson disease: a dose-response meta-analysis. Medicine (Baltimore). 2018;97(37):e12172. doi:10.1097/MD.0000000000012172
43. American Parkinson Disease Association. Medications to be avoided or used with caution in Parkinson’s disease. Available from: https://www.apdaparkinson.org/wp-content/uploads/2018/05/APDA-Meds_to_Avoid.pdf.
44. Yan T, Rizak JD, Yang S, et al. Acute morphine treatments alleviate tremor in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated monkeys. PLoS One. 2014;9(2):e88404. Published 2014 Feb 10. doi:10.1371/journal.pone.0088404
45. Samadi P, Grégoire L, Bédard PJ. The opioid agonist morphine decreases the dyskinetic response to dopaminergic agents in parkinsonian monkeys. Neurobiol Dis. 2004;16(1):246–253. doi:10.1016/j.nbd.2004.02.002
46. Trenkwalder C, Chaudhuri KR, Martinez-Martin P, et al.; PANDA study group. Prolonged-release oxycodone-naloxone for treatment of severe pain in patients with Parkinson’s disease (PANDA): a double-blind, randomised, placebo-controlled trial. Lancet Neurol. 2015;14(12):1161–1170. doi:10.1016/S1474-4422(15)00243-4
47. Antonini A, Tinazzi M. Targeting pain in Parkinson’s disease. Lancet Neurol. 2015;14(12):1144–1145. doi:10.1016/S1474-4422(15)00286-0
48. Madeo G, Schirinzi T, Natoli S, et al. Efficacy and safety profile of prolonged release oxycodone in combination with naloxone (OXN PR) in Parkinson’s disease patients with chronic pain. J Neurol. 2015;262(9):2164–2170. doi:10.1007/s00415-015-7823-3
49. Zesiewicz TA, Hauser RA, Freeman A, Sullivan KL, Miller AM, Halim T. Fentanyl-induced bradykinesia and rigidity after deep brain stimulation in a patient with Parkinson disease. Clin Neuropharmacol. 2009;32(1):48–50. doi:10.1097/WNF.0b013e31817e23e3
50. Cascella M, Forte CA, Bimonte S, et al. Multiple effectiveness aspects of tapentadol for moderate-severe cancer-pain treatment: an observational prospective study. J Pain Res. 2018;12:117–125. eCollection 2019. doi:10.2147/JPR.S181079
51. Abrams SB, Hallett M. Clinical utility of different botulinum neurotoxin preparations. Toxicon. 2013;67:81–86. doi:10.1016/j.toxicon.2012.11.024
52. Jung YJ, Kim HJ, Jeon BS, Park H, Lee WW, Paek SH. An 8-year follow-up on the effect of subthalamic nucleus deep brain stimulation on pain in parkinson disease. JAMA Neurol. 2015;72:504–510. doi:10.1001/jamaneurol.2015.8
53. Honey CR, Stoessl AJ, Tsui JK, Schulzer M, Calne DB. Unilateral pallidotomy for reduction of parkinsonian pain. J Neurosurg. 1999;91:198–201. doi:10.3171/jns.1999.91.2.0198
54. Loher TJ, Burgunder JM, Weber S, Sommerhalder R, Krauss JK. Effect of chronic pallidal deep brain stimulation on off period dystonia and sensory symptoms in advanced Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2002;73:395–399. doi:10.1136/jnnp.73.1.76
55. Hassan S, Amer S, Alwaki A, Elborno A. A patient with Parkinson’s disease benefits from spinal cord stimulation. J Clin Neurosci. 2013;20(8):1155–1156. doi:10.1016/j.jocn.2012.08.018
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.