Back to Journals » Journal of Blood Medicine » Volume 13
Total Knee Arthroplasty: Superiority of Intra-Articular Tranexamic Acid Over Intravenous and Cell Salvage as Blood Sparing Strategy – A Retrospective Study
Authors Coelho M , Bastos C, Figueiredo J
Received 25 November 2021
Accepted for publication 4 February 2022
Published 18 February 2022 Volume 2022:13 Pages 75—82
DOI https://doi.org/10.2147/JBM.S348862
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Martin H Bluth
Miguel Coelho, Catarina Bastos, Jose Figueiredo
Anesthesiology Department, Centro Hospitalar do Baixo Vouga, Aveiro, Portugal
Correspondence: Miguel Coelho, Anesthesiology Department, Centro Hospitalar do Baixo Vouga, Av. Artur Ravara, Aveiro, 3810-164, Portugal, Tel +351 914397295, Email [email protected]
Purpose: Total knee arthroplasty is associated with considerable perioperative hemorrhage. The decrease in hemoglobin concentration and the need for allogenic blood transfusion are related to increased morbidity and mortality. Strategies for minimizing perioperative bleeding are used, such as tranexamic acid and cell salvage. The study aimed to compare intravenous, intra-articular tranexamic acid and cell salvage protocols regarding perioperative hemoglobin variation. Secondary outcomes included blood loss; allogenic transfusions; complications and in-hospital stay.
Patients and Methods: Patients submitted to unilateral total knee arthroplasty between January and December 2018 were retrospectively evaluated. After excluding 62 patients, 204 were subdivided into 3 groups according to the protocol used. Statistical analysis was performed with SPSS version 26.0. One-way ANOVA and Kruskal–Wallis tests were used. Considered a p-value of < 0.05 for statistical significance.
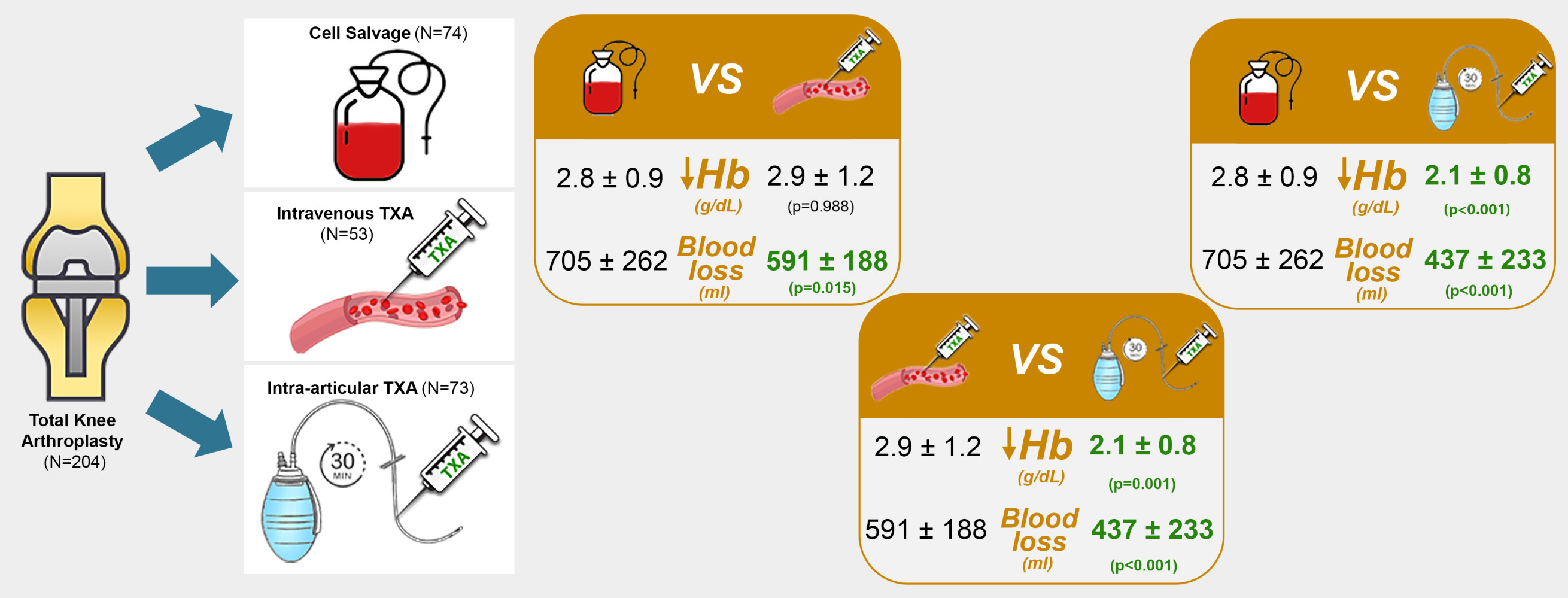
Results: Variation of hemoglobin in the intra-articular tranexamic acid group was significantly lower than that of intravenous (p < 0.001) and cell salvage (p = 0.001) groups. Blood loss, variation of hematocrit, need for blood transfusion and in-hospital stay were also statistically significantly lower in the intra-articular tranexamic acid group. The only related complications were in the intravenous tranexamic acid group. No thromboembolic complications were identified which further solidifies the safety of tranexamic acid administration.
Conclusion: This data shows superiority of the intra-articular administration of tranexamic acid over the other techniques in total knee arthroplasty. We propose this protocol as an efficient, low-risk blood-sparing strategy.
Keywords: arthroplasty, knee, acid tranexamic, salvage, transfusion
Graphical Abstract:
Introduction
Total knee arthroplasty (TKA) is the procedure of choice for the treatment of degenerative joint disease of the knee and is associated with considerable perioperative hemorrhage.1 Decrease in postoperative hemoglobin (Hb) concentration (<7 g/dL or >50% of baseline) is associated with ischemic events and increased morbidity and mortality.2 Previously, more than 20% of TKA required transfusion of blood products in the postoperative period.1,3 With the improvement of surgical technique, the transfusion rate is currently 1–12%.4,5 However, allogenic blood transfusion is associated with allergic reactions,6 increased risk of infection of the surgical incision7,8 or prosthetic joint infection,8 transmission of infectious diseases,6,7 immune modulation/suppression,9 and increased hospitalization time, morbidity, mortality and costs.6–8,10 Strategies focused on minimizing perioperative hemorrhage and the need for transfusion (controlled hypotension, self-transfusion, tourniquet, antifibrinolytics and cell salvage) have been used in orthopedic surgery.10
Tranexamic acid (TXA) is a synthetic lysine analogue that acts as an inhibitor of fibrin degradation through plasminogen.11 It can be administered orally, intravenously or topically.11 It decreases hemorrhage in the perioperative period and transfusion rates in cardiac,11,12 pelvic,11,13 spinal11,14 and orthopedic surgery.7 However, studies differ in doses, methods and routes of administration, use of tourniquet and cut-off values for transfusion. Cell salvage consists of filtering and reinfusion of the blood losses collected by surgical drains.15 Currently, there is no consensus on whether it reduces the transfusion need in TKA.15,16
At our institution, we use protocols for either intravenous TXA (TXA-iv), intra-articular TXA (TXA-art), or cell salvage usage in TKA. The primary outcome of our study is the comparison of these 3 methods in terms of perioperative Hb variation. Secondary outcomes are the comparison of drainage blood losses; number of allogenic transfusions; variation in hematocrit (Htc) and platelets (PLT) values; complications and duration of hospital stay.
Materials and Methods
After obtaining approval from the Institutional Ethics Committee, the medical records of patients undergoing TKA between January and December 2018 were retrospectively evaluated. We analyzed both computer and physical registries to collect clinical and demographic data, perioperative laboratory results and records on postoperative complications. A total of 266 patients were identified, all classified as ASA 2 or 3, submitted to unilateral TKA. We excluded a total of 62 cases, resulting in 204 admitted patients subdivided into 3 groups according to the transfusion-sparing technique used: 74 patients in the Cell salvage group, 53 in the TXA-iv group and 77 in the TXA-art group (Figure 1). Exclusion criteria involved previous surgery on the same limb; incomplete registries; pre-operative hemoglobin <10 g/dL; and history of ischemic cardiac disease.
 |
Figure 1 Exclusion criteria and subdivision of patients included in the study. |
- Cell salvage protocol: the contents of the surgical drain were collected and filtered in the CellTransTM system. Re-administration occurred during the 24-hour post-operative period.
- TXA-iv protocol: 7.5 mg/kg of TXA diluted in 100 mL of NaCl 0.9%. The full dose was slowly administered twice: firstly, 15 minutes before inflating the tourniquet and, secondly, immediately before deflation (total: 15 mg/kg in 200mL of NaCl 0.9%).
- TXA-art protocol: after tourniquet deflation, the surgeon injected 1500 mg of TXA diluted in 20 mL of NaCl 0.9% through the surgical drain, which remained clamped for 30 minutes.
All patients included were operated by different surgeons of the same Orthopedic Department, using the same technique and protocols. All procedures were performed under tourniquet. A classic medial parapatellar approach and a conventional mechanical alignment technique were used in all patients. The same primary prothesis was applied to all patients. The drainage in the postoperative period was recorded during 24 hours by the nursing team.
Blood sampling for complete blood count (CBC) was drawn 24 hours post operatively.
Our hospital uses a Hb transfusion threshold between 8 and 10 g/dL.
The same immediate post-operative care and rehabilitation was applied to all patients included. This consisted of a Fast-Track Protocol which included: passive assisted range-of-movement isometric exercises at 8h to 12h after surgery, followed by active range of movement exercises and walking-assisted gait. Patients were discharged at 3 to 4 days after surgery depending on in-patient rehabilitation results and absence of complications.
Statistical analysis was performed with SPSS version 26.0. The normality of variables was tested with Kolmogorov–Smirnov. For variables with normal distribution, we used the One-Way ANOVA test and the Bonferroni or Games-Howell post-hoc tests, according to homogeneity of variances. For variables without normal distribution, we used the Kruskal–Wallis test. We considered statistical significance with p-value of <0.05.
Results
We started by analyzing the baseline differences between the 3 study groups (Table 1) and did not find statistically significant differences between the means of age (p = 0.757), gender (p = 0.184), BMI (p = 0.219), preoperative Hb (p = 0.522), preoperative Htc (p = 0.512) and preoperative PLT count (p = 0.301). About 24% of patients had preoperative anemia, defined as Hb <13 g/dL. No difference was found between the timing of CBC sampling (p = 0.588).
 |
Table 1 Demographic Characterization and Comparison Between the Cell Salvage, TXA-iv and TXA-art Groups |
Regarding the primary outcome (Table 2), we found that the mean Hb variation (vHb) of TXA-art was significantly lower than that of TXA-iv (p < 0.001) and Cell salvage (p = 0.001). On the other hand, the mean vHb in Cell salvage and TXA-iv groups were not significantly different (p = 0.988). Postoperative anemia was found in 91% of patients.
 |
Table 2 Comparison of Primary and Secondary Outcomes |
Regarding secondary outcomes (Table 2), hematic losses in the TXA-art group in the first postoperative 24 hours were significantly lower than those in the TXA-iv (p < 0.001) and Cell salvage (p < 0.001) groups. We did not find any difference between the TXA-iv and Cell salvage groups (p = 0.150) for this variable. A total of 39 blood units were transfused in 25 patients (23 blood units in the cell salvage group, 14 blood units in the TXA-iv group and 2 blood units in the TXA-art group). The number of transfusions administered did not differ when comparing TXA-art with TXA-iv (p = 0.724) groups. TXA-art did have fewer transfusion needs than Cell Trans (p = 0.044), as did TXA-iv (p = 0.009). The mean variation of Htc (vHtc) in the TXA-art group was significantly lower than that in the TXA-iv (p < 0.001) and Cell salvage groups (p = 0.030). On the contrary, the vHtc of the Cell salvage and TXA-iv groups were not significantly different (p = 0.919).
The mean perioperative variation of PLT (vPLT) was not significantly different between the Cell salvage and TXA-iv groups (p = 0.794), nor between TXA-art and TXA-iv (p = 0.792). vPLT in the TXA-art group was significantly lower than in the Cell salvage group (p = 0.044).
The duration of in-hospital stay was significantly shorter in TXA-art group than in the Cell salvage (p < 0.001) and in TXA-iv groups (p = 0.001). We found no statistically significant difference between the duration of in-hospital stay in the TXA-iv and the cell salvage groups (p = 0.853). We found 2 complications in the TXA-iv group, namely seizures and infection of the surgical wound.
Discussion
We did not find any published studies that directly compared the three methods depicted here in TKA. The use of intra-articular injection of TXA was associated with less decrease in Hb in TKA compared to other two techniques. We established the same superiority regarding blood loss through drainage, vHtc and in-hospital stay time. Injection through the surgical drain allows better exposure to TXA in the surgical site, which may be the reason for decreased blood loss.17 The clamping of the drain further prolongs this contact, which is especially important in TKA in which the operative tourniquet makes the blood loss more evident in the postoperative period.18,19 Recent studies have analyzed the efficacy of the topical/intra-articular route and found a significant decrease in hemorrhage when compared to placebo,20 but not when compared to its intravenous administration.17,20,21 Some studies report that the topical route promotes fewer losses and lower transfusion needs compared to the intravenous route.18
Allogenic transfusions occurred in about 12% of patients, a value similar to that found in the current literature.5 The TXA-art group had reduced need of allogenic transfusions (only 2 patients). Although not statistically significant (p = 0.724), TXA-art seems to be better than TXA-iv in this regard. It has been shown that the benefit of cell salvage decreases when a more restrictive transfusion cut off (<8 g/dL) is used.16
It has been demonstrated that the use of TXA is not associated with increased risk of thromboembolic events in arthroplastic surgery, regardless of personal history7,22–24 and the route of administration.17,18,20,21 However, studies still advise caution for this potential risk.7,24,25 In our study, we did not identify any thromboembolic complications in any group, which further solidifies the safety of TXA administration. In the TXA-iv group, we identified a case of generalized seizures and one case of surgical wound infection. High dose (100 mg/kg) of TXA is associated with perioperative seizures,26 but a single bolus of the usual dose used in orthopedic surgery is considered to be safe in this respect.26 Topical administration of TXA results in higher local levels, lower plasma levels and, theoretically, lower risk of associated complications.17,27
The 24% of preoperative anemia (Hb <13 g/dL) identified is similar to the 12–33% already described in patients undergoing TKA.28,29 Preoperative anemia is the most important independent risk factor for transfusion and perioperative morbidity and mortality.30 Postoperative anemia was 91%, higher than the 82% observed in another study.29
Our study presents some limitations: It is a retrospective study; the choice of blood sparing strategy was made according to surgeon’s preference, which can cause some group biases not analysed by this study; even though the ideal dose of tranexamic acid remains a subject of discussion, the difference between the dose of tranexamic acid in the two protocols (15mg/kg in the TXA-iv group vs 1500mg TXA-art) can contribute as bias of analysis considering patient’s weight. We tried to minimize this by analysing differences in groups’ BMI; the wide interval of transfusion threshold (Hb 8–10g/dL) was based on a subjective clinical evaluation which can translate as a limitation on the interpretation of the presented findings. Nevertheless, as it worked as the same bias in all the 3 groups and the results were of significance, we chose to present them.
Conclusion
Based on the results obtained in this study, our institution assumed the preference for the use of intra-articular tranexamic acid in TKA. It seems to be a low-risk, highly efficient blood-sparing strategy. Prospective studies are needed, with a larger sample that allows a conclusive answer to the differences between intravenous and intra-articular administration of TXA and to evaluate possible associated thromboembolic complications.
Abbreviations
CBC, complete blood count; Hb, hemoglobin; Htc, hematocrit; PLT, platelets; TKA, total knee arthroplasty; TXA, tranexamic acid; TXA-art, intra-articular tranexamic acid; TXA-iv, intravenous tranexamic acid; vHb, hemoglobin variation; vHtc, hematocrit variation; vPLT, platelets variation.
Ethics Approval and Informed Consent
The Comissão de Ética (Institutional Review Board) of the Centro Hospitalar do Baixo Vouga (Aveiro), approved this research study. The review board has waived the need for informed consent as it is a retrospective study, all patients had signed consent for the surgical and anesthetic procedure and blood transfusions and no extraordinary interventions were performed. The data was accessed only by the authors and confidentiality of the patients was maintained at all times, in compliance with the Declaration of Helsinki.
Acknowledgments
No acknowledgements to be stated in terms of contributions, technical help of financial support.
Author Contributions
All authors had an important role in designing the study, presenting it to the ethical committee, gathering information, treating the data, writing, and reviewing the manuscript. All revisions and alterations were agreed upon by all authors. All authors decided together to which journal the study should be sent and are accountable for the contents of the article.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Abdel MP, Chalmers BP, Taunton MJ, et al. Intravenous versus topical tranexamic acid in total knee arthroplasty. J Bone Jt Surg. 2018;100:1023–1029. doi:10.2106/JBJS.17.00908
2. Spolverato G, Kim Y, Ejaz A, et al. Effect of relative decrease in blood hemoglobin concentrations on postoperative morbidity in patients who undergo major gastrointestinal surgery. JAMA Surg. 2015;150:949–956. doi:10.1001/jamasurg.2015.1704
3. Gombotz H, Rehak PH, Shander A, et al. The second Austrian benchmark study for blood use in elective surgery: results and practice change. Transfusion. 2014;54:2646–2657. doi:10.1111/trf.12687
4. Bini SA, Darbinian JA, Brox WT, et al. Risk factors for reaching the post-operative transfusion trigger in a community primary total knee arthroplasty population. J Arthroplasty. 2018;33:711–717. doi:10.1016/j.arth.2017.10.029
5. Klika AK, Small TJ, Saleh A, et al. Primary total knee arthroplasty allogenic transfusion trends, length of stay, and complications: nationwide inpatient sample 2000–2009. J Arthroplasty. 2014;29:2070–2077. doi:10.1016/j.arth.2014.06.018
6. Carson JL, Guyatt G, Heddle NM, et al. Clinical practice guidelines from the AABB. JAMA. 2016;316:2025–2035. doi:10.1001/jama.2016.9185
7. Kim JL, Park J-H, Han S-B, et al. Allogeneic blood transfusion is a significant risk factor for surgical-site infection following total hip and knee arthroplasty: a meta-analysis. J Arthroplasty. 2017;32:320–325. doi:10.1016/j.arth.2016.08.026
8. Everhart JS, Sojka JH, Mayerson JL, et al. Perioperative allogeneic red blood-cell transfusion associated with surgical site infection after total hip and knee arthroplasty. J Bone Jt Surg. 2018;100:288–294. doi:10.2106/JBJS.17.00237
9. Remy KE, Hall MW, Cholette J, et al. Mechanisms of red blood cell transfusion-related immunomodulation. Transfusion. 2018;58:804–815. doi:10.1111/trf.14488
10. Shah A, Palmer AJR, Klein AA. Strategies to minimize intraoperative blood loss during major surgery. Br J Surg. 2020;107:e26–38. doi:10.1002/bjs.11393
11. Draxler DF, Medcalf RL. The fibrinolytic system—more than fibrinolysis? Transfus Med Rev. 2015;29:102–109. doi:10.1016/j.tmrv.2014.09.006
12. Sigaut S, Tremey B, Ouattara A, et al. Comparison of two doses of tranexamic acid in adults undergoing cardiac surgery with cardiopulmonary bypass. Anesthesiology. 2014;120:590–600. doi:10.1097/ALN.0b013e3182a443e8
13. Lundin ES, Johansson T, Zachrisson H, et al. Single-dose tranexamic acid in advanced ovarian cancer surgery reduces blood loss and transfusions: double-blind placebo-controlled randomized multicenter study. Acta Obstet Gynecol Scand. 2014;93:335–344. doi:10.1111/aogs.12333
14. Colomina MJ, Koo M, Basora M, et al. Intraoperative tranexamic acid use in major spine surgery in adults: a multicentre, randomized, placebo-controlled trial. Br J Anaesth. 2017;118:380–390. doi:10.1093/bja/aew434
15. Miao Y, Guo W, An L, et al. Postoperative shed autologous blood reinfusion does not decrease the need for allogeneic blood transfusion in unilateral and bilateral total knee arthroplasty. PLoS One. 2019;14:e0219406. doi:10.1371/journal.pone.0219406
16. van Bodegom-vos L, Voorn VM, So-Osman C, et al. Cell salvage in hip and knee arthroplasty: a meta-analysis of randomized controlled trials. J Bone Jt Surg. 2015;97:1012–1021. doi:10.2106/JBJS.N.00315
17. Liu Y, Meng F, Yang G, et al. Comparison of intra-articular versus intravenous application of tranexamic acid in total knee arthroplasty: a meta-analysis of randomized controlled trials. Arch Med Sci. 2017;3:533–540. doi:10.5114/aoms.2017.67278
18. Xie J, Hu Q, Huang Q, et al. Comparison of intravenous versus topical tranexamic acid in primary total hip and knee arthroplasty: an updated meta-analysis. Thromb Res. 2017;153:28–36. doi:10.1016/j.thromres.2017.03.009
19. Jeon YS, Park JS, Kim MK. Optimal release timing of temporary drain clamping after total knee arthroplasty. J Orthop Surg Res. 2017;12:47. doi:10.1186/s13018-017-0550-y
20. Montroy J, Hutton B, Moodley P, et al. The efficacy and safety of topical tranexamic acid: a systematic review and meta-analysis. Transfus Med Rev. 2018;32:165–178. doi:10.1016/j.tmrv.2018.02.003
21. Wang H, Shen B, Zeng Y. Comparison of topical versus intravenous tranexamic acid in primary total knee arthroplasty: a meta-analysis of randomized controlled and prospective cohort trials. Knee. 2014;21:987–993. doi:10.1016/j.knee.2014.09.010
22. Sabbag OD, Abdel MP, Amundson AW, et al. Tranexamic acid was safe in arthroplasty patients with a history of venous thromboembolism: a matched outcome study. J Arthroplasty. 2017;32:S246–50. doi:10.1016/j.arth.2017.02.008
23. Duncan CM, Gillette BP, Jacob AK, et al. Venous thromboembolism and mortality associated with tranexamic acid use during total hip and knee arthroplasty. J Arthroplasty. 2015;30:272–276. doi:10.1016/j.arth.2014.08.022
24. Fillingham YA, Ramkumar DB, Jevsevar DS, et al. The safety of tranexamic acid in total joint arthroplasty: a direct meta-analysis. J Arthroplasty. 2018;33:3070–82.e1. doi:10.1016/j.arth.2018.03.031
25. Li Z-J, Fu X, Xing D, et al. Is tranexamic acid effective and safe in spinal surgery? A meta-analysis of randomized controlled trials. Eur Spine J. 2013;22:1950–1957. doi:10.1007/s00586-013-2774-9
26. Lecker I, Wang D, Whissell PD, et al. Tranexamic acid–associated seizures: causes and treatment. Ann Neurol. 2016;79:18–26. doi:10.1002/ana.24558
27. Alshryda S, Sukeik M, Sarda P, et al. A systematic review and meta-analysis of the topical administration of tranexamic acid in total hip and knee replacement. Bone Joint J. 2014;96-B:1005–1015. doi:10.1302/0301-620X.96B8.33745
28. Jans Ø, Nielsen CS, Khan N, et al. Iron deficiency and preoperative anaemia in patients scheduled for elective hip- and knee arthroplasty - an observational study. Vox Sang. 2018;113:260–267. doi:10.1111/vox.12630
29. Lloyd TD, Neal-Smith G, Fennelly J, et al. Peri-operative administration of tranexamic acid in lower limb arthroplasty: a multicentre, prospective cohort study. Anaesthesia. 2020;75:1050–1058. doi:10.1111/anae.15056
30. Muñoz M, Acheson AG, Auerbach M, et al. International consensus statement on the peri-operative management of anaemia and iron deficiency. Anaesthesia. 2017;72:233–247. doi:10.1111/anae.13773
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
